Abstract
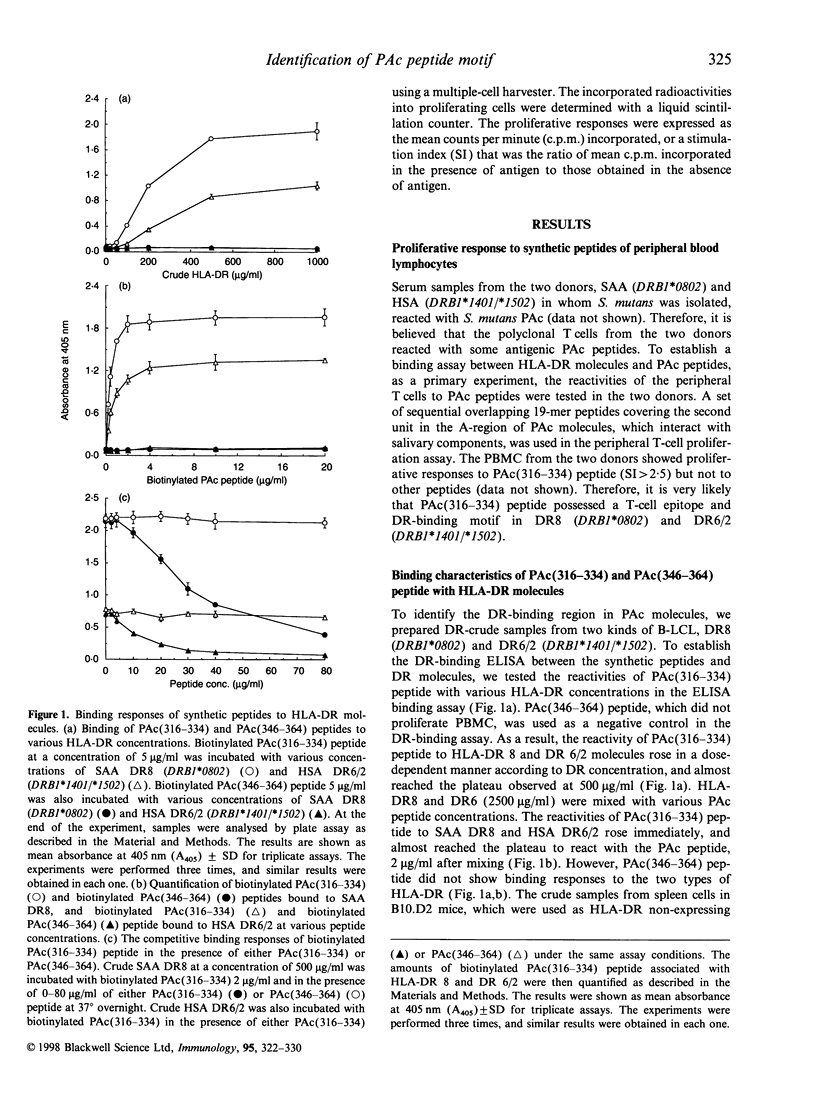
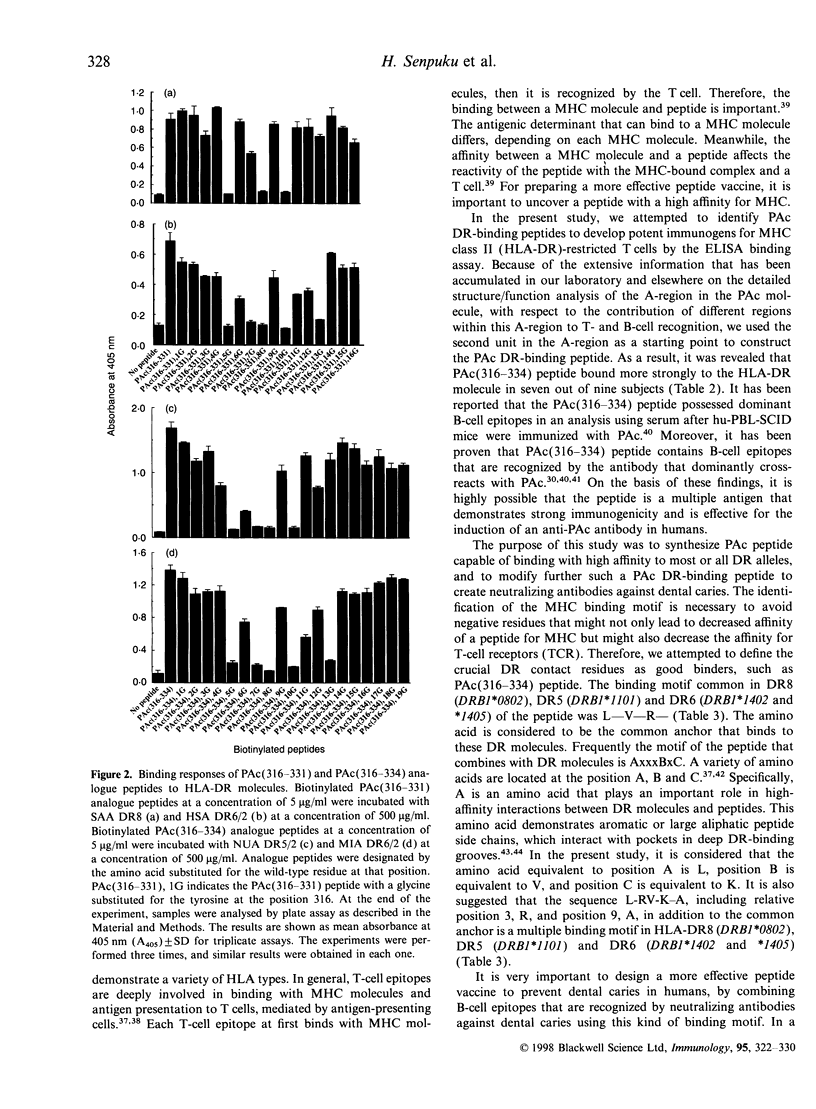
A surface protein antigen (PAc) of Streptococcus mutans, in particular the A-region of this PAc molecule, has been noted as a possible target in research for an effective dental caries vaccine. To identify the antigenic peptide binding to major histocompatibility complex (MHC) class II (HLA-DR) molecules in the A-region, we prepared a panel of overlapping synthetic peptides in the second unit of the A-region, and established that a simple enzyme-linked immunosorbent assay (ELISA) binding assay could be achieved by incubating the DR-crude. Binding to DR molecules of these peptides from nine donors was investigated by using the ELISA binding assay. It was revealed that the PAc(316-334) peptide bound more strongly to the HLA-DR molecule in seven out of nine subjects. In particular, DR8 (DRB1*0802), DR5 (DRB1*1101) and DR6 (DRB1*1402 and *1405), which bound strongly to PAc(316-334) peptide, were identified. Moreover, we synthesized glycine-substituted peptide analogues of the peptide and examined the binding motif of the binding region. As a result, the multiple binding motif in DR8, DR5 and DR6 was found in L-RV-K-A. It is suggested that a peptide vaccine for dental caries that is more effective for humans, with fewer adverse side-effects, could be designed by combining the multiple binding motif with the B-cell epitope to produce only the inhibiting antibody against dental caries. The peptide could therefore be useful for peptide vaccine development in the general human population.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlers J. D., Pendleton C. D., Dunlop N., Minassian A., Nara P. L., Berzofsky J. A. Construction of an HIV-1 peptide vaccine containing a multideterminant helper peptide linked to a V3 loop peptide 18 inducing strong neutralizing antibody responses in mice of multiple MHC haplotypes after two immunizations. J Immunol. 1993 Jun 15;150(12):5647–5665. [PubMed] [Google Scholar]
- Avva R. R., Cresswell P. In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity. 1994 Dec;1(9):763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- Baba E., Nakamura M., Ohkuma K., Kira J., Tanaka Y., Nakano S., Niho Y. A peptide-based human T cell leukemia virus type I vaccine containing T and B cell epitopes that induces high titers of neutralizing antibodies. J Immunol. 1995 Jan 1;154(1):399–412. [PubMed] [Google Scholar]
- Berkower I., Matis L. A., Buckenmeyer G. K., Gurd F. R., Longo D. L., Berzofsky J. A. Identification of distinct predominant epitopes recognized by myoglobin-specific T cells under the control of different Ir genes and characterization of representative T cell clones. J Immunol. 1984 Mar;132(3):1370–1378. [PubMed] [Google Scholar]
- Berzofsky J. A., Pendleton C. D., Clerici M., Ahlers J., Lucey D. R., Putney S. D., Shearer G. M. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and humans of multiple MHC types. J Clin Invest. 1991 Sep;88(3):876–884. doi: 10.1172/JCI115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. G., McLachlan S. M., Britton S. Cyclosporin A promotes spontaneous outgrowth in vitro of Epstein-Barr virus-induced B-cell lines. Nature. 1981 Jan 22;289(5795):300–301. doi: 10.1038/289300a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Demuth D. R., Golub E. E., Malamud D. Streptococcal-host interactions. Structural and functional analysis of a Streptococcus sanguis receptor for a human salivary glycoprotein. J Biol Chem. 1990 May 5;265(13):7120–7126. [PubMed] [Google Scholar]
- Demuth D. R., Lammey M. S., Huck M., Lally E. T., Malamud D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb Pathog. 1990 Sep;9(3):199–211. doi: 10.1016/0882-4010(90)90022-i. [DOI] [PubMed] [Google Scholar]
- Finnegan A., Smith M. A., Smith J. A., Berzofsky J., Sachs D. H., Hodes R. J. The T cell repertoire for recognition of a phylogenetically distant protein antigen. Peptide specificity and MHC restriction of staphylococcal nuclease-specific T cell clones. J Exp Med. 1986 Sep 1;164(3):897–910. doi: 10.1084/jem.164.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Hammer J., Valsasnini P., Tolba K., Bolin D., Higelin J., Takacs B., Sinigaglia F. Promiscuous and allele-specific anchors in HLA-DR-binding peptides. Cell. 1993 Jul 16;74(1):197–203. doi: 10.1016/0092-8674(93)90306-b. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Ogasawara K., Takami K., Gotohda T., Naruse H., Good R. A., Onoé K. Determination of amino acids on agretopes of pigeon cytochrome c-related peptides specifically bound to I-A allelic products. Eur J Immunol. 1994 Jan;24(1):76–83. doi: 10.1002/eji.1830240113. [DOI] [PubMed] [Google Scholar]
- Kelly C. G., Todryk S., Kendal H. L., Munro G. H., Lehner T. T-cell, adhesion, and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect Immun. 1995 Sep;63(9):3649–3658. doi: 10.1128/iai.63.9.3649-3658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985 Dec;50(3):796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Hunjan M., Smith R., Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol. 1989 Sep;77(3):331–337. [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Nisizawa T., Nagaoka S., Kawagoe M., Koga T. Identification of antigenic epitopes in a surface protein antigen of Streptococcus mutans in humans. Infect Immun. 1994 Sep;62(9):4034–4042. doi: 10.1128/iai.62.9.4034-4042.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor D. L., Jr, Kim P. S. Context-dependent secondary structure formation of a designed protein sequence. Nature. 1996 Apr 25;380(6576):730–734. doi: 10.1038/380730a0. [DOI] [PubMed] [Google Scholar]
- Moisset A., Schatz N., Lepoivre Y., Amadio S., Wachsmann D., Schöller M., Klein J. P. Conservation of salivary glycoprotein-interacting and human immunoglobulin G-cross-reactive domains of antigen I/II in oral streptococci. Infect Immun. 1994 Jan;62(1):184–193. doi: 10.1128/iai.62.1.184-193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara K., Maloy W. L., Beverly B., Schwartz R. H. Functional analysis of the antigenic structure of a minor T cell determinant from pigeon cytochrome C. Evidence against an alpha-helical conformation. J Immunol. 1989 Mar 1;142(5):1448–1456. [PubMed] [Google Scholar]
- Ogasawara K., Maloy W. L., Schwartz R. H. Failure to find holes in the T-cell repertoire. 1987 Jan 29-Feb 4Nature. 325(6103):450–452. doi: 10.1038/325450a0. [DOI] [PubMed] [Google Scholar]
- Ogasawara K., Naruse H., Itoh Y., Gotohda T., Arikawa J., Kida H., Good R. A., Onoé K. A strategy for making synthetic peptide vaccines. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8995–8999. doi: 10.1073/pnas.89.19.8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Takahashi I., Nakai M., Senpuku H., Nisizawa T., Koga T. Identification of antigenic epitopes in an alanine-rich repeating region of a surface protein antigen of Streptococcus mutants. Infect Immun. 1993 Apr;61(4):1301–1306. doi: 10.1128/iai.61.4.1301-1306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reay P. A., Wettstein D. A., Davis M. M. pH dependence and exchange of high and low responder peptides binding to a class II MHC molecule. EMBO J. 1992 Aug;11(8):2829–2839. doi: 10.1002/j.1460-2075.1992.tb05350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989 Jun;4(2):106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- Schaeffer E. B., Sette A., Johnson D. L., Bekoff M. C., Smith J. A., Grey H. M., Buus S. Relative contribution of "determinant selection" and "holes in the T-cell repertoire" to T-cell responses. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4649–4653. doi: 10.1073/pnas.86.12.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Senpuku H., Iizima T., Yamaguchi Y., Nagata S., Ueno Y., Saito M., Hanada N., Nisizawa T. Immunogenicity of peptides coupled with multiple T-cell epitopes of a surface protein antigen of Streptococcus mutans. Immunology. 1996 Jun;88(2):275–283. doi: 10.1111/j.1365-2567.1996.tb00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senpuku H., Kato H., Takeuchi H., Noda A., Nisizawa T. Identification of core B cell epitope in the synthetic peptide inducing cross-inhibiting antibodies to a surface protein antigen of Streptococcus mutans. Immunol Invest. 1997 Aug-Dec;26(5-7):531–548. doi: 10.3109/08820139709088538. [DOI] [PubMed] [Google Scholar]
- Senpuku H., Kato H., Todoroki M., Hanada N., Nisizawa T. Interaction of lysozyme with a surface protein antigen of Streptococcus mutans. FEMS Microbiol Lett. 1996 Jun 1;139(2-3):195–201. doi: 10.1111/j.1574-6968.1996.tb08202.x. [DOI] [PubMed] [Google Scholar]
- Senpuku H., Miyauchi T., Hanada N., Nisizawa T. An antigenic peptide inducing cross-reacting antibodies inhibiting the interaction of Streptococcus mutans PAc with human salivary components. Infect Immun. 1995 Dec;63(12):4695–4703. doi: 10.1128/iai.63.12.4695-4703.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senpuku H., Nakai M., Koga T., Hanada N., Nisizawa T. Identification of a repeated epitope recognized by human serum antibodies in a surface protein antigen of Streptococcus mutans. Oral Microbiol Immunol. 1996 Apr;11(2):121–128. doi: 10.1111/j.1399-302x.1996.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Sherman M. A., Weber D. A., Jensen P. E. DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity. 1995 Aug;3(2):197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Matsushita K., Nisizawa T., Okahashi N., Russell M. W., Suzuki Y., Munekata E., Koga T. Genetic control of immune responses in mice to synthetic peptides of a Streptococcus mutans surface protein antigen. Infect Immun. 1992 Feb;60(2):623–629. doi: 10.1128/iai.60.2.623-629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Matsushita K., Tokuda M., Kanamoto T., Munekata E., Russell M. W., Koga T. Immunogenicity and protective effect against oral colonization by Streptococcus mutans of synthetic peptides of a streptococcal surface protein antigen. J Immunol. 1991 Jan 1;146(1):332–336. [PubMed] [Google Scholar]
- Turck C. W. Identification of phosphotyrosine residues in peptides by high performance liquid chromatography on-line derivative spectroscopy. Pept Res. 1992 May-Jun;5(3):156–160. [PubMed] [Google Scholar]


