Abstract
B19 virus is a human virus belonging to the genus Erythrovirus. The genetic diversity among B19 virus isolates has been reported to be very low, with less than 2% nucleotide divergence in the whole genome sequence. We have previously reported the isolation of a human erythrovirus isolate, termed V9, whose sequence was markedly distinct (>11% nucleotide divergence) from that of B19 virus. To date, the V9 isolate remains the unique representative of a new variant in the genus Erythrovirus, and its taxonomic position is unclear. We report here the isolation of 11 V9-related viruses. A prospective study conducted in France between 1999 and 2001 indicates that V9-related viruses actually circulate at a significant frequency (11.4%) along with B19 viruses. Analysis of the nearly full-length genome sequence of one V9-related isolate (D91.1) indicates that the D91.1 sequence clusters together with but is notably distant from the V9 sequence (5.3% divergence) and is distantly related to B19 virus sequences (13.8 to 14.2% divergence). Additional phylogenetic analysis of partial sequences from the V9-related isolates combined with erythrovirus sequences available in GenBank indicates that the erythrovirus group is more diverse than thought previously and can be divided into three well-individualized genotypes, with B19 viruses corresponding to genotype 1 and V9-related viruses being distributed into genotypes 2 and 3.
B19 virus is a small, nonenveloped human virus that belongs to the genus Erythrovirus of the family Parvoviridae (50). B19 virus infection in humans is associated with a wide range of clinical manifestations (see reference 7 for a review). B19 virus is most commonly responsible for mild disease, including erythema infectiosum (fifth disease) in childhood (2) and arthropathy in young women (51). In addition B19 virus can cause more severe disease, such as transient aplastic crisis in patients suffering from chronic hemolytic disorders (38) and fetal infection during pregnancy that can lead to spontaneous abortion, fetal hydrops, or fetal death (9). Acute B19 virus infection is thought to confer a protective, lifelong immunity (27). However, persistent B19 virus infection has been reported to occur not only in immunocompromised patients, who can develop chronic anemia and thrombopenia (29), but also in immunocompetent individuals, who can develop chronic arthropathy (14, 35).
The genome of the B19 virus consists of a single-stranded DNA of 5,596 nucleotides with palindromic inverted terminal repeats at both ends that form hairpin structures (4, 10). A single functionally active promoter, termed p6, is located at the 5′ end and regulates all the viral transcripts (6, 11, 39). The genome contains two main open reading frames (ORFs), one encoding the nonstructural protein NS1, which is involved in viral DNA replication and transcription, and the other encoding both the major VP2 (554 amino acids [aa]) and the minor VP1 (781 aa) structural capsid proteins, with VP1 consisting of a unique sequence of 227 aa (VP1u) followed by the entire sequence of VP2 (4, 42). Overlapping the main ORFs, two additional ORFs encode two small proteins of 7.5 kDa (32) and 11 kDa (43) whose functions are unknown.
The genetic diversity among B19 virus isolates has been reported to be very low, with less than 1 to 2% nucleotide divergence in the whole genome, although full-length sequences are available only for a limited number of isolates (24, 25, 31, 42). Partial sequence data from different coding regions of the viral genome have confirmed this high degree of similarity with a larger number of isolates (12, 15, 23, 25, 48). For instance, sequence variation of the VP1/VP2 gene has been reported to be very low among B19 virus isolates obtained from a single community-wide outbreak (0 to 0.6% base substitutions) and only slightly greater among B19 virus isolates obtained from distinct epidemiological settings and geographical area, ranging between 0.5 and 4.8% for the most distant isolates (12). B19 virus genomes recovered from synovial tissue during persistent infection have also been reported to be very similar to those recovered from the same tissue during acute infection and to those recovered from blood or bone marrow (25). However, some isolates obtained from patients with persistent B19 virus infection have been reported to exhibit a higher degree of variability in some parts of the genome, with the VP1 unique region being the most variable at both the DNA and protein levels with up to 4 and 8% divergence, respectively (23). Although different genome types have been described based on restriction analysis of the B19 virus genome (33, 34, 46, 47) sequence analysis has not allowed the identification of phylogenetic clusters with well-resolved nodes within the B19 viruses (25, 31).
In contrast with the high sequence homology observed among B19 virus isolates, we have previously reported the isolation, from a child with transient aplastic anemia, of a human erythrovirus isolate termed V9, whose VP1u sequence was markedly distinct (>11% nucleotide divergence) from that of B19 virus (36, 37). The almost-full-length sequence of the V9 genome was subsequently determined (5, 19), and the genetic variability was found to extend outside the VP1u region with more than 12% nucleotide divergence between the entire genomes of V9 and B19 virus isolates (19). With the exception of one erythrovirus isolate (R1) which we have previously found to be related to V9 according to sequence homology on 346 bp of the VP1u region (36), no other V9-related isolate has been reported to date; however, only a limited number of studies have been conducted to search for such isolates (19, 21, 26). Thus, since it is the unique representative of a new B19 virus variant, the taxonomic position of the V9 isolate remains unclear, even though Lukashov and Goudsmit (31) have suggested that, based on phylogenetic analysis, separation between B19 virus and V9 was probably an ancient event.
This study was conducted to evaluate the possible circulation, the relative frequency, and the clinical presentation of V9-related viruses in different groups of patients and to specify the taxonomic grouping of these viruses. By using a consensus PCR assay designed for detection of and discrimination between B19 virus and V9 DNAs, 11 V9-related viruses were isolated. Phylogenetic analysis of full-length and partial sequences from these isolates combined with erythrovirus sequences available in GenBank indicated that the human erythrovirus group was actually more diverse than thought previously and could be divided into three well-individualized genotypes.
MATERIALS AND METHODS
Clinical samples.
The study included various clinical samples obtained from different sources. The samples were classified into five panels (A to E) according to their origin. Group A (n = 21) comprised sera collected in 1992 to 1997 (Hôpital Rothschild, Paris, France) from human immunodeficiency virus (HIV)-infected adult patients with chronic anemia lasting for more than 3 months. Group B (n = 73) comprised amniotic fluid or fetal blood samples collected in 1995 to 1997 (Hôpital St Vincent de Paul, Paris, France) from pregnant women with fetal hydrops. Group C (n = 270) included sera provided by the Centers for Disease Control and Prevention (Atlanta, Ga.) and collected from patients with B19 virus-related symptoms (erythema infectiosum, aplastic crisis, arthropathy, or fetal hydrops) or from pregnant women exposed to B19 virus. Group D (n = 87) included sera collected in 1972 to 1999 and previously found to be positive for B19 virus antigen by counterimmunoelectrophoresis. These samples were provided by the Institut National de Transfusion Sanguine (Paris, France). Group E (n = 633) included clinical samples (serum, whole blood, bone marrow, or amniotic fluid samples) collected from March 1999 to September 2001 and sent to the virology laboratory at Hôpital Trousseau (Paris, France) for B19 virus diagnostic laboratory testing. Clinical samples were screened for the presence of erythrovirus DNA by PCR either retrospectively (groups A to D) or prospectively (group E).
DNA was extracted from clinical samples (except for serum samples) by a standard phenol-chloroform procedure. DNA extraction from serum was performed with the QIAamp DNA minikit (Qiagen, Courtaboeuf, France) according to the manufacturer's instructions. All of the DNA extracts were stored at −80°C prior to PCR testing.
B19 virus serological assays.
Testing for B19 virus-specific antibodies was performed with a commercial assay (parvovirus B19 immunoglobulin G [IgG] or IgM enzyme immunoassay [third generation]; Biotrin, Dublin, Ireland) according to the manufacturer's recommendations.
PCR procedures. (i) Consensus PCR for erythrovirus DNA detection.
B19 virus sequences in GenBank were aligned with the V9 sequence (5) in order to define consensus primer pairs located in conserved regions and surrounding divergent regions of the viral genome. The consensus primers shown in Table 1 allowed amplification of B19 and V9 virus DNAs and discrimination between the B19 and V9 viruses by restriction length polymorphism analysis of the PCR product.
TABLE 1.
Primers used in PCRs
| Gene | Primer namea | Nucleotide sequence (5′-3′) | Locationb (nucleotides) |
|---|---|---|---|
| NS1 | e1905f | TGCAGATGCCCTCCACCCA | 1905-1923 |
| e1987r | GCTGCTTTCACTGAGTTCTTC | 1987-2007 | |
| VP1u | e2717f | CATGCCTTATCATCCAGTA | 2717-2735 |
| e2901r | TTGGCTATACCTAAAGTCAT | 2901-2920 | |
| NS1/VP1u | e1855f | CACTATGAAAACTGGGCAA | 1855-1873 |
| e2960r | ACAATTCTTCATCTGCTAC | 2960-2978 | |
| e1863f | AAACTGGGCAATAAACTACAC | 1863-1883 | |
| e2953r | CTTCATCTGCTACCGTCCAA | 2953-2972 | |
| Noncoding region | e0001f | CCGGAATTCGACGTCACAGGAAATGACGc | 1-19 |
| e5011r | CCGGAATTCGACGTCACAGGAAATGACc | 5011-5028 |
Screening of clinical samples for the presence of erythrovirus DNA was performed with a consensus PCR assay using primers e1905f and e1987r located in the NS1 gene (NS1-PCR). Five microliters of DNA was added to a reaction mixture (in a final volume of 50 μl) containing 15 mM Tris-HCl (pH 8), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 12.5 pmol of each primer, and 1.5 U of AmplitaqGold (Applied Biosystems, Villebon, France). Hot-start amplification was performed on a Gene Amp PCR System 9700 (Applied Biosystems) under the following conditions: 1 cycle of 94°C for 6 min; 5 cycles of 94°C for 30 s, 55°C for 1 min, and 72°C for 1 min; 45 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s; and a final elongation step at 72°C for 7 min. The PCR products were analyzed by electrophoresis in 2% agarose gels. To confirm the positive results found by the NS1-PCR, a second consensus PCR assay using primers e2717f and e2901r located in the VP1 unique region (VP1u-PCR) was developed. The VP1u-PCR was performed under the same conditions as the NS1-PCR except that 2 mM MgCl2 was used and amplification consisted of 1 cycle of 94°C for 6 min; 5 cycles of 94°C for 30 s, 45°C for 30 s, and 72°C for 30 s; 45 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and a final elongation step at 72°C for 7 min.
Discrimination between the B19 and V9 viruses was done by MfeI and ApaI restriction of the NS1-PCR and VP1u-PCR products, respectively. Five microliters of the PCR products was digested for 2 h with 10 U of MfeI or ApaI (New England Biolabs, Ozyme, St. Quentin en Yvelines, France), respectively, at 37 or 25°C and was analyzed by electrophoresis in 2.5% agarose gels. MfeI restriction of the NS1-PCR product gave two fragments of 36 and 67 bp for B19 virus and a 103-bp uncleaved fragment for V9. Similarly, ApaI restriction of the VP1u-PCR product gave two fragments of 149 and 55 bp for B19 virus and a 204-bp uncleaved fragment for V9.
Each PCR and restriction fragment length polymorphism experiment included negative controls (H2O and cellular DNA from human fibroblasts) and positive controls. A serum sample containing a B19 virus isolate, as confirmed by DNA sequencing of 1,100 bp, was used as the B19 virus positive control. A V9 plasmid (5) was used as the V9 positive control. The V9 plasmid consisted of the entire V9 genome except the 3′- and 5′-terminal hairpins (5,028 bp) cloned into the AatII site of a modified version of pcDNAII (Invitrogen, Groningen, The Netherlands).
(ii) Nested PCR for DNA sequencing.
Viral DNA (B19 or V9) for sequencing was prepared by nested-PCR amplification of a 1,100-bp region spanning the NS1-VP1u junction with the primers described in Table 1. The primary PCR round was performed in a 100-μl reaction mixture containing 10 μl of DNA, 15 mM Tris-HCl (pH 8), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 25 pmol of each primer (e1855f and e2960r), and 3 U of AmplitaqGold (Applied Biosystems) The reaction was performed with 1 cycle at 94°C for 6 min; 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min; and 1 cycle at 72°C for 7 min. The primary PCR product (2 μl) was then amplified with primers e1863f and e2953r under the same conditions except that 3 mM MgCl2 and 50 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 50 s were used. The PCR products were analyzed by electrophoresis in 1% agarose gels and were subsequently purified from the gel slice with the QIAquick gel extraction and QIAquick PCR purification kits (Qiagen) prior to sequencing.
Cloning of the D91.1 genome.
Erythrovirus isolate D91.1 was detected in a panel D serum sample collected from a child with aplastic crisis. This sample was positive by the NS1-PCR assay, and the amplicon had a V9 type according to the MfeI restriction profile. DNA of the D91.1 isolate was extracted with the QIAamp DNA minikit (Qiagen) from 0.2 ml of serum. The DNA was then amplified by PCR with the Expand High Fidelity PCR system (Roche Molecular Biochemicals, Meylan, France) and the primer pair e0001f-e5011r (Table 1) to generate a 5,029-bp product containing the 3′ end of the p6 promoter and the entire coding sequence (nucleotides 329 to 4887). The 5,029-bp PCR product was cloned into the EcoRI site of the pcDNA3.1 HisB plasmid (Invitrogen), and the recombinant plasmid was propagated in Escherichia coli TOP10F′ (Invitrogen). Three independent bacterial clones containing inserts originating from three independent PCRs were selected for sequencing.
DNA sequencing.
Sequencing was carried out by the dideoxynucleotide chain termination method on an ABI Prism 377 automatic sequencer (Applied Biosystems), using the ABI PRISM DyeTerminator cycle sequencing Ready Reaction kit. Nucleotide sequences were determined on both strands, either from two independent PCR products for the NS1-VP1u partial sequence or from plasmid DNAs of two independent clones for the D91.1 genome. Plasmid DNA from a third clone was partially sequenced to eliminate three sequence ambiguities between the first two clones. As a control, the NS1-VP1u 994-bp partial sequence of D91.1 was also determined directly from the PCR products and was found identical to the sequence obtained from cloned PCR products.
Sequence analysis and phylogenetic analysis.
The D91.1 sequence was used as the query sequence in Blast searches of the GenBank, EMBL, and DDBJ data banks (1). The sequences were aligned by using ClustalW software (45). Phylogenetic analysis was performed by using two methods. Distances between sequences were analyzed using the neighbor-joining algorithm in the Phylip package based on the Kimura 2 parameter distance estimation method for nucleotides and the Dayhoff PAM matrix for amino acids (13). The second method was maximum parsimony with heuristic or branch-and-bound search using Paup 4.0b6 (44). Bootstrap resampling (1,000 replicates) was performed for analysis of full-length sequences and of NS1-VP1u sequences. For the NS1, VP1, VP2, and VP1u coding regions, nonsynonymous nucleotide substitutions (resulting in amino acid changes) and synonymous substitutions (not resulting in amino acid changes) were analyzed by using the MEGA 2.1 software (28). The Nei-Gojobori method was used to estimate synonymous nucleotide distances (Ds) and nonsynonymous nucleotide distances (Dns).
Nucleotide sequence accession numbers.
The nucleotide sequences of erythrovirus isolates D91.1, CDC105, CDC156, A95.1, E99.2, E99.3, E99.4, E00.2, and E01.1 are available under GenBank accession no. AY083234 through AY083242.
RESULTS
V9-related viruses circulate along with B19 viruses.
To evaluate the possible circulation of V9-related strains and their relative frequency with respect to B19 virus strains, we developed two consensus erythrovirus PCR assays (NS1-PCR and VP1u-PCR) designed to detect B19 and V9 virus DNAs and to discriminate between both viruses. These assays were first evaluated with serial dilutions of DNA extracted from a B19 virus-positive serum sample and from the V9 plasmid containing 5,028 bp of the V9 genome. The NS1-PCR assay was able to detect B19 virus DNA from the 10−9-fold serum dilution and V9 plasmid DNA diluted to 10−11 (approximately one plasmid copy per PCR) with 100% sensitivity. Based on the results obtained with the V9 plasmid, the assay sensitivity threshold was considered to be 200 copies of erythrovirus DNA per ml of sample. No amplification was observed when the specificity of the primers for human fibroblast DNA was tested. MfeI restriction of the 103-bp NS1-PCR product allowed a clear distinction between B19 virus DNA (resulting in two fragments of 36 and 67 bp) and V9 DNA (no cleavage). Similar results in terms of sensitivity, specificity, and strain distinction were obtained with the VP1u-PCR assay (data not shown).
The NS1-PCR assay was subsequently used to screen clinical samples for the presence and typing of erythrovirus DNA, either retrospectively for panels A to D or prospectively for panel E (Table 2). Samples from panels A and C were also tested by the VP1u-PCR assay and gave identical results. In all, 396 out 1,084 samples were positive for erythrovirus DNA detection, among which 385 (97.2%) contained a B19-type virus and 11 (2.7%) contained a V9-type virus. The relative frequencies of the B19- and V9-type viruses were unequally distributed between the different panels. In panel C (U.S. population), all of the 204 detected viruses had a B19 type, while in the four other panels (French population), 11 (5.7%) of the 192 detected viruses had a V9 type (chi-square test, P = 0.0005). This result indicated that V9-type viruses, if any, were infrequent among the U.S. population, as their relative frequency with respect to B19 viruses could not have exceeded 0.5%. In panel E, which was prospectively tested for the presence of erythrovirus DNA, the relative frequency of V9-type viruses (9 out of 79 strains; 11.4%) was much higher than that in panel D (1 out of 87 strains; 1.1%) (P = 0.005). However, as samples from panel D were collected over a long time period (1972 to 1999), only a few samples (median, 3; range, 1 to 13) from each year's epidemics were analyzed. This might explain the low rate of detection of V9 viruses in panel D, considering the 11.4% relative frequency of these viruses in panel E. To determine whether the B19-V9 virus distribution could be different in specific clinical groups, samples from HIV-positive patients with chronic anemia (panel A) and samples from pregnant women with fetal hydrops (panel B) were evaluated. The single erythrovirus strain found in panel A had a V9 type, and all of the 16 strains in panel B had a B19 virus type. However, due to the small number of strains, the B19-V9 virus distribution in these groups was not significantly different from that observed in panel E (P > 0.1). Panel B included samples that had previously tested B19 virus positive (n = 15) and samples that had previously tested B19 virus negative (n = 58) by a standard B19 virus PCR assay that might have missed the V9-type viruses. When tested with the NS1-PCR assay, a B19-type virus was detected in all B19 virus-positive samples and additionally in one B19 virus-negative sample, but no V9-type virus was identified, indicating that V9 virus was not an underestimated cause of fetal hydrops in this panel.
TABLE 2.
Results of the erythrovirus consensus PCR assay
| Panela | Total | No. of samples
|
||
|---|---|---|---|---|
| PCR positive | B19 virus profile | V9 virus profile | ||
| A (HIV patients, France) | 21 | 1 | 0 | 1 |
| B (Fetal hydrops, France) | 73 | 16 | 16 | 0 |
| C (B19 virus-related symptoms, USA) | 270 | 204 | 204 | 0 |
| D (B19 virus-antigen positive, France) | 87 | 87 | 86 | 1 |
| E (Prospective study, France) | 633 | 88 | 79 | 9 |
| Total | 1,084 | 396 | 385 | 11 |
See Materials and Methods for details about panels.
The main characteristics of the 11 patients with a V9 infection are shown in Table 3. V9 viruses were detected since 1991, and in the prospective study their detection rates were similar during the years 1999 and 2000. V9 viruses were isolated from two children and nine adults, including six immunocompromised patients. In panel E, patients infected with a V9 virus were older than those infected with a B19 virus (median ages of 38 and 17 years, respectively; Mann-Whitney U test, P = 0.02), and five out of nine V9-infected patients were immunocompromised. B19 virus serological testing was available for 9 out of 11 patients with V9 infection: 4 patients had B19 virus-specific IgM, which is typical of acute B19 virus infection; 3 immunocompromised patients had IgG but no IgM, which is compatible with a chronic infection; and 2 patients had no detectable B19 virus antibodies. For these last two cases, one could be related to an impaired immune response in an HIV patient and the other could be related to a serum sampling that was too early to detect B19 virus antibodies.
TABLE 3.
Clinical characteristics of patients infected with a V9-related virus
| Strain | Sample | Yr | Age (yr) of patient | Clinical signsa | B19 virus antibodies
|
|
|---|---|---|---|---|---|---|
| IgM | IgG | |||||
| A95.1 | Serum | 1995 | 48 | Chronic anemia, HIV positive | − | − |
| D91.1 | Serum | 1991 | 8 | Aplastic crisis, G6PD deficit, minor thalassemia | − | − |
| E99.1 | Blood | 1999 | 33 | Chronic anemia, HIV positive | − | + |
| E99.2 | Bone marrow | 1999 | 48 | Pancytopenia, HCV positive | NAb | NA |
| E99.3 | Serum | 1999 | 38 | Rash | + | + |
| E99.4 | Serum | 1999 | 5 | Anemia, immunocompromised | + | + |
| E00.1 | Bone marrow | 2000 | 79 | Aplastic crisis, Waldenstrom disease | NA | NA |
| E00.2 | Serum | 2000 | 28 | Pregnant woman, fever | + | + |
| E00.3 | Bone marrow | 2000 | 58 | Chronic anemia, HIV positive | − | + |
| E00.4 | Serum | 2000 | 38 | Anemia, immunocompromised | − | + |
| E01.1 | Serum | 2001 | 59 | Aplastic crisis, immunocompetent | + | − |
G6PD, glucose-6-phosphate dehydrogenase; HCV, hepatitis C virus.
NA, not available.
Taken together, these results indicated that V9-type viruses circulated among the French population, could be responsible for symptomatic infection that was clinically and serologically undistinguishable from B19 virus infection, and could represent up to 11.4% of the erythrovirus isolates. The detection of 11 V9-related strains provided the opportunity for evaluating the genetic diversity and the phylogenetic relationships within the Erythrovirus genus. For this purpose, we first attempted to determine the full-length genome sequence of the V9-related strain D91.1, for which a sufficient amount of material was available.
The erythrovirus strain D91.1 clusters with but is notably distant from V9.
The V9-related strain D91.1 was recovered from a serum sample collected during a transient aplastic crisis in an 8-year-old boy with a glucose-6-phosphate dehydrogenase defect and minor thalassemia. The almost-full-length genome of the D91.1 strain was cloned into the pcDNA3.1 HisB plasmid, and its 5,029-bp sequence, including the 3′ end of the p6 promoter and the complete coding sequence (nucleotides 329 to 4887), was further determined. The organization of the D91.1 genome was found to be identical to those of the B19 virus and V9 genomes, with the same number, ordering, and length of ORFs.
To determine the phylogenetic relationship of the D91.1 strain within the genus Erythrovirus, the D91.1 sequence was aligned with those of other erythroviruses for which nearly full-length genome sequences were available in GenBank. These sequences, which are listed in Table 4, included 12 distinct B19 virus sequences, an individual erythrovirus sequence (strain Lali) recently made available in GenBank (accession no AY044266; K. Hokynar et al., unpublished data), the V9 sequence, and 3 simian parvovirus sequences (rhesus macaque, pig-tailed macaque, and long-tailed macaque parvoviruses) which were recently reported to be phylogenetically related to the human erythroviruses (31). The chipmunk parvovirus sequence (52) was used as an outlier for the purpose of rooting the tree. Phylogenetic analyses were based on nucleotide distances and, for coding regions, on amino acid distances.
TABLE 4.
Erythrovirus sequences used in this study
| Strain | GenBank accession no. | Reference |
|---|---|---|
| Human erythroviruses | ||
| Pvbaua | M13178 | 42 |
| Pvb19nsvp | Z68146 | 24 |
| Pvb19x528 | Z70528 | 23 |
| Pvb19x560 | Z70560 | 23 |
| Pvb19x599 | Z70599 | 23 |
| Pvbpro | M24682 | 6 |
| HV | AF162273 | G. Gallinella et al., unpub.,a 1999 |
| N8 | AB030673 | K. Ishii et al., unpub., 1999 |
| MI | AB030693 | Ishii et al., unpub., 1999 |
| RM | AB030694 | Ishii et al., unpub., 1999 |
| AF113323 | AF113323 | 22 |
| AY028237 | AY028237 | T. Tolfvenstam et al., unpub., 2001 |
| EVZ95625 | Z95625 | 16 |
| EVZ95627 | Z95627 | 16 |
| EVZ95631 | Z95631 | 16 |
| EVZ95632 | Z95632 | 16 |
| EVZ95633 | Z95633 | 16 |
| R43 | AJ249435 | Q. T. Nguyen, unpub., 2001 |
| R227 | AJ249432 | Nguyen, unpub., 2001 |
| R308 | AJ249433 | Nguyen, unpub., 2001 |
| V9 | AX003421 | 5 |
| R1 | AJ249430 | Nguyen, unpub., 2001 |
| R225 | AJ249431 | Nguyen, unpub., 2001 |
| R322 | AJ249434 | Nguyen, unpub., 2001 |
| Lali | AY044266 | K. Hokynar et al., unpub., 2001 |
| HaAM | AY044268 | Hokynar et al., unpub., 2001 |
| CDC105 | AY083235 | This study |
| CDC156 | AY083236 | This study |
| A95.1 | AY083237 | This study |
| D91.1 | AY083234 | This study |
| E99.2 | AY083238 | This study |
| E99.3 | AY083239 | This study |
| E99.4 | AY083240 | This study |
| E00.2 | AY083241 | This study |
| E01.1 | AY083242 | This study |
| Animal parvovirusesb | ||
| RMPV | AF221122 | 18 |
| PTMPV | AF221123 | 18 |
| LTMPV | U26342 | 8 |
| ChPV | U86868 | 52 |
unpub., unpublished data.
RMPV, PTMPV, and LTMPV: rhesus, pig-tailed, and long-tailed macaque parvoviruses, respectively; ChPV, chipmunk parvovirus.
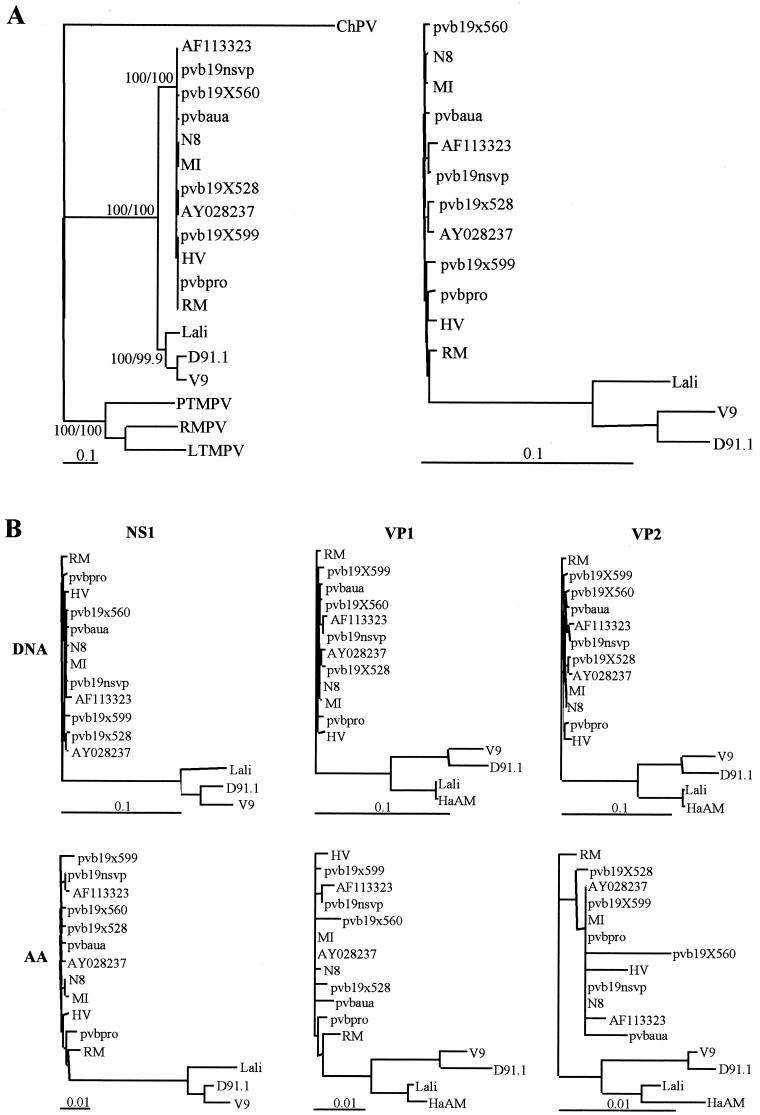
The phylogenetic tree (Fig. 1A ) based on a sequence analysis of 4,339 bp (corresponding to nucleotides 425 to 4763 of the B19 Pvbaua sequence) showed that the D91.1 strain clustered together with V9 virus and was as distantly related to B19 viruses (13.8 to 14.2% divergence) as was the V9 virus (13.7 to 14.2% divergence). Interestingly, D91.1 was more distant from V9 (5.3% divergence) than B19 virus sequences were divergent among themselves (0.2 to 1.1% divergence), with the exception of the Lali strain, which was clearly distinct from both the B19 virus cluster (11.6 to 12% divergence) and the V9-D91.1 cluster (9.2 to 9.4% divergence). Compared to simian parvovirus sequences, D91.1 and V9 sequences clustered separately from these sequences (>62% divergence) and together with Lali and B19 virus sequences. Although the nucleotide or amino acid distances between erythrovirus sequences could vary according to the genome region (Table 5), phylogenetic trees based on nucleotide and amino acid distances of the NS1, VP1, and VP2 genes had topologies similar to those observed with full-length sequences (Fig. 1B). In all cases D91.1 clustered with but was notably distant from V9.
FIG.1.
Phylogenetic relationships among erythroviruses. (A) Phylogenetic relationships based on full-length genome sequences (4,339 bp). Phylogenetic analysis was performed by using the neighbor-joining algorithm based on the Kimura two-parameter distance estimation method (similar results were obtained by using the maximum-parsimony method). Significant bootstrap values (in percentages) are indicated for 103 neighbor-joining/103 maximum-parsimony replicates. In the left panel simian parvovirus sequences (rhesus macaque parvovirus [RMPV], pig-tailed macaque parvovirus [RTMPV], and long-tailed macaque parvovirus [LTMPV]) are included and the chipmunk parvovirus (ChPV) sequence is used as an outlier. The right panel shows the nucleotide distances between human erythroviruses only. (B) Phylogenetic relationships among human erythroviruses based on nucleotide (DNA) and amino acid (AA) distances for the NS1, VP1, and VP2 genes. Distances between sequences were analyzed by using the neighbor-joining algorithm in the Phylip package (based on the Kimura 2 parameter distance estimation method for nucleotides and Dayhoff PAM matrix for amino acids). The phylogenetic trees have topologies similar to those observed with full-length sequences.
TABLE 5.
Genetic distances between B19 virus, D91.1, V9, and Lali sequences
| Genome region | Location in B19 genomea (nucleotides) | % Divergence range (DNA/protein)
|
|||||
|---|---|---|---|---|---|---|---|
| B19 vs B19 | D91.1 vs B19 | V9 vs B19 | Lali vs B19 | D91.1 vs V9 | D91.1/V9 vs Lali | ||
| Allb | 425-4763 | 0.2-1.1/NAd | 13.8-14.2/NA | 13.7-14.2/NA | 11.6-12/NA | 5.3/NA | 9.2-9.4/NA |
| 3′ p6 | 144-434 | 0-1.7/NA | 19.8-21.3/NA | 23.3-25.5/NA | 23.5-25.7/NA | 9/NA | 12.4-12.6/NA |
| NS1 | 436-2451 | 0.2-1.1/0.1-1.1 | 13.9-14.2/5.1-5.6 | 14.8-15.1/5.6-6.2 | 14.1-14.5/5.9-6.4 | 4.6/1.1 | 7.5-8/2.6-2.9 |
| VP1c | 2444-4789 | 0.1-3.7/0-1.6 | 13.1-13.7/3.4-3.9 | 12.5-13.2/2.9-3.4 | 9.4-9.9/1.9-2.4 | 5.5/1.4 | 10.2-10.9/2.6-3.4 |
| VP2c | 3125-4789 | 0.1-1.3/0-1 | 14.4-15.2/1-2.3 | 13.7-14.6/1-2.1 | 11.5-12.2/1-2.1 | 6.2/0.3 | 10.8-11.7/1.2-1.9 |
| VP1u | 2444-3124 | 0.1-1.3/0-1.6 | 10.1-10.7/7.8-8.7 | 9.1-9.9/6.4-7.4 | 4.3-5.3/3.8-4.7 | 3.9/3.3 | 8.7-8.9/6.1-6.9 |
| 7.5-kDa protein | 1910-2128 | 0-0.9/0-1 | 3.2-3.7/6.9-8.4 | 3.7-4.2/8.4-9.8 | 5.2-5.7/9.7-11.2 | 0.4/1.3 | 2.3-2.7/2.6-3.9 |
| 11-kDa protein | 4710-4994 | 0.3-1.4/1-2.6 | 20.1-21/19.3-22.4 | 18.7-20.1/18.9-23.2 | NA | 4.7/5.5 | NA |
| X | 2694-2939 | 0-1.6/0-3.9 | 5.4-6.3/10.9-13.9 | 4.1-5/8.1-11 | 2.4-3.3/2.6-5.3 | 2/2.6 | 4.1-4.5/6.7-9.5 |
Nucleotide position refers to the Pvbaua sequence (GenBank accession no. M13178).
Restricted to the largest region available for all of the nearly complete genome sequences.
The last 25 nucleotides of the VP1/VP2 gene are not available for the Lali sequence.
NA, not applicable.
Regarding the two main viral ORFs, NS1 and VP1/VP2, nucleotide distances between D91.1 and B19 virus, V9, or Lali were markedly higher than amino acid distances (Table 5), indicating that most of nucleotide substitutions were synonymous. This was confirmed by analyzing the Ds and Dns with the MEGA software. The mean Ds between the D91.1 and B19 virus sequences were 0.473, 0.455, and 0.519, whereas the mean Dns were 0.028, 0.021, and 0.012, resulting in mean Ds/Dns ratios of 16.9, 21.6, and 43.2 for the NS1, VP1, and VP2 genes, respectively. Similar Ds/Dns ratios were observed for pairwise comparisons of these genes between Lali and B19 virus (Ds/Dns ratios of 14, 26.9, and 36, respectively) or between D91.1 and V9 (Ds/Dns ratios, 26, 26.1, and 48.4, respectively). Compared to other parts of the genome, the VP1 unique regions (VP1u) of D91.1, V9, and Lali had a higher degree of amino acid divergence while being less divergent at the nucleotide level. For instance, the mean Ds/Dns ratio for pairwise comparison of VP1u between D91.1 and B19 virus was 0.281/0.042 = 6.6. In VP1u, most of the amino acid substitutions between B19 virus and the variants were observed in the N-terminal part (aa 1 to 123). Conversely, the VP1u domain spanning positions 130 to 195, which has been reported to have phospholipase A2 activity (53), was highly conserved among B19 virus and variants sequences. In this region two amino acid changes were observed (S144N and V192A), but all of the amino acid residues reported by Zadori et al. (53) as being essential for phospholipase A2 activity were conserved, in particular those located in the catalytic site and in the calcium binding loop, thus confirming the critical role of these residues.
The two small ORFs coding for the 7.5- and 11-kDa nonstructural proteins were maintained in the D91.1 as well as in the V9 sequence, indicating that these proteins might actually be functionally important for the virus. Additionally, a third small ORF overlapping the VP1 gene (nucleotides 2694 to 2939) was also maintained among these viruses, and the possibility that it might code for a putative 9-kDa protein, which we propose to term X, should be considered. Interestingly, amino acid distances between the small ORFs of D91.1 and B19 virus were higher than those observed between the main ORFs. Indeed, nucleotide substitutions observed between D91.1 and B19 virus in the three regions where a common nucleotide sequence is shared by two overlapping ORFs (NS1/7.5 kDa, VP1/X and VP2/11 kDa) always resulted in amino acid changes that were more frequent in the small protein (five, eight, and three changes in the 7.5-kDa, X, and 11-kDa proteins, respectively) than in the large protein (two, six, and zero changes in the NS1, VP1, and VP2 proteins, respectively). This might indicate that during evolution the virus was constrained to maintain the large structural and nonstructural proteins rather than the small nonstructural polypeptides.
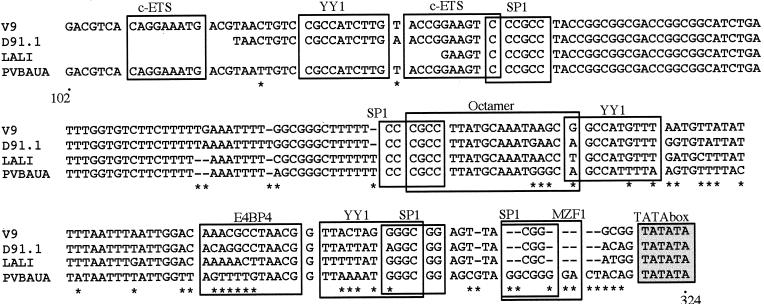
Unexpectedly, the p6 promoter region, which has been reported to be highly conserved among B19 viruses (16), was markedly divergent between D91.1 and B19 virus (19.8 to 21.3% divergence in a 291-bp sequence corresponding to nucleotides 144 to 434 of the Pvbaua sequence), as well as between V9 and B19 virus, between Lali and B19 virus, and even between D91.1 and V9 (Table 5). Compared to the B19 virus p6 promoter sequence, the fourth GC box, a putative binding site for the cellular factor Sp1 located just upstream of the TATA box, was deleted (Fig. 2). Within the p6 region analyzed, the other identified binding motifs for transcription factors were preserved, although some of them contained a significant number of nucleotide substitutions.
FIG. 2.
Sequence alignment in the p6 promoter region (nucleotides 102 to 324 of the Pvbaua sequence). Major potential binding sites for transcription factors are boxed as described by Gareus et al. (16). Compared to the B19 virus p6 sequence, the D91.1, V9, and Lali sequences contain numerous substitutions, and deletions are observed in the fourth GC box located just upstream of the TATA box.
Taken together, these results indicated that the D91.1 and V9 sequences, although notably divergent, belonged to the same cluster, which was clearly distinct from the B19 virus cluster within the human erythrovirus phylogenetic group. Additionally, the Lali sequence, which branched out between these two clusters, might be representative of a third cluster. To specify the genetic diversity within these tentative clusters, the genomes of additional B19 virus and V9-related isolates were partly sequenced for further phylogenetic analyses.
Identification of three genotypes within the human erythroviruses.
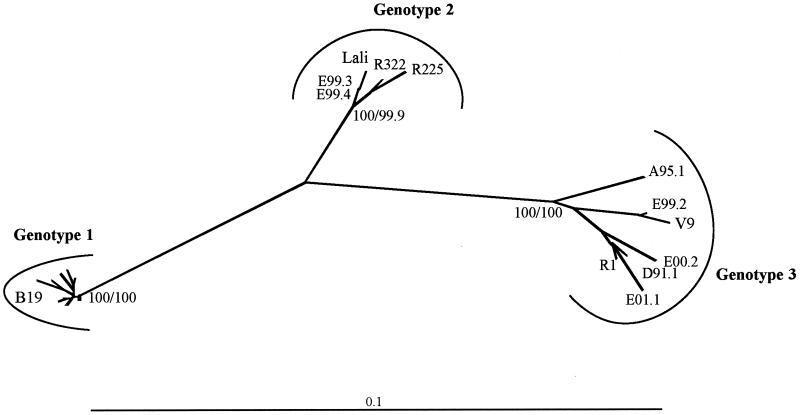
To specify the results obtained with the D91.1 sequence, we chose to analyze a partial 994-bp sequence spanning the NS1-VP1u junction for the V9-related strains that were detected by the consensus NS1-PCR assay (listed in Table 3). The NS1-VP1u region was considered suitable for phylogenetic analysis because it covers nearly one-fifth of the viral genome and includes genetic information on three viral proteins (NS1, 7.5-kDa protein, and VP1u, spanning two recognized major neutralizing epitopes). Sequence analysis was restricted to 6 of the 10 V9-related strains because in four cases nested PCR amplification failed to produce a NS1-VP1u amplicon. As a control, the NS1-VP1u sequence was determined for two strains typed as B19 virus by the NS1-PCR assay (strains CDC105 and CDC156). These NS1-VP1u sequences were then aligned with those of other erythrovirus strains listed in Table 4 (including V9, D91.1, Lali, and 15 additional erythrovirus sequences available in GenBank), and phylogenetic analysis was performed by using both the neighbor-joining and the maximum-parsimony methods.
Irrespective of the phylogenetic model used, the human erythrovirus sequences analyzed were distributed into three distinct clusters with bootstrap values of 100 (Fig. 3). Among the six V9-related strains, four clustered together with V9 and D91.1, while two unexpectedly clustered with the Lali strain. Additionally, among sequences from GenBank, one (R1) clustered with V9 and two (R225 and R322, referenced in GenBank as erythrovirus VX) clustered with Lali. The nucleotide distances observed between the B19-V9, V9-Lali, and B19-Lali clusters were 10.5 to 12%, 7.6 to 8.9%, and 6.4 to 7.9%, respectively, and were higher than the maximum nucleotide distance observed between two sequences belonging to a same cluster (1.1, 1.7, and 4.3% within the B19 virus, Lali, and V9 clusters, respectively). These results confirmed those obtained by analysis of the full-length sequences and showed that genetic diversity was higher among V9-related viruses than among B19-related viruses. We thus propose that the human erythroviruses should be classified into three distinct genotypes, based on the phylogenetic analysis of the NS1-VP1u sequences. According to this classification, B19-related viruses will correspond to genotype 1 (prototype strain Pvbaua), whereas V9-related viruses will be divided into genotype 2 (prototype strain Lali) and genotype 3 (prototype strain V9).
FIG. 3.
Phylogenetic relationships among B19 virus and V9-related viruses based on the NS1-VP1u sequences (994 bp). Phylogenetic analysis was performed by using the neighbor-joining algorithm based on the Kimura 2 parameter distance estimation method. Significant bootstrap values (in percentages) are indicated for 103 neighbor-joining/103 maximum-parsimony replicates. The human erythrovirus sequences are distributed into three distinct clusters corresponding to genotype 1 (prototype strain Pvbaua), genotype 2 (prototype strain Lali), and genotype 3 (prototype strain V9). For clarity, the names of the B19 virus sequences included in genotype 1 (Pvbaua, Pvb19nsvp, Pvb19×528, Pvb19×560, Pvb19×599, Pvbpro, HV, R43, R227, R308, CDC105, and CDC156) are omitted from the figure.
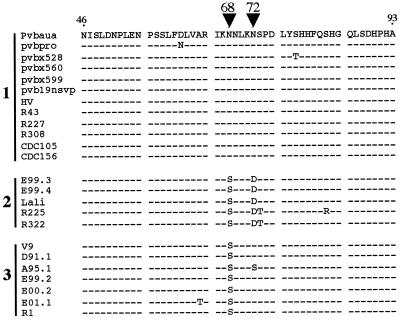
The significance of these three genotypes in terms of pathogenic and antigenic properties remains to be established. However, it is noteworthy that one of the two recognized major neutralization epitopes located in the VP1u sequence we analyzed (aa 46 to 93) was well conserved among genotype 1 viruses, whereas an N68S substitution was shared by genotype 2 and genotype 3 viruses and an additional N72D substitution was observed for genotype 2 viruses (Fig. 4).
FIG. 4.
Amino acid alignment of the VP1u neutralizing epitope (aa 46 to 93 of the Pvbaua sequence). Numbers indicate genotypes as defined in Fig. 3. Compared to genotype 1 viruses (B19 virus strains), an N68S substitution is shared by genotype 2 (prototype strain Lali) and genotype 3 (prototype strain V9) viruses, and an additional N72D substitution is shared by genotype 2 viruses (arrowheads).
DISCUSSION
In this study we report the isolation of 11 V9-related strains, the majority of which were isolated during a prospective study conducted on a French population between 1999 and 2001. The relative frequency of the V9-related isolates is significant, as they account for approximately 11% of the erythroviruses isolated during this period. These results indicate that V9-related viruses circulate in France along with B19 viruses. Until now, only a limited number of studies have been conducted in order to specifically detect V9-related viruses (19, 21, 26), and none of these studies has reported the detection of V9 strains. For instance, V9 DNA was not detected by PCR in 22 serum samples that were unclassifiable by B19 virus serology (26). In a larger study, Heegaard et al. reported that with a nested PCR assay, V9 DNA could not be detected in 100 B19 virus IgM-positive serum samples or in plasma pools representing 100,000 Danish blood donor units (19). The discrepancy between the apparent absence of V9 viruses reported in these studies and the somewhat high prevalence of V9 isolates observed in our study might be due to several factors. On one hand, circulation of V9 virus might be epidemiologically restricted to a certain geographic area (i.e., to France at the present time), and our findings that no V9 virus could be detected in a panel of 270 serum samples from a U.S. population might favor this hypothesis. On the other hand, PCR assays designed for specific detection of V9 DNA might have failed to reliably detect all of the V9-related strains. Indeed, sequence analysis of the NS1-VP1u region reveals that sequence divergence is greater among the V9 cluster (up to 4.3% divergence) than among the B19 virus cluster. Moreover, sequence variation has been observed in genome regions corresponding to primers used for V9 detection. For instance, the V9-specific reverse primer (nucleotide positions 4361 to 4342) described by Heegaard et al. (19) has four and five mismatches with the D91.1 and Lali sequences, respectively. Additionally, the reverse primers (e2953r and e2960r) used in our NS1-VP1u nested PCR assay have two mismatches and one mismatch with the D91.1 and Lali sequences, respectively. Variation within the sequence recognized by these primers might explain why we failed to obtain an NS1-VP1u PCR product for 4 of the 11 V9-related isolates that were detected in the NS1 PCR assay. Thus, the DNA sequence variability among the V9 group might be greater than what we have observed in this study, and the hypothesis that V9 viruses with more divergent sequences might exist, especially in different geographic locations, should be considered. When more V9-related sequences are available, new primers could be designed to enhance erythrovirus detection. Alternatively, although it would be less sensitive, a non-PCR-based assay, such as hybridization, could be used for broader detection of erythrovirus DNA.
Although only a limited number of V9 viruses have been characterized in this study, our data indicate that genetic diversity within the erythrovirus group is drastically higher than thought previously. In addition, phylogenetic analysis has allowed us to specify the taxonomic position of V9 viruses. These viruses clearly belong to the Erythrovirus genus, sharing the same genetic organization as B19 viruses, including conservation of the minor ORFs. On the other hand, B19 and V9 viruses account for distinct lineages within the Erythrovirus genus. Regarding the evolutionary relationships among these viruses, the high ratio of synonymous to nonsynonymous substitutions might be indicative of an ancient separation between the B19 and V9 lineages, as suggested by Lukashov and Goudsmit (31). As reported by those authors for parvoviruses (31) and for HIV type 1 (17, 30), synonymous substitutions, which accumulate with time and are not subjected to selection pressure, are thought to reflect long-term evolution, while nonsynonymous substitutions, which change amino acids and are subjected to strong selection pressure, rather reflect short-term intrahost evolution. Indeed, the evolutionary events that resulted in the separation between the B19 and V9 virus lineages appear to be clearly distinct from those reported to occur for B19 viruses during persistent infection (23). In this event, most of the substitutions resulted in amino acid changes, especially in the VP1u region, which is known to play a crucial part in eliciting a neutralizing antibody response (40, 41) and probably reflects the selective pressure of the immune system over a long period of viral replication in the host. Lastly, the finding that the V9 lineage includes other isolates whose sequences are notably distant from the V9 sequence suggests that V9-type viruses are ancient viruses which have evolved beside and independently from B19 virus rather than recently emerging viruses. On the other hand, it could be argued that the origin of V9-type viruses remains uncertain and that such viruses might have arisen as a result of human intervention, such as exposure to mutagenic agents. However, it is unlikely that variant erythroviruses have arisen as a result of the procedures used to inactivate viruses from plasma-derived products such as heating or solvent or detergent treatment, which are not known to induce nucleotide modifications.
Our analysis of the NS1-VP1u sequences indicates that erythrovirus sequences are distributed into three well-resolved phylogenetic clusters. We therefore propose a new classification that distinguishes three genotypes within the Erythrovirus genus, with B19-related viruses corresponding to genotype 1 (prototype strain Pvbaua) and V9-related viruses being divided into genotype 2 (prototype strain Lali) and genotype 3 (prototype strain V9). Like for other DNA or RNA viruses for which sequence-based genotypes have been defined (see reference 3 for a review), it seems to us more appropriate to refer to V9-related viruses as genotypes rather than to propose V9 virus as a tentative new species in the Erythrovirus genus. Indeed, according to the guidelines for species definition (49), significant phenotypic differences between B19 and V9 viruses, such as antigenic and pathogenic properties, should be demonstrated in addition to genotypic differences before they are distinguished as different species.
Regarding antigenic properties, the high degree of homology of the VP2 capsid protein between genotype 1 (B19), genotype 2 (Lali), and genotype 3 (D91.1, V9) viruses should have resulted in a high level of antigenic cross-reactivity between these viruses. Some of our results favor this hypothesis: first, a B19 virus-specific IgM antibody response was detected by a commercial enzyme-linked immunosorbent assay (ELISA) with B19 virus VP2 as the antigen in four patients infected with a genotype 2 virus (E99.3 and E99.4) or a genotype 3 virus (E00.2 and E01.1); second, the D91.1 isolate (genotype 3) was initially detected by counterimmunoelectrophoresis with a B19 virus-specific serum. Additionally, it has been recently reported by others (20) that serological screening by ELISA with the baculovirus recombinant VP2 protein of V9 as the antigen gives results that are 100% concordant with those obtained by ELISA with B19 virus VP2 as the antigen, indicating a serological cross-reactivity between B19 virus and V9. On the other hand, variation at the amino acid level, which was observed between the three genotypes in the VP1u region and particularly in one of the major neutralization epitopes, might lead to differences in the neutralizing antibody response. Peptide-based assays might be useful to address this question. On this assumption, infection by a virus belonging to a given genotype might fail to ensure cross-protective immunity against viruses belonging to a different genotype. It is noteworthy that in our study most of genotype 2 and 3 viruses were isolated in patients older than those infected with a genotype 1 virus, frequently in association with immunodeficiency. Although this could be due to a recruitment bias, the hypothesis that adult patients with impaired immunity, who are likely to have experienced a previous genotype 1 virus infection, might have been reinfected by a genotype 2 or 3 virus should be considered.
Regarding pathogenic properties, the clinical spectrum associated with genotype 2 or 3 virus infection has been found to be similar to that observed with genotype 1 (B19 virus) infection. This was expected, since our study aimed to detect V9-related isolates in groups of patients with symptoms suggestive of B19 virus infection. Whether genotype 2 or 3 viruses could be responsible for other diseases in humans remains to be determined. Interestingly, the p6 promoter has been found to be markedly divergent between the different genotypes, and this could suggest that pathogenic properties might differ according to the genotype. Indeed, it has been reported that the p6 promoter might be important for viral cell tropism, since its activity was found to vary in different cell lines (16, 39). In particular, the deletion of the fourth GC box in the p6 promoters of genotype 2 and 3 viruses might have some effects, as it has been reported that the mutation of the fourth GC box has a negative effect on promoter strength and transactivation by the NS1 protein (16) and that the GC boxes adjacent to the TATA box interact with the cellular factors Sp1 and Sp3, which might regulate the promoter activity depending on the cell type (39).
In conclusion, our results indicate that the human erythrovirus group is actually more diverse than thought previously and can be divided into three distinct genotypes, with the separation between these genotypes probably being an ancient event. Further studies are needed in order to establish a new classification of the Erythrovirus genus based on genotypes and to evaluate the biological and medical significance of these genotypes.
Acknowledgments
We are grateful to Fiona Manning (Biotrin) for providing the panel of sera from the Centers for Disease Control and Prevention and to Pierre Lebon for providing the amniotic fluid samples. We thank Paul Dény and Christophe Goujon for advice on phylogenetic methods and constructive comments. We are indebted to Alexandra Binet, Sabine Ducret, Annie Girault, and Anne-Laurence Josnin for excellent technical assistance.
This work was supported in part by a grant from the Fondation pour la Recherche Médicale and by European Commission Parvovirus Project grant QLK2-CT-2001-00877.
REFERENCES
- 1.Altschul, S., T. Madden, A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped Blast and PSI-Blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed]
- 2.Anderson, M. J., S. E. Jones, S. P. Fisher-Hoch, E. Lewis, S. M. Hall, C. L. Bartlett, B. J. Cohen, P. P. Mortimer, and M. S. Pereira. 1983. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet i:1378. [DOI] [PubMed]
- 3.Arens, M. 2001. Clinically relevant sequence-based genotyping of HBV, HCV, CMV, and HIV. J. Clin. Virol. 22:11-29. [DOI] [PubMed] [Google Scholar]
- 4.Astell, C. R., W. Luo, J. Brunstein, and J. St. Amand. 1997. B19 parvovirus: biochemical and molecular features, p. 16-41. In L. J. Anderson and N. S. Young (ed.), Human parvovirus B19, 1st ed., vol. 20. Karger, New York, N.Y.
- 5.Auguste, V., A. Garbarg-Chenon, and Q. T. Nguyen. June 1999. Erythrovirus and its applications. French patent WO9928439.
- 6.Blundell, M. C., C. Beard, and C. R. Astell. 1987. In vitro identification of a B19 parvovirus promoter. Virology 157:534-538. [DOI] [PubMed] [Google Scholar]
- 7.Brown, K. E. 1997. Human parvovirus B19 epidemiology and clinical manifestations, p. 42-55. In L. J. Anderson and N. S. Young (ed.), Human parvovirus B19, 1st ed., vol. 20. Karger, New York, N.Y.
- 8.Brown, K. E., S. W. Green, M. G. O'Sullivan, and N. S. Young. 1995. Cloning and sequencing of the simian parvovirus genome. Virology 210:314-322. [DOI] [PubMed] [Google Scholar]
- 9.Brown, T., A. Anand, L. D. Ritchie, J. P. Clewley, and T. M. S. Reid. 1984. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet ii:1033-1034. [DOI] [PubMed]
- 10.Deiss, V., J. D. Tratschin, M. Weitz, and G. Siegl. 1990. Cloning of the human parvovirus B19 genome and structural analysis of its palindromic termini. Virology 175:247-254. [DOI] [PubMed] [Google Scholar]
- 11.Doerig, C., B. Hirt, J. P. Antonietti, and P. Beard. 1990. Nonstructural protein of parvoviruses B19 and minute virus of mice controls transcription. J. Virol. 15:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdman, D. D., E. L. Durigon, Q. Y. Wang, and L. J. Anderson. 1996. Genetic diversity of human parvovirus B19: sequence analysis of the VP1/VP2 gene from multiple isolates. J. Gen. Virol. 77:2767-2774. [DOI] [PubMed] [Google Scholar]
- 13.Felsenstein, J. 1989. PHYLIP—Phylogeny Interference Package. Cladistics 5:164-166. [Google Scholar]
- 14.Foto, F., K. G. Sang, L. L. Scharosch, E. J. Howard, and S. J. Naides. 1993. Parvovirus B19-specific DNA in bone marrow from B19 arthropathy patients: evidence for B19 virus persistence. J. Infect. Dis. 167:744-748. [DOI] [PubMed] [Google Scholar]
- 15.Gallinella, G., S. Venturoli, G. Gentilomi, M. Musiani, and M. Zerbini. 1995. Extent of sequence variability in a genomic region coding for capsid proteins of B19 parvovirus. Arch. Virol. 140:1119-1125. [DOI] [PubMed] [Google Scholar]
- 16.Gareus, R., A. Gigler, A. Hemauer, M. Leruez-Ville, F. Morinet, H. Wolf, and S. Modrow. 1998. Characterization of cis-acting and NS1 protein-responsive elements in the p6 promoter of parvovirus B19. J. Virol. 72:609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goudsmit, J., and V. V. Lukashov. 1999. Dating the origin of HIV-1 subtypes. Nature 400:325-326. [DOI] [PubMed] [Google Scholar]
- 18.Green, S. W., I. Malkovska, M. G. O'Sullivan, and K. E. Brown. 2000. Rhesus and pig-tailed macaque parvoviruses: identification of two new members of the erythrovirus genus in monkeys. Virology 269:105-112. [DOI] [PubMed] [Google Scholar]
- 19.Heegaard, E. D., I. Panum Jensen, and J. Christensen. 2001. Novel PCR assay for differential detection and screening of erythrovirus B19 and erythrovirus V9. J. Med. Virol. 65:362-367. [DOI] [PubMed] [Google Scholar]
- 20.Heegaard, E. D., K. Qvortrup, and J. Christensen. 2002. Baculovirus expression of erythrovirus V9 capsids and screening by ELISA: serologic cross-reactivity with erythrovirus B19. J. Med. Virol. 66:246-252. [DOI] [PubMed] [Google Scholar]
- 21.Heegaard, E. D., and E. B. Taaning. 2002. Parvovirus B19 and parvovirus V9 are not associated with Henoch-Schönlein purpura in children. Pediatr. Infect. Dis. J. 21:31-34. [DOI] [PubMed] [Google Scholar]
- 22.Hemauer, A., K. Beckenlehner, H. Wolf, B. Lang, and S. Modrow. 1999. Acute parvovirus B19 infection in connection with a flare of systemic lupus erythematodes in a female patient. J. Clin. Virol. 14:73-77. [DOI] [PubMed] [Google Scholar]
- 23.Hemauer, A., A. von Poblotzki, A. Gigler, P. Cassinotti, G. Siegl, H. Wolf, and S. Modrow. 1996. Sequence variability among different parvovirus B19 isolates. J. Gen. Virol. 77:1781-1785. [DOI] [PubMed] [Google Scholar]
- 24.Hicks, K. E., R. C. Cubel, B. J. Cohen, and J. P. Clewley. 1996. Sequence analysis of a parvovirus B19 isolate and baculovirus expression of the non-structural protein. Arch. Virol. 141:1319-1327. [DOI] [PubMed] [Google Scholar]
- 25.Hokynar, K., J. Brunstein, M. Soderlund-Venermo, O. Kiviluoto, E. K. Partio, Y. Konttinen, and K. Hedman. 2000. Integrity and full coding sequence of B19 virus DNA persisting in human synovial tissue. J. Gen. Virol. 81:1017-1025. [DOI] [PubMed] [Google Scholar]
- 26.Kaikkonen, L., M. Soderlund-Venermo, J. Brunstein, O. Schou, I. Panum Jensen, S. Rousseau, E. Owen Caul, B. Cohen, M. Valle, L. Hedman, and K. Hedman. 2001. Diagnosis of human parvovirus B19 infections by detection of epitope-type-specific VP2 IgG. J. Med. Virol. 64:360-365. [DOI] [PubMed] [Google Scholar]
- 27.Kajigaya, S., and M. Momoeda. 1997. Immune response to B19 infection, p. 121-136. In L. J. Anderson and N. S. Young (ed.), Human parvovirus B19, 1st ed., vol. 20. Karger, New York, N.Y.
- 28.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 29.Kurtzman, G. J., B. Cohen, P. Meyers, A. Amunullah, and N. S. Young. 1988. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet ii:1159-1162. [DOI] [PubMed]
- 30.Lukashov, V. V., and J. Goudsmit. 1997. Evolution of the human immunodeficiency virus type 1 subtype-specific V3 domain is confined to a sequence space with a fixed distance to the subtype consensus. J. Virol. 71:6332-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukashov, V. V., and J. Goudsmit. 2001. Evolutionary relationships among parvoviruses: virus-host coevolution among autonomous primate parvoviruses and links between adeno-associated and avian parvoviruses. J. Virol. 75:2729-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo, W., and C. R. Astell. 1993. A novel protein encoded by small RNAs of parvovirus B19. Virology 195:448-455. [DOI] [PubMed] [Google Scholar]
- 33.Mori, J., P. Beattie, D. W. Melton, B. Cohen, and J. P. Clewley. 1987. Structure and mapping of the DNA of human parvovirus B19. J. Gen. Virol. 68:2797-2806. [DOI] [PubMed] [Google Scholar]
- 34.Morinet, F., J. D. Tratschin, and Y. Perol. 1986. Comparison of 17 isolates of the human parvovirus B19 by restriction enzyme analysis. Arch. Virol. 90:165-172. [DOI] [PubMed] [Google Scholar]
- 35.Naides, S. J. 1993. Parvovirus B19 infection. Rheum. Dis. Clin. N. Am. 19:457-475. [PubMed] [Google Scholar]
- 36.Nguyen, Q. T., C. Sifer, V. Schneider, X. Allaume, A. Servant, F. Bernaudin, V. Auguste, and A. Garbarg-Chenon. 1999. Novel human erythrovirus associated with transient aplastic anemia. J. Clin. Microbiol. 37:2483-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen, Q. T., C. Sifer, V. Schneider, F. Bernaudin, V. Auguste, and A. Garbarg-Chenon. 1998. Detection of an erythrovirus sequence distinct from B19 in a child with acute anemia. Lancet 352:1524.. [DOI] [PubMed] [Google Scholar]
- 38.Pattison, J. R., S. E. Jones, J. Hodgson, L. R. Davis, J. M. White, C. E. Stroud, and L. Murtaza. 1981. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet i:664-665. [DOI] [PubMed]
- 39.Raab, U., B. Bauer, A. Gigler, K. Beckenlehner, H. Wolf, and S. Modrow. 2001. Cellular transcription factors that interact with p6 promoter elements of parvovirus B19. J. Gen. Virol. 82:1473-1480. [DOI] [PubMed] [Google Scholar]
- 40.Rosenfeld, S. J., N. S. Young, D. Alling, J. Ayub, and C. Saxinger. 1994. Subunit interaction in B19 parvovirus empty capsids. Arch. Virol. 136:9-18. [DOI] [PubMed] [Google Scholar]
- 41.Saikawa, T., S. Anderson, M. Momoeda, S. Kajigaya, and N. S. Young. 1993. Neutralizing linear epitopes of B19 parvovirus cluster in the VP1 unique and VP1-VP2 junction regions. J. Virol. 67:3004-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shade, R. O., M. C. Blundell, S. F. Cotmore, P. Tattersall, and C. R. Astell. 1986. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J. Virol. 58:921-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St. Amand, J., and C. R. Astell. 1993. Identification and characterization of a family of 11-kDa proteins encoded by the human parvovirus B19. Virology 192:121-131. [DOI] [PubMed] [Google Scholar]
- 44.Swofford, D. 1998. PAUP: phylogenetic analysis using parsimony (and other methods), version 4.0b6. Sinauer Associates, Sunderland, Mass.
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed]
- 46.Umene, K., and T. Nunoue. 1990. The genome type of human parvovirus B19 strains isolated in Japan during 1981 differs from types detected in 1986 to 1987: a correlation between genome type and prevalence. J. Gen. Virol. 71:983-986. [DOI] [PubMed] [Google Scholar]
- 47.Umene, K., and T. Nunoue. 1991. Genetic diversity of human parvovirus B19 determined using a set of restriction endonucleases recognizing four or five base pairs and partial nucleotide sequencing: use of sequence variability in virus classification. J. Gen. Virol. 72:1997-2001. [DOI] [PubMed] [Google Scholar]
- 48.Umene, K., and T. Nunoue. 1993. Partial nucleotide sequencing and characterization of human parvovirus B19 genome DNAs from damaged human fetuses and from patients with leukemia. J. Med. Virol. 39:333-339. [DOI] [PubMed] [Google Scholar]
- 49.Van Regenmortel, M. H., D. H. Bishop, C. M. Fauquet, M. A. Mayo, J. Maniloff, and C. H. Calisher. 1997. Guidelines to the demarcation of virus species. Arch Virol. 142:1505-1518. [PubMed] [Google Scholar]
- 50.Van Regenmortel, M. H. V., C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.). 2000. Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 51.White, D. G., A. D. Woolf, P. P. Mortimer, B. J. Cohen, D. R. Blake, and P. A. Bacon. 1985. Human parvovirus arthropathy. Lancet i:419-421. [DOI] [PubMed]
- 52.Yoo, B. C., D. H. Lee, S. M. Park, C. Y. Kim, H. Lee, J. S. Seo, K. J. Park, and W. Ryull. 1999. A novel parvovirus isolated from Manchurian chipmunks. Virology 253:250-258. [DOI] [PubMed] [Google Scholar]
- 53.Zadori, Z., J. Szelei, M. C. Lacoste, Y. Li, S. Gariepy, P. Raymond, M. Allaire, I. R. Nabi, and P. Tijssen. 2001. A viral phospholipase A2 is required for parvovirus infectivity. Dev. Cell 1:291-302. [DOI] [PubMed] [Google Scholar]