Abstract
Non-obese diabetic (NOD) mice spontaneously develop insulin-dependent diabetes mellitus (IDDM) as a consequence of autoimmune aggression of beta cells of the endocrine pancreas by T cells. T lymphocytes of NOD mice are resistant to apoptosis induced by glucocorticoids, or by starving or DNA-damaging treatments, a feature that was interpreted as being linked to escape of autoreactive T cells from thymic negative selection. c-myc is one of the gene targets of glucocorticoids (GC), its expression being down-regulated by the activated GC-GC receptor complex. We investigated here whether expression of Myc protein, in response to dexamethasone stimulation, was the same in NOD mice and in non-autoimmune strains, namely NON, BALB/c and C57Bl.6. We found a consistent increase in the levels of Myc protein after GC-treatment of lymphocytes of NOD mice, a finding that was in contrast to the down-regulation of c-myc that we observed in lymphocytes from mice not prone to diabetes. We also report that, rather than a absolute resistance to GC-induced cell death, NOD mice display a delayed apoptotic response to GC. We propose that the resistance of NOD mice lymphocytes to GC-induced apoptosis is because of inhibition of the repressive action of GC-GR complexes at the level of c-myc transcription. This deficient action of GC-GR results in increased production of nuclear Myc protein, peculiar to NOD mice cells, following their treatment with GC.
Full text
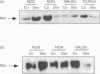
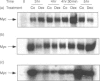
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colucci F., Cilio C. M., Lejon K., Gonçalves C. P., Bergman M. L., Holmberg D. Programmed cell death in the pathogenesis of murine IDDM: resistance to apoptosis induced in lymphocytes by cyclophosphamide. J Autoimmun. 1996 Apr;9(2):271–276. doi: 10.1006/jaut.1996.0034. [DOI] [PubMed] [Google Scholar]
- Delovitch T. L., Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997 Dec;7(6):727–738. doi: 10.1016/s1074-7613(00)80392-1. [DOI] [PubMed] [Google Scholar]
- Eastman-Reks S. B., Vedeckis W. V. Glucocorticoid inhibition of c-myc, c-myb, and c-Ki-ras expression in a mouse lymphoma cell line. Cancer Res. 1986 May;46(5):2457–2462. [PubMed] [Google Scholar]
- Fischer G., Kent S. C., Joseph L., Green D. R., Scott D. W. Lymphoma models for B cell activation and tolerance. X. Anti-mu-mediated growth arrest and apoptosis of murine B cell lymphomas is prevented by the stabilization of myc. J Exp Med. 1994 Jan 1;179(1):221–228. doi: 10.1084/jem.179.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsthoefel A. M., Thompson E. A. Glucocorticoid regulation of transcription of the c-myc cellular protooncogene in P1798 cells. Mol Endocrinol. 1987 Dec;1(12):899–907. doi: 10.1210/mend-1-12-899. [DOI] [PubMed] [Google Scholar]
- Garchon H. J., Luan J. J., Eloy L., Bédossa P., Bach J. F. Genetic analysis of immune dysfunction in non-obese diabetic (NOD) mice: mapping of a susceptibility locus close to the Bcl-2 gene correlates with increased resistance of NOD T cells to apoptosis induction. Eur J Immunol. 1994 Feb;24(2):380–384. doi: 10.1002/eji.1830240217. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck S., Kullmann M., Gast A., Ponta H., Rahmsdorf H. J., Herrlich P., Cato A. C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994 Sep 1;13(17):4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmberg A., Auphan N., Caelles C., Karin M. Glucocorticoid-induced apoptosis of human leukemic cells is caused by the repressive function of the glucocorticoid receptor. EMBO J. 1995 Feb 1;14(3):452–460. doi: 10.1002/j.1460-2075.1995.tb07021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibner U., Benhamou L. E., Haury M., Cazenave P. A., Sarthou P. Signaling of programmed cell death induction in WEHI-231 B lymphoma cells. Eur J Immunol. 1993 Nov;23(11):2821–2825. doi: 10.1002/eji.1830231115. [DOI] [PubMed] [Google Scholar]
- Hutchings P. R., Simpson E., O'Reilly L. A., Lund T., Waldmann H., Cooke A. The involvement of Ly2+ T cells in beta cell destruction. J Autoimmun. 1990 Apr;3 (Suppl 1):101–109. doi: 10.1016/s0896-8411(09)90018-x. [DOI] [PubMed] [Google Scholar]
- Iseki R., Mukai M., Iwata M. Regulation of T lymphocyte apoptosis. Signals for the antagonism between activation- and glucocorticoid-induced death. J Immunol. 1991 Dec 15;147(12):4286–4292. [PubMed] [Google Scholar]
- Kiefer J., Okret S., Jondal M., McConkey D. J. Functional glucocorticoid receptor expression is required for cAMP-mediated apoptosis in a human leukemic T cell line. J Immunol. 1995 Nov 15;155(10):4525–4528. [PubMed] [Google Scholar]
- King L. B., Ashwell J. D. Signaling for death of lymphoid cells. Curr Opin Immunol. 1993 Jun;5(3):368–373. doi: 10.1016/0952-7915(93)90055-w. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Behrmann I., Daniel P., Dhein J., Debatin K. M. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994 Apr;6(2):279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Leijon K., Hammarström B., Holmberg D. Non-obese diabetic (NOD) mice display enhanced immune responses and prolonged survival of lymphoid cells. Int Immunol. 1994 Feb;6(2):339–345. doi: 10.1093/intimm/6.2.339. [DOI] [PubMed] [Google Scholar]
- Ma T., Mahajan P. B., Thompson E. A. Glucocorticoid regulation of c-myc promoter utilization in P1798 T-lymphoma cells. Mol Endocrinol. 1992 Jun;6(6):960–968. doi: 10.1210/mend.6.6.1495494. [DOI] [PubMed] [Google Scholar]
- Martins T. C., Aguas A. P. Changes in B and T lymphocytes associated with mycobacteria-induced protection of NOD mice from diabetes. J Autoimmun. 1996 Aug;9(4):501–507. doi: 10.1006/jaut.1996.0067. [DOI] [PubMed] [Google Scholar]
- McCormack J. E., Pepe V. H., Kent R. B., Dean M., Marshak-Rothstein A., Sonenshein G. E. Specific regulation of c-myc oncogene expression in a murine B-cell lymphoma. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5546–5550. doi: 10.1073/pnas.81.17.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller B. J., Appel M. C., O'Neil J. J., Wicker L. S. Both the Lyt-2+ and L3T4+ T cell subsets are required for the transfer of diabetes in nonobese diabetic mice. J Immunol. 1988 Jan 1;140(1):52–58. [PubMed] [Google Scholar]
- Rhee K., Bresnahan W., Hirai A., Hirai M., Thompson E. A. c-Myc and cyclin D3 (CcnD3) genes are independent targets for glucocorticoid inhibition of lymphoid cell proliferation. Cancer Res. 1995 Sep 15;55(18):4188–4195. [PubMed] [Google Scholar]
- Rocha B., Larsson E. L., Freitas A. A. Effects of hydroxyurea on concanavalin-A-induced T-cell proliferation. Depletion of T-cell growth factor-reactive and -producing T lymphocytes. Scand J Immunol. 1984 Apr;19(4):315–321. doi: 10.1111/j.1365-3083.1984.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Telford W. G., King L. E., Fraker P. J. Rapid quantitation of apoptosis in pure and heterogeneous cell populations using flow cytometry. J Immunol Methods. 1994 Jun 3;172(1):1–16. doi: 10.1016/0022-1759(94)90373-5. [DOI] [PubMed] [Google Scholar]
- Thompson E. B. Apoptosis and steroid hormones. Mol Endocrinol. 1994 Jun;8(6):665–673. doi: 10.1210/mend.8.6.7935482. [DOI] [PubMed] [Google Scholar]
- Thulasi R., Harbour D. V., Thompson E. B. Suppression of c-myc is a critical step in glucocorticoid-induced human leukemic cell lysis. J Biol Chem. 1993 Aug 25;268(24):18306–18312. [PubMed] [Google Scholar]
- Ucker D. S., Hebshi L. D., Blomquist J. F., Torbett B. E. Physiological T-cell death: susceptibility is modulated by activation, aging, and transformation, but the mechanism is constant. Immunol Rev. 1994 Dec;142:273–299. doi: 10.1111/j.1600-065x.1994.tb00893.x. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Strasser A. The molecular biology of apoptosis. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2239–2244. doi: 10.1073/pnas.93.6.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H., Matsumoto M., Kunimoto K., Kawaguchi J., Makino S., Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992 Sep;22(9):2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]