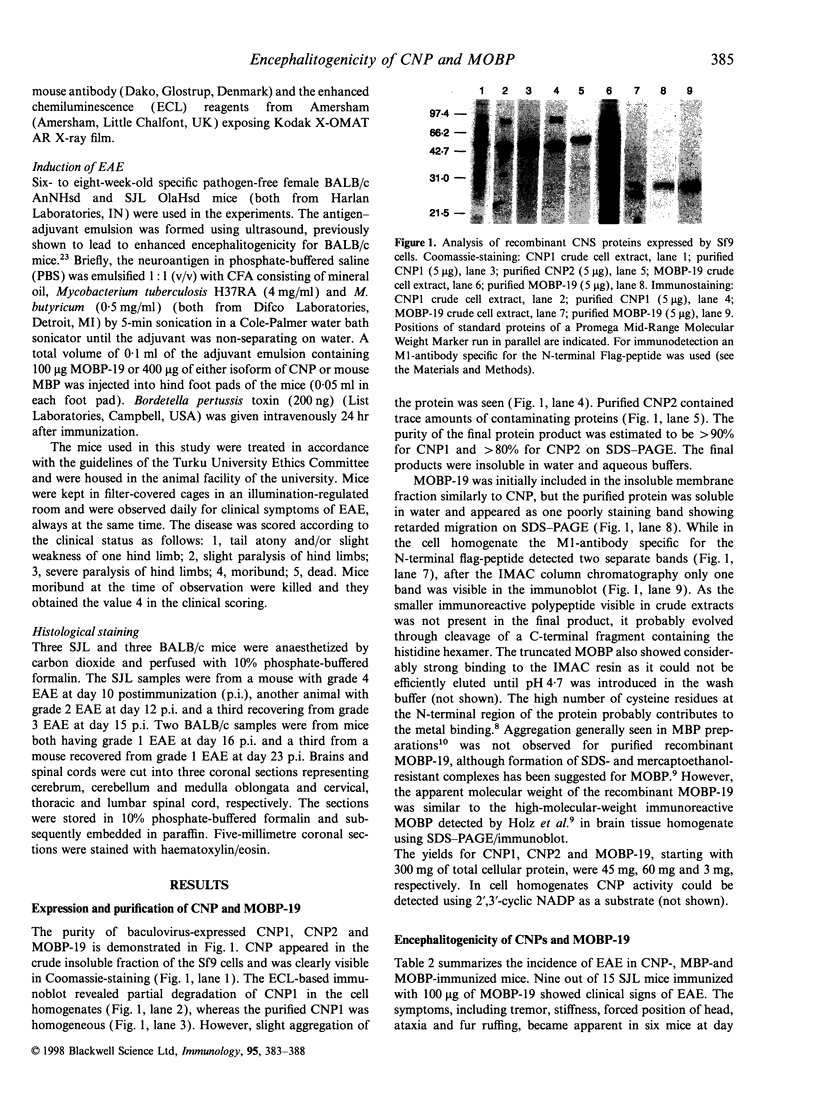
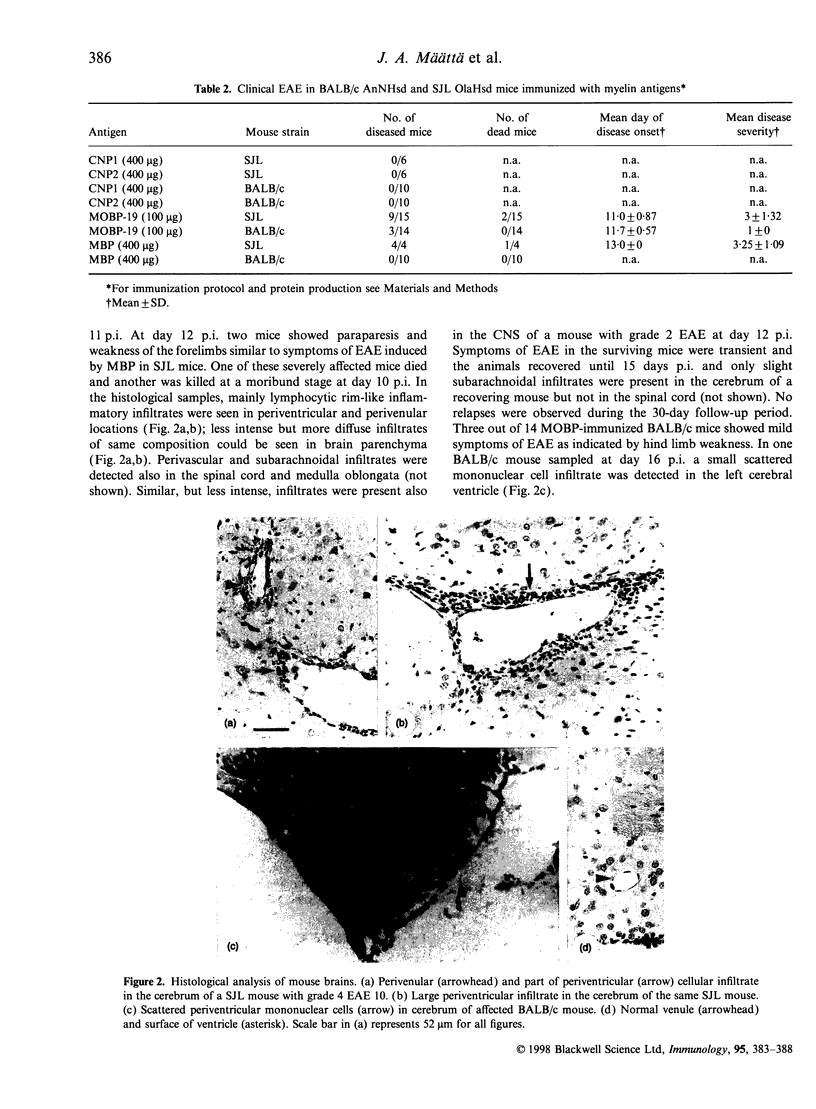
Abstract
In search of new encephalitogenic myelin antigens, the 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNP) and 19 000 MW isoform of myelin-associated oligodendrocytic basic protein (MOBP) were obtained as recombinant proteins by the baculovirus expression system in Spodoptera frugiperda cells and purified to homogeneity by immobilized metal chelate affinity chromatography (IMAC). The purified MOBP was soluble in water and showed retarded migration on sodium dodecyl sulphate-polyacrylamide gel electrophoresis similar to myelin basic protein (MBP). MOBP induced experimental autoimmune encephalomyelitis (EAE) in nine of 15 susceptible SJL OlaHsd mice, causing death in two animals, whereas three of 14 BALB/c mice showed mild symptoms of EAE, manifested as transient weakness of hind limbs. In both mouse strains, periventricular infiltrates of mononuclear cells were observed. In addition, both 46 000 MW and 48 000 MW CNP isoforms were shown to be non-encephalitogenic for both mouse strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aanstoot H. J., Kang S. M., Kim J., Lindsay L. A., Roll U., Knip M., Atkinson M., Mose-Larsen P., Fey S., Ludvigsson J. Identification and characterization of glima 38, a glycosylated islet cell membrane antigen, which together with GAD65 and IA2 marks the early phases of autoimmune response in type 1 diabetes. J Clin Invest. 1996 Jun 15;97(12):2772–2783. doi: 10.1172/JCI118732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abromson-Leeman S., Alexander J., Bronson R., Carroll J., Southwood S., Dorf M. Experimental autoimmune encephalomyelitis-resistant mice have highly encephalitogenic myelin basic protein (MBP)-specific T cell clones that recognize a MBP peptide with high affinity for MHC class II. J Immunol. 1995 Jan 1;154(1):388–398. [PubMed] [Google Scholar]
- Abromson-Leeman S., Hayashi M., Martin C., Sobel R., al-Sabbagh A., Weiner H., Dorf M. E. T cell responses to myelin basic protein in experimental autoimmune encephalomyelitis-resistant BALB/c mice. J Neuroimmunol. 1993 Jun;45(1-2):89–101. doi: 10.1016/0165-5728(93)90168-x. [DOI] [PubMed] [Google Scholar]
- Amor S., Groome N., Linington C., Morris M. M., Dornmair K., Gardinier M. V., Matthieu J. M., Baker D. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J Immunol. 1994 Nov 15;153(10):4349–4356. [PubMed] [Google Scholar]
- Bernier L., Alvarez F., Norgard E. M., Raible D. W., Mentaberry A., Schembri J. G., Sabatini D. D., Colman D. R. Molecular cloning of a 2',3'-cyclic nucleotide 3'-phosphodiesterase: mRNAs with different 5' ends encode the same set of proteins in nervous and lymphoid tissues. J Neurosci. 1987 Sep;7(9):2703–2710. doi: 10.1523/JNEUROSCI.07-09-02703.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum G., Kotilinek L., Schlievert P., Clark H. B., Trotter J., Horvath E., Gao E., Cox M., Braun P. E. Heat shock proteins and experimental autoimmune encephalomyelitis (EAE): I. Immunization with a peptide of the myelin protein 2',3' cyclic nucleotide 3' phosphodiesterase that is cross-reactive with a heat shock protein alters the course of EAE. J Neurosci Res. 1996 May 15;44(4):381–396. doi: 10.1002/(SICI)1097-4547(19960515)44:4<381::AID-JNR10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheifetz S., Moscarello M. A. Effect of bovine basic protein charge microheterogeneity on protein-induced aggregation of unilamellar vesicles containing a mixture of acidic and neutral phospholipids. Biochemistry. 1985 Apr 9;24(8):1909–1914. doi: 10.1021/bi00329a016. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Endoh M., Rapoport S. I., Tabira T. Studies of experimental allergic encephalomyelitis in old mice. J Neuroimmunol. 1990 Sep-Oct;29(1-3):21–31. doi: 10.1016/0165-5728(90)90144-c. [DOI] [PubMed] [Google Scholar]
- Endoh M., Tabira T., Kunishita T., Sakai K., Yamamura T., Taketomi T. DM-20, a proteolipid apoprotein, is an encephalitogen of acute and relapsing autoimmune encephalomyelitis in mice. J Immunol. 1986 Dec 15;137(12):3832–3835. [PubMed] [Google Scholar]
- Holz A., Frank M., Copeland N. G., Gilbert D. J., Jenkins N. A., Schwab M. E. Chromosomal localization of the myelin-associated oligodendrocytic basic protein and expression in the genetically linked neurological mouse mutants ducky and tippy. J Neurochem. 1997 Nov;69(5):1801–1809. doi: 10.1046/j.1471-4159.1997.69051801.x. [DOI] [PubMed] [Google Scholar]
- Holz A., Schaeren-Wiemers N., Schaefer C., Pott U., Colello R. J., Schwab M. E. Molecular and developmental characterization of novel cDNAs of the myelin-associated/oligodendrocytic basic protein. J Neurosci. 1996 Jan 15;16(2):467–477. doi: 10.1523/JNEUROSCI.16-02-00467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury S. J., Sayegh M. H., Hancock W. W., Gallon L., Carpenter C. B., Weiner H. L. Acquired tolerance to experimental autoimmune encephalomyelitis by intrathymic injection of myelin basic protein or its major encephalitogenic peptide. J Exp Med. 1993 Aug 1;178(2):559–566. doi: 10.1084/jem.178.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara T., Monoh K., Sakimura K., Takahashi Y. Alternative splicing of mouse brain 2',3'-cyclic-nucleotide 3'-phosphodiesterase mRNA. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1074–1081. doi: 10.1016/0006-291x(90)90502-e. [DOI] [PubMed] [Google Scholar]
- Luckow V. A., Lee S. C., Barry G. F., Olins P. O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993 Aug;67(8):4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin W. B., Weill C. L. Appearance of myelin proteins during development in the chick central nervous system. Dev Neurosci. 1985;7(3):170–178. doi: 10.1159/000112285. [DOI] [PubMed] [Google Scholar]
- Montague P., Dickinson P. J., McCallion A. S., Stewart G. J., Savioz A., Davies R. W., Kennedy P. G., Griffiths I. R. Developmental expression of the murine Mobp gene. J Neurosci Res. 1997 Jul 15;49(2):133–143. doi: 10.1002/(sici)1097-4547(19970715)49:2<133::aid-jnr2>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Mättä J. A., Coffey E. T., Hermonen J. A., Salmi A. A., Hinkkanen A. E. Detection of myelin basic protein isoforms by organic concentration. Biochem Biophys Res Commun. 1997 Sep 18;238(2):498–502. doi: 10.1006/bbrc.1997.7318. [DOI] [PubMed] [Google Scholar]
- Prickett K. S., Amberg D. C., Hopp T. P. A calcium-dependent antibody for identification and purification of recombinant proteins. Biotechniques. 1989 Jun;7(6):580–589. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B. D., Bernier L., Andrews S. B., Colman D. R. Cellular and subcellular distribution of 2',3'-cyclic nucleotide 3'-phosphodiesterase and its mRNA in the rat central nervous system. J Neurochem. 1988 Sep;51(3):859–868. doi: 10.1111/j.1471-4159.1988.tb01822.x. [DOI] [PubMed] [Google Scholar]
- Tuohy V. K., Sobel R. A., Lees M. B. Myelin proteolipid protein-induced experimental allergic encephalomyelitis. Variations of disease expression in different strains of mice. J Immunol. 1988 Mar 15;140(6):1868–1873. [PubMed] [Google Scholar]
- Yamamoto Y., Mizuno R., Nishimura T., Ogawa Y., Yoshikawa H., Fujimura H., Adachi E., Kishimoto T., Yanagihara T., Sakoda S. Cloning and expression of myelin-associated oligodendrocytic basic protein. A novel basic protein constituting the central nervous system myelin. J Biol Chem. 1994 Dec 16;269(50):31725–31730. [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]