Abstract
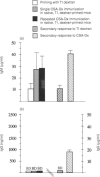
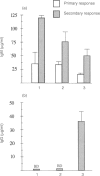
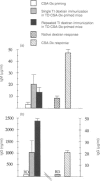
The bacterial carbohydrate dextran B512 is a thymus-independent (TI) antigen and a poor immunogen. Humoral responses consist primarily of IgM and no memory response is observed; rather, secondary responses to native dextran are similar to or suppressed compared with primary responses. However, immune responses to dextran can be enhanced. In this study we have used a protein-dextran conjugate that elicits a thymus-dependent (TD) immune response against dextran. Furthermore, we used the potent adjuvant cholera toxin (CT) for the dextran immunizations. This enables us to re-evaluate the phenomenon of poor secondary response to dextran and whether it can be abrogated. We show that native dextran-primed mice were not able to mount IgG anti-dextran antibody responses after repeated immunizations with the TD, protein-dextran conjugate. This was also apparent in the spleen, where almost no dextran-specific germinal centres were detected. However, the anti-protein antibody response was normal in these mice, demonstrating that it is only the anti-dextran-responding cells that are affected. The effect of CT adjuvant on these events was also evaluated. CT enhanced the humoral IgM anti-dextran responses as well as the splenic responses to dextran. But, the isotype profile was not altered, still no IgG was produced. In contrast, mice primed with the TD conjugate and repeatedly re-immunized with native, TI, dextran generated IgG anti-dextran responses. Our results indicate that it is probable that the lack of proper costimulation in the initiation of the response to dextran causes the suppressed secondary dextran responses. Furthermore, these results suggest that TI and TD forms of dextran activate the same type of B cells, since TI dextran-priming abrogated TD dextran IgG responses. The importance of the priming event for the induction of a classical memory response to carbohydrate antigens and the implications for vaccination strategies, are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agren L. C., Ekman L., Löwenadler B., Lycke N. Y. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J Immunol. 1997 Apr 15;158(8):3936–3946. [PubMed] [Google Scholar]
- Berek C., Berger A., Apel M. Maturation of the immune response in germinal centers. Cell. 1991 Dec 20;67(6):1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- Billian G., Bella C., Mondière P., Defrance T. Identification of a tonsil IgD+ B cell subset with phenotypical and functional characteristics of germinal center B cells. Eur J Immunol. 1996 Aug;26(8):1712–1719. doi: 10.1002/eji.1830260808. [DOI] [PubMed] [Google Scholar]
- Coutinho A., Möller G., Richter W. Molecular basis of B-cell activation. I. Mitogenicity of native and substituted dextrans. Scand J Immunol. 1974;3(3):321–328. doi: 10.1111/j.1365-3083.1974.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Ammann A. J., Wara D. W., Howie V. M., Schultz L., Doyle N., Kaplan M. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978 Nov;62(5):721–727. [PubMed] [Google Scholar]
- Dintzis R. Z., Okajima M., Middleton M. H., Greene G., Dintzis H. M. The immunogenicity of soluble haptenated polymers is determined by molecular mass and hapten valence. J Immunol. 1989 Aug 15;143(4):1239–1244. [PubMed] [Google Scholar]
- Feldmann M., Easten A. The relationship between antigenic structure and the requirement for thymus-derived cells in the immune response. J Exp Med. 1971 Jul 1;134(1):103–119. doi: 10.1084/jem.134.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Antigen-induced strain-specific autoantiidiotypic antibodies modulate the immune response to dextran B 512. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5944–5947. doi: 10.1073/pnas.76.11.5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Immunological unresponsiveness to thymus-independent antigens: two fundamentally different genetic mechanisms of B-cell unresponsiveness to dextran. J Exp Med. 1977 Dec 1;146(6):1663–1677. doi: 10.1084/jem.146.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Irreversible immunological tolerance to thymus-independent antigens is restricted to the clone of B cells having both Ig and PBA receptors for the tolerogen. Scand J Immunol. 1978;7(2):137–144. doi: 10.1111/j.1365-3083.1978.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Möller G. Serum antibody and cellular immune response in mice to dextran B512. Cell Immunol. 1990 Nov;131(1):41–51. doi: 10.1016/0008-8749(90)90233-h. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Möller G. The influence of T cells on the immunoglobulin repertoire and the affinity maturation of the immune response against dextran B512 in C57BL/6 mice. Scand J Immunol. 1991 Mar;33(3):307–317. doi: 10.1111/j.1365-3083.1991.tb01776.x. [DOI] [PubMed] [Google Scholar]
- Fernandez C., Sverremark E. Immune responses to bacterial polysaccharides: terminal epitopes are more immunogenic than internal structures. Cell Immunol. 1994 Jan;153(1):67–78. doi: 10.1006/cimm.1994.1006. [DOI] [PubMed] [Google Scholar]
- Förster I., Rajewsky K. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol. 1987 Apr;17(4):521–528. doi: 10.1002/eji.1830170414. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Allen P. M., Unanue E. R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. G., Courtenay B. M. Induction of B cell tolerance to polysaccharides by exhaustive immunization and during immunosuppression with cyclophosphamide. Eur J Immunol. 1974 Sep;4(9):603–608. doi: 10.1002/eji.1830040905. [DOI] [PubMed] [Google Scholar]
- Ishioka G. Y., Lamont A. G., Thomson D., Bulbow N., Gaeta F. C., Sette A., Grey H. M. MHC interaction and T cell recognition of carbohydrates and glycopeptides. J Immunol. 1992 Apr 15;148(8):2446–2451. [PubMed] [Google Scholar]
- Jacob J., Kassir R., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991 May 1;173(5):1165–1175. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob J., Kelsoe G., Rajewsky K., Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991 Dec 5;354(6352):389–392. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- Jacob J., Przylepa J., Miller C., Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)acetyl. III. The kinetics of V region mutation and selection in germinal center B cells. J Exp Med. 1993 Oct 1;178(4):1293–1307. doi: 10.1084/jem.178.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb C., Fuchs B., Weiler E. The thymus-independent antigen alpha(1-3) dextran elicits proliferation of precursors for specific IgM antibody-producing cells (memory cells), which are revealed by LPS stimulation in soft agar cultures and detected by immunoblot. Eur J Immunol. 1993 Nov;23(11):2959–2966. doi: 10.1002/eji.1830231135. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Zhang J., Lane P. J., Chan E. Y., MacLennan I. C. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991 Dec;21(12):2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- Maizels N., Lau J. C., Blier P. R., Bothwell A. The T-cell independent antigen, NP-ficoll, primes for a high affinity IgM anti-NP response. Mol Immunol. 1988 Dec;25(12):1277–1282. doi: 10.1016/0161-5890(88)90042-9. [DOI] [PubMed] [Google Scholar]
- Mond J. J., Lees A., Snapper C. M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Möller G., Fernandez C. Immunological tolerance to the thymus-independent antigen dextran can be abrogated by thymus-dependent dextran conjugates: evidence against clonal deletion as the mechanism of tolerance induction. Scand J Immunol. 1978;8(1):29–37. doi: 10.1111/j.1365-3083.1978.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Seppälä I., Mäkelä O. Antigenicity of dextran-protein conjugates in mice. Effect of molecular weight of the carbohydrate and comparison of two modes of coupling. J Immunol. 1989 Aug 15;143(4):1259–1264. [PubMed] [Google Scholar]
- Stedra J., Cerny J. Distinct pathways of B cell differentiation. I. Residual T cells in athymic mice support the development of splenic germinal centers and B cell memory without an induction of antibody. J Immunol. 1994 Feb 15;152(4):1718–1726. [PubMed] [Google Scholar]
- Sverremark E., Fernandez C. Germinal center formation following immunization with the polysaccharide dextran B512 is substantially increased by cholera toxin. Int Immunol. 1998 Jul;10(7):851–859. doi: 10.1093/intimm/10.7.851. [DOI] [PubMed] [Google Scholar]
- Sverremark E., Fernandez C. Immunogenicity of bacterial carbohydrates: cholera toxin modulates the immune response against dextran B512. Immunology. 1997 Sep;92(1):153–159. doi: 10.1046/j.1365-2567.1997.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Morita C. T., Tanaka Y., Nieves E., Brenner M. B., Bloom B. R. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995 May 11;375(6527):155–158. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- Wang D., Wells S. M., Stall A. M., Kabat E. A. Reaction of germinal centers in the T-cell-independent response to the bacterial polysaccharide alpha(1-->6)dextran. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2502–2506. doi: 10.1073/pnas.91.7.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker L. S., Scher I. X-linked immune deficiency (xid) of CBA/N mice. Curr Top Microbiol Immunol. 1986;124:87–101. doi: 10.1007/978-3-642-70986-9_6. [DOI] [PubMed] [Google Scholar]
- Willers J., Weiler E., Kolb C. Stimulation of the same B-cell population by thymus-independent dextran and by thymus-dependent oligosaccharide-carrier. Scand J Immunol. 1995 Sep;42(3):345–352. doi: 10.1111/j.1365-3083.1995.tb03666.x. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu Y. J., MacLennan I. C., Gray D., Lane P. J. B cell memory to thymus-independent antigens type 1 and type 2: the role of lipopolysaccharide in B memory induction. Eur J Immunol. 1988 Sep;18(9):1417–1424. doi: 10.1002/eji.1830180918. [DOI] [PubMed] [Google Scholar]