Abstract
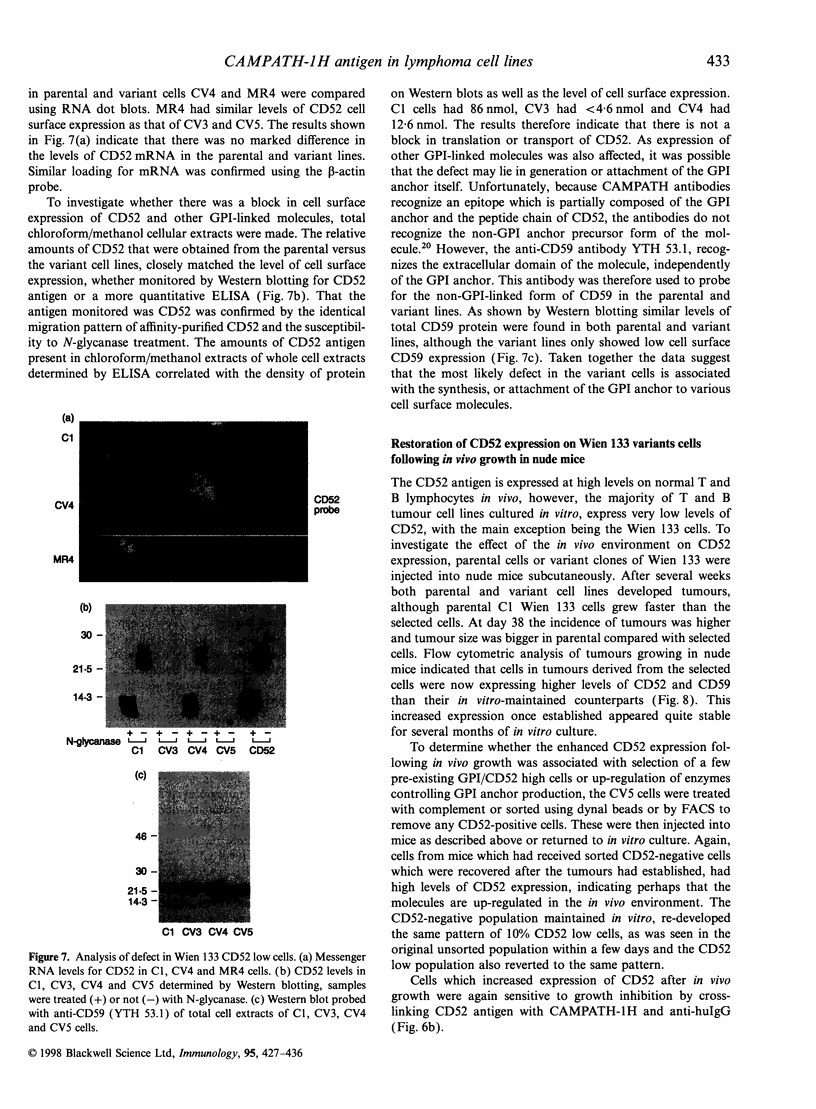
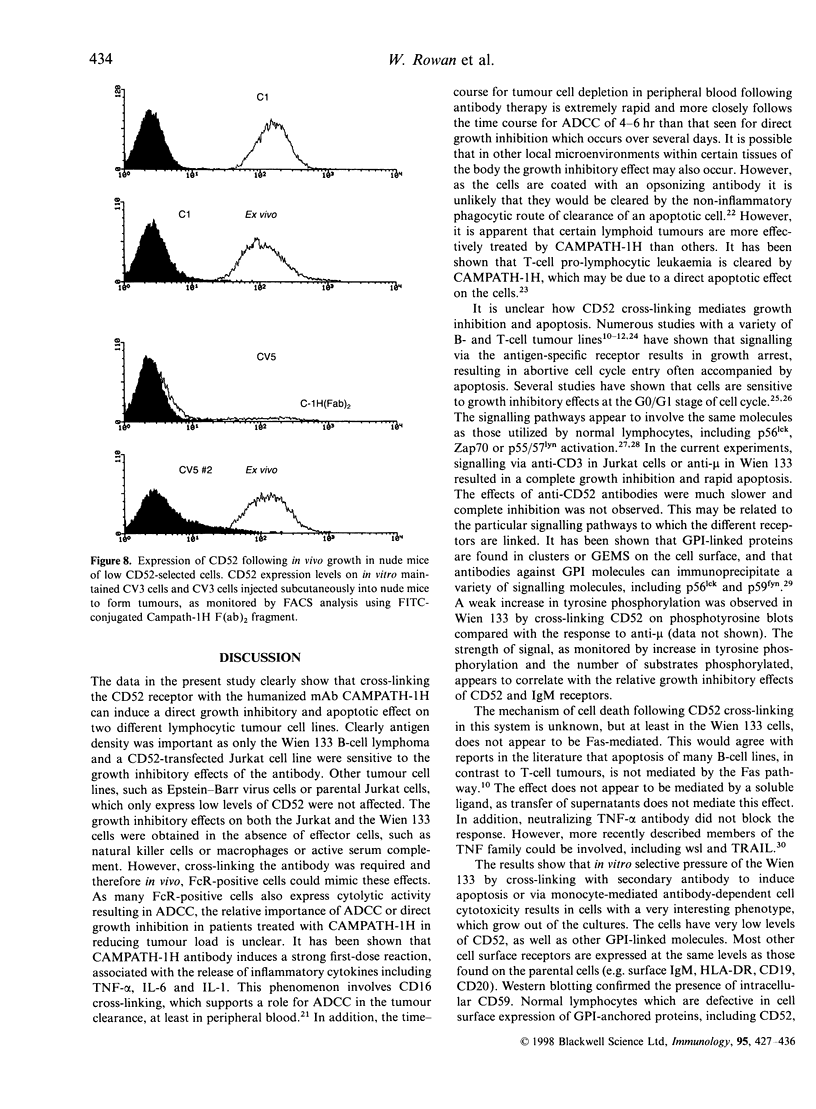
The CAMPATH-1H (CD52) antigen is a 21 000-28 000 MW glycopeptide antigen that is highly expressed on T and B lymphocytes and is coupled to the membrane by a glycosylphosphatidylinositol (GPI) anchoring structure. The humanized CAMPATH-1H anti-CD52 antibody is extremely effective at mediating depletion of both normal and tumorigenic lymphocytes in vivo and has been used in clinical trials for lymphoid malignancy and rheumatoid arthritis. Cross-linking GPI-anchored molecules, including CD52, on the surface of T lymphocytes in the presence of phorbol 12-myristate 13-acetate or anti-CD3, results in cellular activation. In the present study we have investigated the functional effects of cross-linking CD52 on T and B tumour cell lines. Cross-linking CD52 on either a B-cell line, Wien 133, which expresses high levels of endogenous CD52 or Jurkat T cells transfected and selected to express high levels of CD52 resulted in growth inhibition. This effect showed slower kinetics and occurred in a lower percentage of cells than growth inhibition stimulated via T- or B-cell receptors. Growth inhibition of the Wien 133 line was followed by the induction of apoptosis, which appeared independent of the Fas/Fas L pathway. Wien 133 cells surviving anti-CD52 treatment were selected and cloned and found to have down-regulated CD52 expression, with a characteristic biphasic pattern of 10% CD52-positive, 90% negative by fluorescence-activated cell sorter analysis. Interestingly, surface expression of other GPI-linked molecules, such as CD59 and CD55, was also down-regulated, but other transmembrane molecules such as surface IgM, CD19, CD20, HLA-DR were unaffected. The present study and previous work show that this is due to a defect in the synthesis of mature GPI precursors. Separation of CD52-positive and negative populations in vitro resulted in a rapid redistribution to the mixed population. Injection of CD52-negative cells into nude mice to form a subcutaneous tumour resulted in a substantial increase in expression of CD52. These results suggest that the defect in the Wien 133 cells is reversible, although the molecular mechanism is not clear. These observations have relevance to the clinical situation as a similar GPI-negative phenotype has been reported to occur in lymphocytes following CAMPATH-1H treatment in vivo.
Full text
PDF


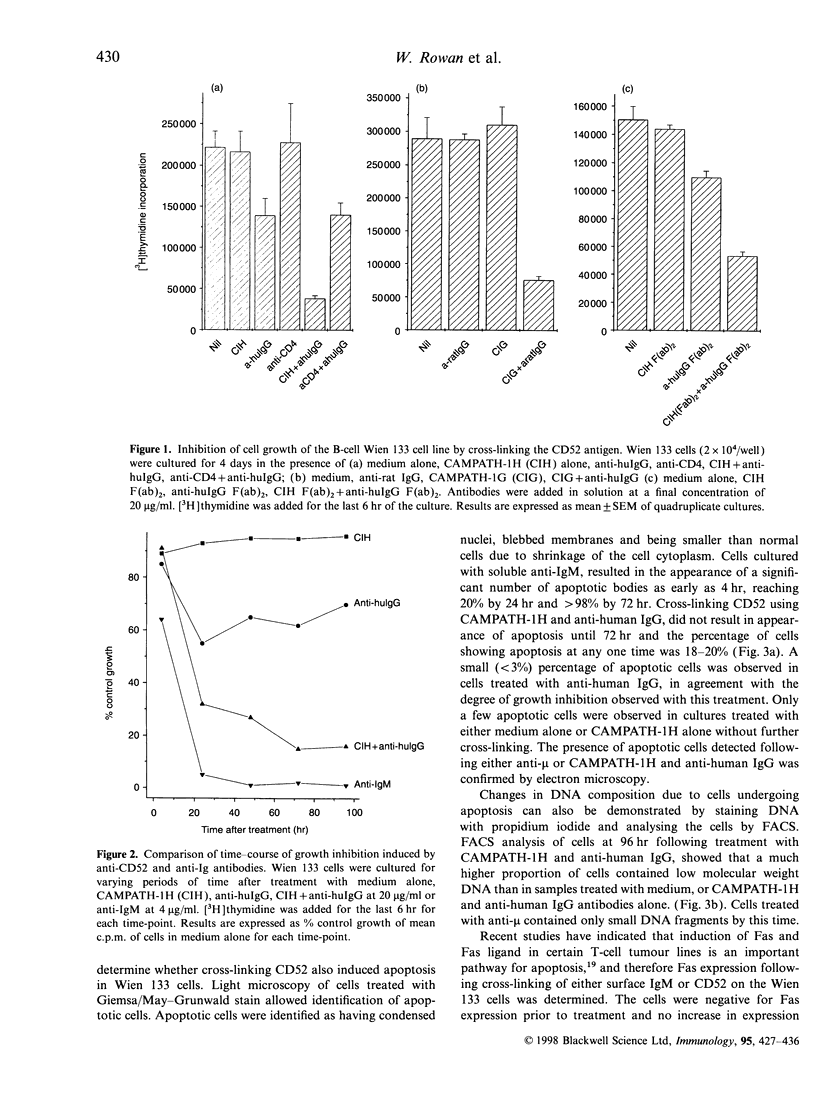
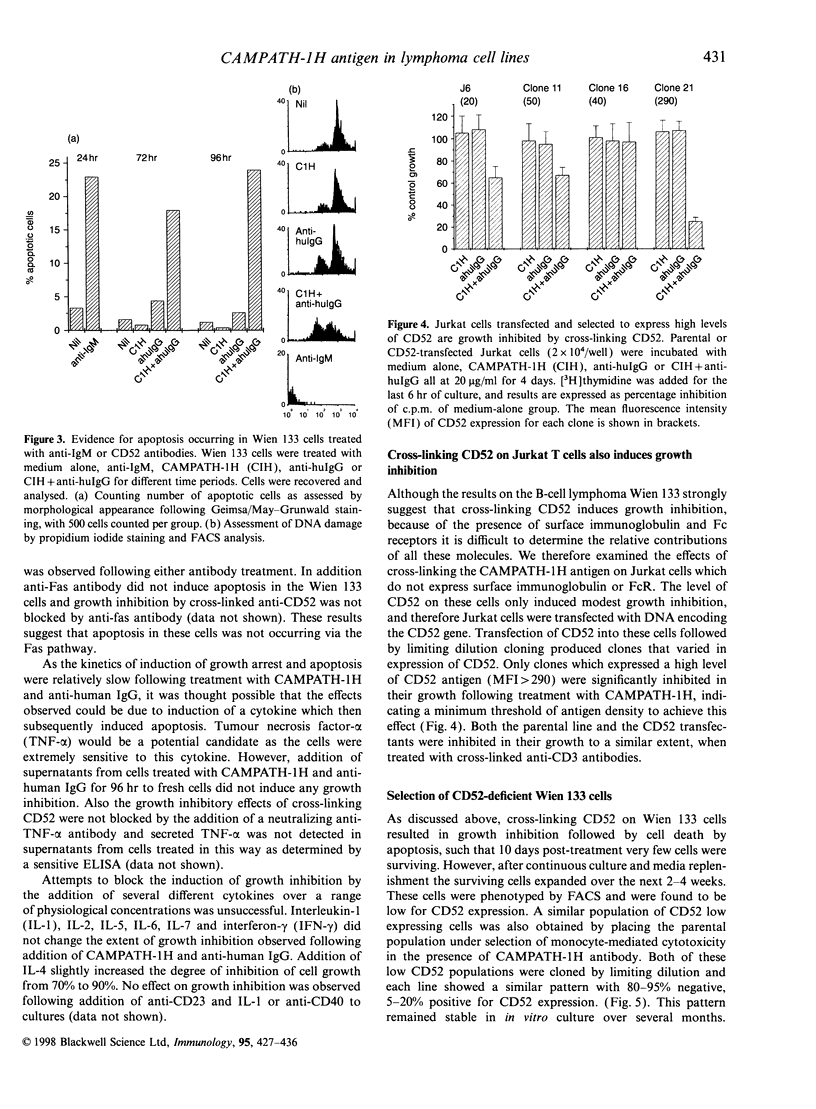
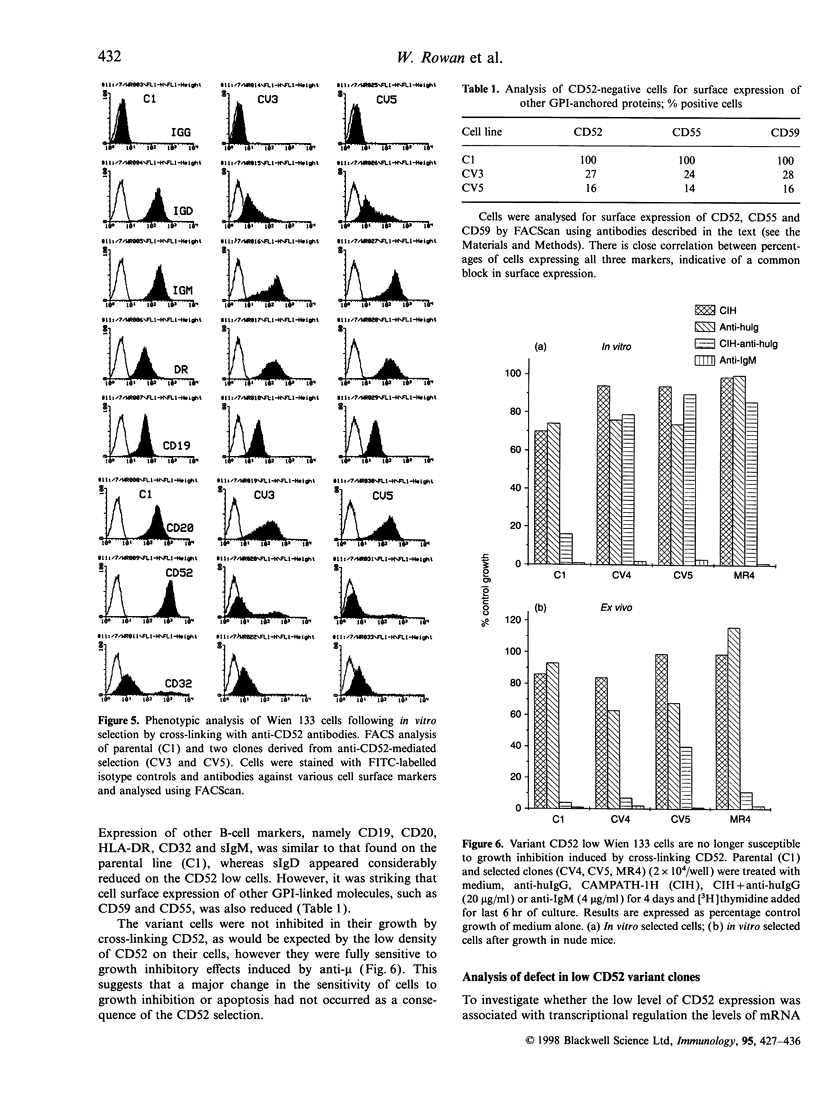


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindon C. I., Hale G., Waldmann H. Importance of antigen specificity for complement-mediated lysis by monoclonal antibodies. Eur J Immunol. 1988 Oct;18(10):1507–1514. doi: 10.1002/eji.1830181006. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Baxter G., Cooper H., Rowan W., Regan T., Tite J., Rapson N. Emergence of CD52-, glycosylphosphatidylinositol-anchor-deficient lymphocytes in rheumatoid arthritis patients following Campath-1H treatment. Int Immunol. 1996 Mar;8(3):325–334. doi: 10.1093/intimm/8.3.325. [DOI] [PubMed] [Google Scholar]
- Bryson G. J., Harmon B. V., Collins R. J. A flow cytometric study of cell death: failure of some models to correlate with morphological assessment. Immunol Cell Biol. 1994 Feb;72(1):35–41. doi: 10.1038/icb.1994.6. [DOI] [PubMed] [Google Scholar]
- Crowe J. S., Hall V. S., Smith M. A., Cooper H. J., Tite J. P. Humanized monoclonal antibody CAMPATH-1H: myeloma cell expression of genomic constructs, nucleotide sequence of cDNA constructs and comparison of effector mechanisms of myeloma and Chinese hamster ovary cell-derived material. Clin Exp Immunol. 1992 Jan;87(1):105–110. doi: 10.1111/j.1365-2249.1992.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Dyer M. J., Hale G., Hayhoe F. G., Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989 May 1;73(6):1431–1439. [PubMed] [Google Scholar]
- Eischen C. M., Williams B. L., Zhang W., Samelson L. E., Lynch D. H., Abraham R. T., Leibson P. J. ZAP-70 tyrosine kinase is required for the up-regulation of Fas ligand in activation-induced T cell apoptosis. J Immunol. 1997 Aug 1;159(3):1135–1139. [PubMed] [Google Scholar]
- Ezhevsky S. A., Toyoshima H., Hunter T., Scott D. W. Role of cyclin A and p27 in anti-IgM induced G1 growth arrest of murine B-cell lymphomas. Mol Biol Cell. 1996 Apr;7(4):553–564. doi: 10.1091/mbc.7.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale C., Bartholomew M., Taylor V., Stables J., Topley P., Tite J. Recognition of CD52 allelic gene products by CAMPATH-1H antibodies. Immunology. 1996 Jun;88(2):183–190. doi: 10.1111/j.1365-2567.1996.tb00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale G., Cobbold S. P., Waldmann H., Easter G., Matejtschuk P., Coombs R. R. Isolation of low-frequency class-switch variants from rat hybrid myelomas. J Immunol Methods. 1987 Oct 23;103(1):59–67. doi: 10.1016/0022-1759(87)90242-0. [DOI] [PubMed] [Google Scholar]
- Hale G., Dyer M. J., Clark M. R., Phillips J. M., Marcus R., Riechmann L., Winter G., Waldmann H. Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988 Dec 17;2(8625):1394–1399. doi: 10.1016/s0140-6736(88)90588-0. [DOI] [PubMed] [Google Scholar]
- Hale G. Synthetic peptide mimotope of the CAMPATH-1 (CD52) antigen, a small glycosylphosphatidylinositol-anchored glycoprotein. Immunotechnology. 1995 Dec;1(3-4):175–187. doi: 10.1016/1380-2933(95)00017-8. [DOI] [PubMed] [Google Scholar]
- Hale G., Xia M. Q., Tighe H. P., Dyer M. J., Waldmann H. The CAMPATH-1 antigen (CDw52). Tissue Antigens. 1990 Mar;35(3):118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Hasbold J., Klaus G. G. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990 Aug;20(8):1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- Hertenstein B., Wagner B., Bunjes D., Duncker C., Raghavachar A., Arnold R., Heimpel H., Schrezenmeier H. Emergence of CD52-, phosphatidylinositolglycan-anchor-deficient T lymphocytes after in vivo application of Campath-1H for refractory B-cell non-Hodgkin lymphoma. Blood. 1995 Aug 15;86(4):1487–1492. [PubMed] [Google Scholar]
- Ishigami T., Kim K. M., Horiguchi Y., Higaki Y., Hata D., Heike T., Katamura K., Mayumi M., Mikawa H. Anti-IgM antibody-induced cell death in a human B lymphoma cell line, B104, represents a novel programmed cell death. J Immunol. 1992 Jan 15;148(2):360–368. [PubMed] [Google Scholar]
- Nacheva E., Fischer P., Karpas A., Sherrington P., Hayhoe F. G., Manolov G., Manolova Y., Ferstl G., Haas O., Gadner H. Complex translocation t(8;12;14) in a cell line derived from a child with nonendemic Burkitt-type acute lymphoblastic leukemia. Cancer Genet Cytogenet. 1987 Sep;28(1):145–153. doi: 10.1016/0165-4608(87)90364-5. [DOI] [PubMed] [Google Scholar]
- Nakashima I., Pu M. Y., Hamaguchi M., Iwamoto T., Rahman S. M., Zhang Y. H., Kato M., Ohkusu K., Katano Y., Yoshida T. Pathway of signal delivery to murine thymocytes triggered by co-crosslinking CD3 and Thy-1 for cellular DNA fragmentation and growth inhibition. J Immunol. 1993 Oct 1;151(7):3511–3520. [PubMed] [Google Scholar]
- Pawson R., Dyer M. J., Barge R., Matutes E., Thornton P. D., Emmett E., Kluin-Nelemans J. C., Fibbe W. E., Willemze R., Catovsky D. Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol. 1997 Jul;15(7):2667–2672. doi: 10.1200/JCO.1997.15.7.2667. [DOI] [PubMed] [Google Scholar]
- Peter M. E., Dhein J., Ehret A., Hellbardt S., Walczak H., Moldenhauer G., Krammer P. H. APO-1 (CD95)-dependent and -independent antigen receptor-induced apoptosis in human T and B cell lines. Int Immunol. 1995 Nov;7(11):1873–1877. doi: 10.1093/intimm/7.11.1873. [DOI] [PubMed] [Google Scholar]
- Riechmann L., Clark M., Waldmann H., Winter G. Reshaping human antibodies for therapy. Nature. 1988 Mar 24;332(6162):323–327. doi: 10.1038/332323a0. [DOI] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Scott D. W., Livnat D., Pennell C. A., Keng P. Lymphoma models for B cell activation and tolerance. III. Cell cycle dependence for negative signalling of WEHI-231 B lymphoma cells by anti-mu. J Exp Med. 1986 Jul 1;164(1):156–164. doi: 10.1084/jem.164.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanová I., Horejsí V., Ansotegui I. J., Knapp W., Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991 Nov 15;254(5034):1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- Takeda J., Miyata T., Kawagoe K., Iida Y., Endo Y., Fujita T., Takahashi M., Kitani T., Kinoshita T. Deficiency of the GPI anchor caused by a somatic mutation of the PIG-A gene in paroxysmal nocturnal hemoglobinuria. Cell. 1993 May 21;73(4):703–711. doi: 10.1016/0092-8674(93)90250-t. [DOI] [PubMed] [Google Scholar]
- Taylor V. C., Sims M., Brett S., Field M. C. Antibody selection against CD52 produces a paroxysmal nocturnal haemoglobinuria phenotype in human lymphocytes by a novel mechanism. Biochem J. 1997 Mar 15;322(Pt 3):919–925. doi: 10.1042/bj3220919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D., Boldin M., Varfolomeev E., Beyaert R., Vandenabeele P., Fiers W. Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett. 1997 Jun 23;410(1):96–106. doi: 10.1016/s0014-5793(97)00553-x. [DOI] [PubMed] [Google Scholar]
- Wing M. G., Moreau T., Greenwood J., Smith R. M., Hale G., Isaacs J., Waldmann H., Lachmann P. J., Compston A. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest. 1996 Dec 15;98(12):2819–2826. doi: 10.1172/JCI119110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M. Q., Hale G., Lifely M. R., Ferguson M. A., Campbell D., Packman L., Waldmann H. Structure of the CAMPATH-1 antigen, a glycosylphosphatidylinositol-anchored glycoprotein which is an exceptionally good target for complement lysis. Biochem J. 1993 Aug 1;293(Pt 3):633–640. doi: 10.1042/bj2930633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M. Q., Tone M., Packman L., Hale G., Waldmann H. Characterization of the CAMPATH-1 (CDw52) antigen: biochemical analysis and cDNA cloning reveal an unusually small peptide backbone. Eur J Immunol. 1991 Jul;21(7):1677–1684. doi: 10.1002/eji.1830210714. [DOI] [PubMed] [Google Scholar]