Abstract
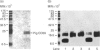
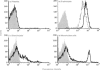
CD59 is the sole characterized regulator of the complement membrane attack complex in humans. It is very widely and abundantly distributed, being present on all circulating cells, endothelia and epithelia, and in most tissues. CD59 analogues in rodents are distributed similarly. Interest in complement regulation in the pig has developed out of the current enthusiasm to exploit this species as a donor in xenotransplantation of organs to humans. We have recently isolated and cloned the pig analogue of human CD59. We here report the development and characterization of monoclonal antibodies against pig CD59. We have used these antibodies to develop efficient methods for the purification of pig CD59 to homogeneity from erythrocyte membranes and have obtained new information on the structure and function of the purified protein. The antibodies were found to function well in immunohistochemistry and have been used to perform a comprehensive survey of the expression and distribution of pig CD59 on cells and in organs of normal pigs. Pig CD59, like human CD59, is broadly expressed but there are some striking differences in tissue distribution, notably the apparent lack of pig CD59 on circulating platelets and on a subset of leucocytes in blood and lymphoid organs. The reported findings have important implications for the current approaches to avoiding complement-mediated hyperacute rejection in pig-to-human xenografts.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraha A., Morgan B. P., Luzio J. P. The preparation and characterization of monoclonal antibodies to human complement component C8 and their use in purification of C8 and C8 subunits. Biochem J. 1988 Apr 1;251(1):285–292. doi: 10.1042/bj2510285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington C. A., Richards A. C., van den Bogaerde J., Tucker A. W., White D. J. Complement activation, its consequences, and blockade by gene transfer. World J Surg. 1997 Nov-Dec;21(9):907–912. doi: 10.1007/s002689900325. [DOI] [PubMed] [Google Scholar]
- Davies A., Simmons D. L., Hale G., Harrison R. A., Tighe H., Lachmann P. J., Waldmann H. CD59, an LY-6-like protein expressed in human lymphoid cells, regulates the action of the complement membrane attack complex on homologous cells. J Exp Med. 1989 Sep 1;170(3):637–654. doi: 10.1084/jem.170.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke R., Mannuss B., Trautwein G. Immunological and enzyme histochemical identification of lymphocyte subpopulations in pig lymphoreticular tissues. Zentralbl Veterinarmed B. 1985 Oct;32(9):660–678. doi: 10.1111/j.1439-0450.1985.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Diamond L. E., McCurry K. R., Martin M. J., McClellan S. B., Oldham E. R., Platt J. L., Logan J. S. Characterization of transgenic pigs expressing functionally active human CD59 on cardiac endothelium. Transplantation. 1996 Apr 27;61(8):1241–1249. doi: 10.1097/00007890-199604270-00021. [DOI] [PubMed] [Google Scholar]
- Fodor W. L., Rollins S. A., Bianco-Caron S., Burton W. V., Guilmette E. R., Rother R. P., Zavoico G. B., Squinto S. P. Primate terminal complement inhibitor homologues of human CD59. Immunogenetics. 1995;41(1):51–51. doi: 10.1007/BF00188435. [DOI] [PubMed] [Google Scholar]
- Funabashi K., Okada N., Matsuo S., Yamamoto T., Morgan B. P., Okada H. Tissue distribution of complement regulatory membrane proteins in rats. Immunology. 1994 Mar;81(3):444–451. [PMC free article] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Hughes T. R., Piddlesden S. J., Williams J. D., Harrison R. A., Morgan B. P. Isolation and characterization of a membrane protein from rat erythrocytes which inhibits lysis by the membrane attack complex of rat complement. Biochem J. 1992 May 15;284(Pt 1):169–176. doi: 10.1042/bj2840169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S., Waldmann H., Lachmann P. J. Distribution of protectin (CD59), a complement membrane attack inhibitor, in normal human tissues. Lab Invest. 1991 Nov;65(5):532–537. [PubMed] [Google Scholar]
- Morgan B. P. Isolation and characterization of the complement-inhibiting protein CD59 antigen from platelet membranes. Biochem J. 1992 Mar 1;282(Pt 2):409–413. doi: 10.1042/bj2820409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ninomiya H., Sims P. J. The human complement regulatory protein CD59 binds to the alpha-chain of C8 and to the "b"domain of C9. J Biol Chem. 1992 Jul 5;267(19):13675–13680. [PubMed] [Google Scholar]
- Powell M. B., Marchbank K. J., Rushmere N. K., van den Berg C. W., Morgan B. P. Molecular cloning, chromosomal localization, expression, and functional characterization of the mouse analogue of human CD59. J Immunol. 1997 Feb 15;158(4):1692–1702. [PubMed] [Google Scholar]
- Rudd P. M., Morgan B. P., Wormald M. R., Harvey D. J., van den Berg C. W., Davis S. J., Ferguson M. A., Dwek R. A. The glycosylation of the complement regulatory protein, human erythrocyte CD59. J Biol Chem. 1997 Mar 14;272(11):7229–7244. doi: 10.1074/jbc.272.11.7229. [DOI] [PubMed] [Google Scholar]
- Rushmere N. K., Tomlinson S., Morgan B. P. Expression of rat CD59: functional analysis confirms lack of species selectivity and reveals that glycosylation is not required for function. Immunology. 1997 Apr;90(4):640–646. doi: 10.1046/j.1365-2567.1997.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon K. R., Chan M., Finberg R. W. Expression of GPI-anchored complement regulatory proteins CD55 and CD59 differentiates two subpopulations of human CD56+ CD3- lymphocytes (NK cells). Cell Immunol. 1995 Oct 15;165(2):294–301. doi: 10.1006/cimm.1995.1217. [DOI] [PubMed] [Google Scholar]
- Terstappen L. W., Nguyen M., Lazarus H. M., Medof M. E. Expression of the DAF (CD55) and CD59 antigens during normal hematopoietic cell differentiation. J Leukoc Biol. 1992 Dec;52(6):652–660. doi: 10.1002/jlb.52.6.652. [DOI] [PubMed] [Google Scholar]
- van den Berg C. W., Harrison R. A., Morgan B. P. A rapid method for the isolation of analogues of human CD59 by preparative SDS-PAGE: application to pig CD59. J Immunol Methods. 1995 Feb 27;179(2):223–231. doi: 10.1016/0022-1759(94)00288-8. [DOI] [PubMed] [Google Scholar]
- van den Berg C. W., Harrison R. A., Morgan B. P. The sheep analogue of human CD59: purification and characterization of its complement inhibitory activity. Immunology. 1993 Mar;78(3):349–357. [PMC free article] [PubMed] [Google Scholar]
- van den Berg C. W., Morgan B. P. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994 Apr 15;152(8):4095–4101. [PubMed] [Google Scholar]