Abstract
The metabolic and cell cycle status of primary T lymphocytes conditions their susceptibility to human immunodeficiency virus (HIV) and HIV-derived vectors. While in fully quiescent T lymphocytes the reverse transcription and nuclear import of these retroelements are impaired, leading to an abortive infection, various stimuli can induce a state of virus permissiveness. Here, we studied the modalities by which interleukin-7 (IL-7), an important controller of T-cell homeostasis, exerts this effect. IL-7-exposed cord blood T lymphocytes proliferated and were efficiently transduced by HIV-derived vectors. In contrast, similarly treated adult peripheral blood (PB) T lymphocytes failed to divide, and only a subset of these cells became infectible. HIV-resistant and -sensitive subsets of IL-7-treated PB T lymphocytes differed in cell cycle status but not in naïve, memory, or activation phenotypes. Nuclear factor of activated T cells was not induced by IL-7, and cyclosporine did not prevent HIV-mediated gene transfer. Furthermore, the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin blocked IL-7-induced cell survival and Bcl-2 synthesis but had no effect on the acquisition of HIV susceptibility, suggesting that IL-7-induced HIV type 1 permissiveness is not mediated by the PI-3 K pathway and that, perhaps, the Jak/STAT5 pathway, the other known mediator of IL-7-triggered signaling in T cells, governs this process.
The preintegration complex of lentiviruses such as human immunodeficiency virus (HIV) is actively transported to the nucleus, allowing infection of nondividing cells such as terminally differentiated macrophages (3, 42). Correspondingly, lentivirus vectors can transduce nonmitotic targets such as adult neurons (25). Nevertheless, quiescent T lymphocytes do not support HIV replication and cannot be transduced with HIV-derived vectors. The block resides at an early postentry stage of the virus life cycle, includes a failure to complete reverse transcription, and is likely multifactorial (20, 35, 36, 43, 44). Addition of nucleosides to resting T lymphocytes indeed restores viral DNA synthesis, but infection remains nonproductive, pointing to additional impedimenta (19).
Most circulating peripheral T lymphocytes and many in lymphoid tissues are in a G0 resting state. Various stimuli, including growth factors and antigen-mediated T-cell activation, can induce their progression through the cell cycle. Treatment with anti-CD3 antibodies alone leads T lymphocytes only into G1a, where they remain refractory to HIV infection (20). Costimulation through the CD28 receptor is accompanied by a transition into G1b and by the establishment of a state of permissiveness to HIV and HIV-based vectors. Similarly, the constitutive expression of an activated form of nuclear factor of activated T cells (NFAT) or treatment with cytokines such as interleukin-2 (IL-2), IL-4, IL-7, or IL-15 can render T lymphocytes susceptible to HIV-mediated transduction (18, 38). However, what particular changes in the intracellular milieu are responsible for inducing HIV permissiveness in T cells remain undefined. The question is of importance, because one of the primary in vivo targets of HIV type 1 (HIV-1) is a T cell that fails to exhibit the hallmarks of a fully activated lymphocyte (48).
IL-7 is a lymphokine critical for T-lymphocyte development, which also promotes the growth and survival of naïve and antigen-stimulated mature T cells (16). In HIV-infected individuals, circulating levels of IL-7 are strongly correlated with CD4+-T-cell loss, reflecting at least in part an increased production of this cytokine by dendritic cells within lymphocyte-depleted tissues (26). While this probably represents a homeostatic response to lymphopenia, it may contribute to increased viral burden and accelerated disease progression. Higher circulating levels of IL-7 are indeed associated with greater viral loads, corroborating the stimulating effect of IL-7 on HIV-1 replication in vitro (5, 34).
With the long-term goal of characterizing the molecular events that render resting T cells supportive of HIV-1 replication and of identifying cellular cofactors required for the immediate early steps of viral replication, we investigated the modalities through which IL-7 exert this influence. The results of our experiments shed light on the signaling events that govern IL-7-induced HIV susceptibility of primary T cells. Furthermore, our data reveal the existence of subset-specific differences in the cytokine response, hence HIV permissiveness, of mature T lymphocytes.
MATERIALS AND METHODS
Preparation of primary human T cells.
Peripheral blood (PB) mononuclear cells were isolated from buffy coat of PB from adult healthy donors or from cord blood (CB) by separation over a Ficoll-Hypaque (Pharmacia) gradient. Monocytes were removed by plastic adherence for 2 h at 37°C. B cells, NK cells, macrophages, activated T lymphocytes, and, for some experiments, CD8 cells were removed by preincubation with antibodies against CD19, CD16, CD14, HLA-DR, CD25, CD69, and, if needed, CD8 (Pharmingen), followed by magnetic removal of antibody-coated cells with Dynabeads (Dynal). This protocol typically resulted in a population of resting T cells with a degree of purity superior to 99.5%, as determined by postpurification fluorescence-activated cell sorter (FACS) analysis. Cells were cultured in RPMI 1640 supplemented with 10% fetal calf serum, penicillin and streptomycin at 5 mM (GIBCO BRL), and 2 mM glutamine (GIBCO BRL). For stimulation, T cells were treated with phytohemagglutinin (Sigma) at 3 μg/ml and then maintained in medium containing recombinant IL-2 (10 U/ml; Sigma). Alternatively, cells were incubated with IL-7 (25 ng/ml; R&D System).
Flow cytometry.
For analysis of surface protein expression (CD25, CD69, HLA-DR, and IL-7 receptor α [IL-7Rα] and common γ [γc] chains), cells were incubated with the appropriate phycoerythrin-conjugated monoclonal antibody (purchased from Immunotech or Pharmingen) on ice for 30 min, washed two times, and fixed in phosphate-buffered saline-0.1% bovine serum albumin with 1% formaldehyde. Background fluorescence was measured by using an immunoglobulin isotype control antibody. Staining of green fluorescent protein (GFP) or yellow fluorescent protein (YFP) expression were analyzed on a FACScan using the CellQuest software (Becton Dickinson). Live cells were gated based on side scatter. When indicated, cells were sorted using a FACSVantage (Becton Dickinson).
Virus production and infections.
Replication-defective lentivirus vector particles pseudotyped with G protein of vesicular stomatitis virus (VSV G) envelope were produced by 3-plasmid transient cotransfection into 293T epithelial cell lines as previously described (25). The HIV-derived packaging construct was pCMVΔR8.2 or pCMVΔR8.91 (49). The HIV vector plasmids were ploxEF-GFP with the posttranscriptional regulatory element of woodchuck hepatitis virus (32) with or without the central polypurine tract (33, 47). Wild-type virus was produced by 293T cells transfection with R9, an X4-tropic HIV-1 molecular clone (15). Transfection medium was replaced after 16 h with fresh culture medium. Viral supernatants were harvested at 48 h posttransfection, filtered through 0.45-μm-pore-size nitrocellulose membranes, ultracentrifuged through a 20% sucrose cushion, resuspended in a minimal volume of medium, aliquoted, and stored frozen at −80°C until use. Vector titers were determined by infecting HeLa cells with serial dilutions of viral supernatants followed by flow cytometry analysis of GFP-positive cells 48 h after transduction. The titers of wild-type HIV-1 stocks were determined on long terminal repeat-lacZ-containing CD4+ HeLa P4 cells. Transduction was performed at a multiplicity of infection (MOI) of 1 to 300 at 3 or 4 days post-phytohemagglutinin (PHA) stimulation or IL-7 treatment. For dual transduction experiments, GFP and/or YFP vectors were used at the same time to infect the indicated cell types. The viral supernatant was washed off after 12 to 16 h, and transgene expression was analyzed by FACS 5 to 6 days later. The percentage of expected dually transduced cells has been calculated according to the following formula: [(GFPTotal + 100] YFPTotal. HIV-1 infections were conducted and monitored as previously described (1).
For cyclosporine (CSA) and wortmannin experiments, the drugs were added, respectively, 30 min and 1 h before PHA stimulation or IL-7 treatment.
PCR amplification.
Cells were sorted for GFP expression 5 days after the last round of infection with DNase-treated viral supernatants and lysed using the DNeasy kit lysis buffer (Qiagen AG). PCR amplification was performed using HIV- and β-globin-specific primers as previously described (1).
Amplified products were resolved by agarose gel electrophoresis.
Cell cycle analyses.
RNA and DNA content were measured as previously described (19, 20). Briefly, a total of 5 × 105 cells were suspended in a buffer containing 0.03% saponin (Sigma), and 7-amino-actinomycin D (7-AAD) (Sigma) was added at a final concentration of 20 μM. The cells were incubated at room temperature for 30 min and cooled on ice for at least 5 min before adding pyronin (PY) Y (Polysciences) at a final concentration of 5 μM. After incubation for an additional 10 min on ice, cells were analyzed with the CellQuest program.
Protein analyses.
Western blotting was performed as previously described (18), using an NFATc-specific mouse monoclonal antibody (7A6) and a Bcl-2-reactive rabbit polyclonal antibody (both from Santa Cruz Biotechnology).
RESULTS
IL-7-treated T cells become differentially permissive to lentivirus vector-mediated transduction and to HIV infection.
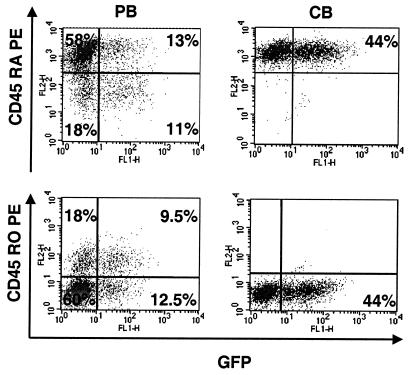
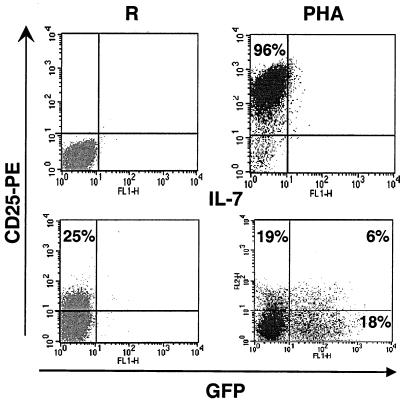
Highly purified quiescent T lymphocytes were obtained from adult PB or CB by negative selection of cells expressing a series of non-T-cell markers (CD19, CD11b, CD16, and CD14) as well as CD69, CD25, and HLA-DR. In most experiments, purified CD4 T cells served as targets, but when mixed populations of resting T lymphocytes were tested, no difference was observed between CD4 and CD8 cells. When kept in culture in the absence of cytokine, primary T lymphocytes died within a few days. In contrast, the addition of IL-7 induced the proliferation of about 20% of the CB T cells and the long-term survival of adult PB T lymphocytes, albeit without significant cell division for the latter cells. Transduction experiments were performed with lentivirus particles produced from a packaging construct that either contained all the HIV-1 genes except for envelope (for initial experiments) or had additional deletions in the virus for accessory genes (nef, vif, vpr, and vpu) (for subsequent manipulations) and with vectors expressing the enhanced GFP sequence downstream of the elongation factor 1-α. VSV G was used to pseudotype the particles. The targets were either fully quiescent T lymphocytes, PHA/IL-2-activated cells, or cells maintained in the presence of IL-7 for various lengths of time. Transduction efficiency was assessed at regular intervals, and at day 6 postinfection transgene expression was noted to be robust and stable. At an MOI that allowed transduction of more than 80% of PHA/IL-2-treated adult T lymphocytes, <0.1% GFP-positive cells could be detected in a resting population, whether or not accessory genes were present in the packaging construct (not illustrated). With IL-7, a significant proportion of adult T cells could be transduced with the HIV-based vector, irrespective of their naïve (CD45 RA+ RO−) or memory (CD45 RO+ RA−) phenotype, even though memory cells were more sensitive (Fig. 1, left panels). CB T lymphocytes, which are almost exclusively of the naïve type, were highly susceptible to HIV transduction (Fig. 1, right panels) and HIV-1 infection (see Fig. 3B). IL-7-treated adult T cells were refractory to an oncoretrovirus vector derived from murine leukemia virus, consistent with their nondividing state (not illustrated). Of note, 4 days of cytokine exposure was required to render PB T cells fully susceptible to HIV transduction, while only 1 to 2 days of PHA/IL-2 treatment was sufficient.
FIG. 1.
HIV-1-mediated transduction of IL-7-treated lymphocytes. CD4+ T cells purified from either PB (left panels) or CB (right panels) were treated with IL-7 for 4 days and exposed to a VSV-G-pseudotyped GFP-expressing HIV-based vector. Six days postinfection, cells were labeled with CD45RO- or RA-specific antibodies and analyzed for GFP expression by FACS. Results shown are representative of at least three independent experiments.
FIG. 3.
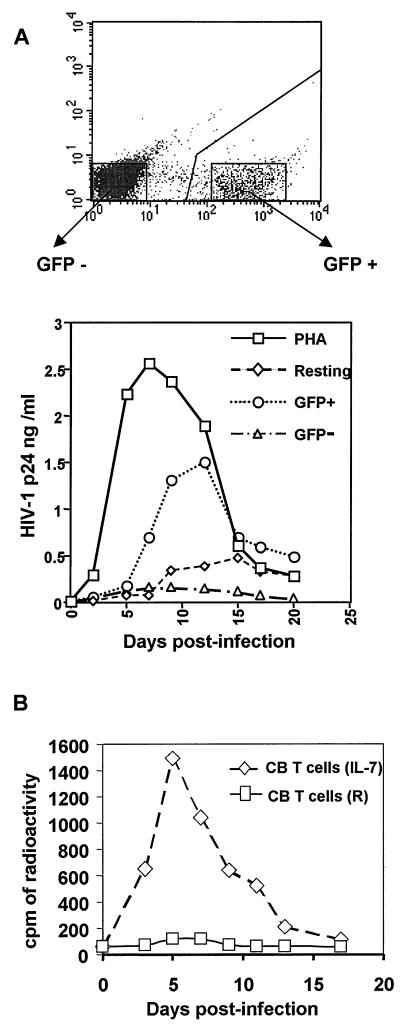
HIV-1 replication in PB and CB CD4 T lymphocytes. (A) HIV vector-resistant PB T cells do not support wild-type HIV-1 replication. IL-7-treated PB CD4+ T cells subjected to saturating rounds of transduction with a GFP vector were sorted for GFP expression and exposed with wild-type HIV-1. Untreated quiescent or PHA-activated cells served as controls. Viral replication was monitored by measuring p24 capsid production in the supernatant. RT measurements (not illustrated) gave similar results. (B) CB T cells are HIV-1 permissive. Resting or IL-7-treated CD4+ T cells were infected with wild-type HIV-1, and RT activity was assessed.
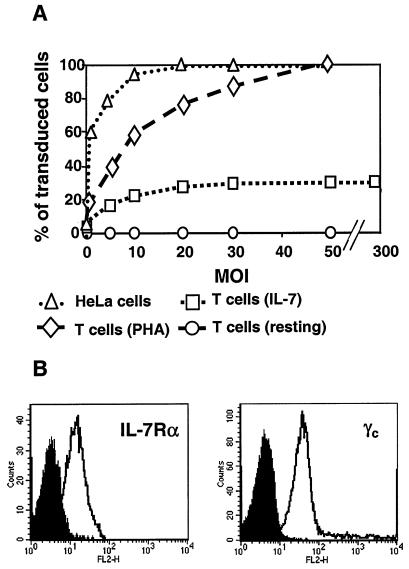
In spite of using an increasingly high MOI, the fraction of IL-7-treated PB T cells that ultimately could be transduced with the HIV-based vector reached a plateau at about 30 to 35% (Fig. 2A), even though all cells in the population expressed both the α and γ chains of the IL-7R (Fig. 2B). Moreover, adding IL-4 or IL-15 to IL-7 did not increase the final percentage of cells that could be infected (data not shown). To confirm the existence of differentially susceptible subsets of cells, we performed a dual transduction experiment by inoculating the lymphocytes with two different vectors, one expressing GFP and the other its yellow derivative YFP. Even at a low MOI of one of the vectors, the percentage of doubly transduced cells was significantly higher than expected if all targets in the culture had been equally susceptible, in sharp contrast with results obtained in HeLa or 293T cells (Table 1).
FIG. 2.
Limited transduction susceptibility of IL-7-treated PB T cells. (A) Increasing amounts of GFP vector were used to transduce PB T cells left quiescent (circles), activated with PHA (diamonds), or treated with IL-7 for 4 days (squares). HeLa cells (triangles) served as a control, and GFP expression was assessed by FACS analysis. (B) The expression of the IL-7Rα (left panel) and γc (right panel) chains (right panel) was analyzed by FACS on highly purified resting CD4+ T cells, using the corresponding phycoerythrin-conjugated monoclonal antibodies (open histogram) or an immunoglobulin isotype control (filled histogram). Upon IL-7 treatment, both subunits were downregulated (not shown).
TABLE 1.
Dual transduction experiment
| Vector cells | % Observed
|
% Expecteda
|
||
|---|---|---|---|---|
| GFP | YFP | GFP + YFP | GFP + YFP | |
| 293 T | 7.81 | 3.32 | 0.27 | 0.28 |
| Hela | 12.55 | 1.59 | 0.26 | 0.24 |
| IL-7 T | 15.4 | 0.61 | 2.4 | 0.53 |
Percentage expected if the process had been random; for calculation, see Materials and Methods.
While at a given time only a fraction of the cytokine-treated T cells was transduction-sensitive, one could not exclude that this subset turned over with time so that finally all cells within the population became infectible. To address this point, IL-7-treated T cells were repetitively challenged over a period of 2 weeks with increasing amounts of a vector containing a central polypurine tract, a modification previously shown to improve the efficiency of some early postentry steps of the virus life cycle (13, 46) (Table 2). In spite of such repetitive infections, the percentage of transduced cells remained no higher than 30 to 35%, indicating that following exposure to IL-7 the relative permissiveness of a given cell is established once and for all.
TABLE 2.
HIV-1 vector susceptibility of IL-7-treated PB T cells is fixed over time
| Dose of vectora (day) | % of transduced cells |
|---|---|
| 1× (D 4) | 4 |
| 2× (D 6) | 8.3 |
| 3× (D 8) | 15 |
| 4× (D 10) | 22 |
| 5× (D 12) | 30.3 |
| 6× (D 14) | 31.2 |
1×, MOI = 3; 2×, MOI = 6; 3×, MOI = 12; 4×, MOI = 25; 5×, MOI = 50; 6×, MOI = 100.
Finally, IL-7-treated CD4+ PB T cells that had been exposed to saturating amounts of an HIV-based vector were sorted according to the expression of its GFP transgene and used as targets for infection with wild-type HIV-1. While the virus efficiently replicated in the GFP+ cells, it failed to spread in the GFP− population (Fig. 3A). It is noteworthy that wild-type HIV-1 efficiently replicated in IL-7-treated CD4+ T lymphocytes isolated from CB (Fig. 3B), which is in full agreement with the data obtained with the HIV-based vector (Fig. 1).
Reverse transcription is blocked in IL-7-treated HIV-resistant T lymphocytes.
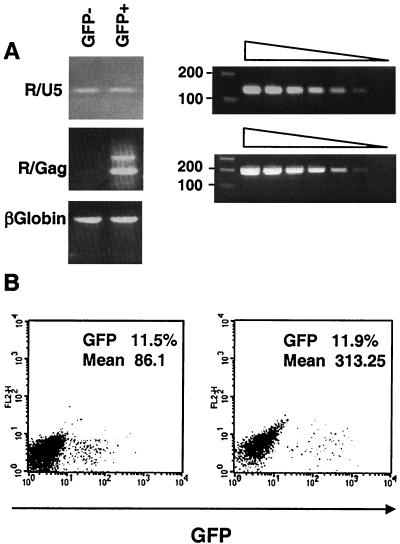
HIV infection of resting T lymphocytes is blocked at several preintegration levels, including reverse transcription (36, 43, 44). To examine the fate of the virus in IL-7-exposed, transduction-resistant adult PB T cells, the presence of early and late viral reverse transcripts was examined in GFP+ and GFP− cells sorted 5 days after inoculation with saturating levels of a GFP-expressing HIV vector (Fig. 4A). As expected, both types of reverse transcriptase (RT) products were found in GFP+ cells. While early reverse transcripts were also detected in cells that remained GFP negative, indicating that the virus had entered, they did not contain late reverse transcript. Thus, from a virological standpoint, these cells exhibited a phenotype very reminiscent of that observed in quiescent T cells. Further strengthening this parallel, when either resting or IL-7-exposed PB T cells were treated with PHA and IL-2 even as shortly as 24 h after inoculation, no increase in the percentage of transduced cells was observed (Fig. 4B and data not shown). Therefore, in none of these two situations is a stable infection intermediate established.
FIG. 4.
Block to reverse transcription in HIV-resistant IL-7-treated T cells. (A) Cells exposed to saturating rounds of transduction were sorted according to GFP expression and subjected to PCR analysis with primers specific for early (R/U5) or late (R/Gag) viral reverse transcripts or for β-globin cellular DNA. Sensitivities of the two primer pairs were controlled using the same fivefold dilutions of vector plasmid DNA. (B) Resting T cells were treated with IL-7 for 4 days and transduced with a GFP vector. Cells were maintained in the presence of the cytokine or additionally treated with PHA 24 h posttransduction. Six days later GFP expression was measured by FACS analysis. Percentages and mean cell fluorescence are indicated inside each panel. The absence of change in the fraction of GFP+ cells after PHA treatment indicates that infection was irreversibly blocked in vector-exposed, GFP-negative cells. The increased mean fluorescence of the positive cells likely results from the transcriptional activation of the integrated proviruses.
IL-7-induced HIV permissiveness does not correlate with expression of T-cell activation markers.
In search of predictors of HIV permissiveness, we examined the expression of prototypic cell surface activation antigens on cells maintained for 4 days in the presence of the cytokine. Throughout treatment, the cells remained negative for HLA-DR and CD69 (data not shown) but about 25% of them expressed low yet detectable levels of the IL-2Rα chain CD25. However, this molecule was not a predictive marker of lentivector susceptibility, as transduced cells appeared as often CD25− as CD25+ (Fig. 5). Furthermore, when we attempted to sort CD25 positive and negative cells prior to transduction with a HIV-based vector, both subpopulations appeared transducible, although the low levels of CD25 expression prevented a clear-cut separation of the two subpopulations (data not shown). Correlating this induction of CD25, about 20% of the cells started to divide when IL-2 was added to IL-7 (not illustrated). This clearly distinct phenotype, compared with that of T cells treated with IL-7 only, further indicates that IL-7 does not induce HIV permissiveness through the induction of CD25 expression and IL-2 secretion.
FIG. 5.
Lack of correlation between CD25 expression and HIV permissiveness. PB-derived primary T cells were either left quiescent (upper-left panel), stimulated with PHA (upper-right panel) or cultured in IL-7 for 4 days. IL-7-treated cells were either mock-infected (lower-left panel) or exposed to a GFP vector (lower-right panel). Six days later, cells were labeled with a phycoerythrin-conjugated CD25-specific antibody (anti-CD25PE) and analyzed by FACS.
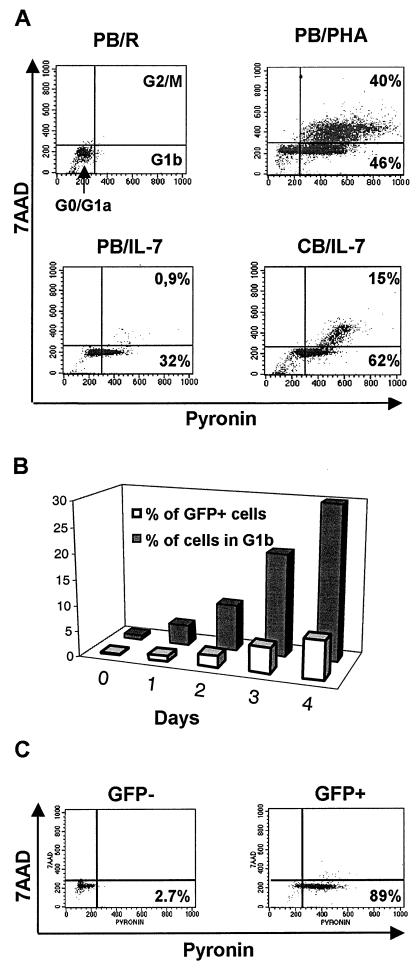
HIV permissiveness of IL-7-treated PB T lymphocytes coincides with progression into the early phase of the cell cycle.
The cell cycle distribution of quiescent, PHA-activated and IL-7-treated T cells was determined as previously described (19, 20). In brief, PY was used to measure increases in the amounts of cellular RNA that occur when cells progress from G0 to G1a and more prominently to G1b, and 7-AAD was used to monitor the accumulation of DNA that takes place in G2/M (Fig. 6A). Untreated resting T cells all resided in G0/G1a (Fig. 6A, upper-left panel). Consistent with the proliferation-inducing effect of IL-7 on CB T lymphocytes, these cells were found at all stages of the cycle (Fig. 6A, lower-right panel), which was reminiscent of PHA-treated PB T cells (Fig. 6A, upper-right panel). In contrast, at steady state, about 30% of IL-7-treated adult PB T lymphocytes were in G1b, while less than 1% was in G2/M (Fig. 6A, lower-left panel). It is noteworthy that the kinetics of entry into G1b and acquisition of HIV transduction permissiveness exhibited similar slopes, reaching maxima after 4 days for both parameters (Fig. 6B and data not shown).
FIG. 6.
HIV susceptibility of IL-7-treated cells correlates with their cell cycle status. (A) Cell cycle analysis of resting (upper-left panel), PHA-stimulated (upper-right panel), or IL-7-treated PB (lower-left panel) or CB (lower-right panel) T cells. Cells were stained for DNA and RNA levels using 7-AAD and PY, respectively, and analyzed by FACS. FACS-quadrant correspondence of different cell cycle phases is shown in the upper-left panel. Percentages of cells having exited G0 are indicated in other panels. (B) Kinetics of entry in G1b and acquisition of transduction permissiveness to a GFP vector (MOI of 4) of resting T cells or cells cultured for 1, 2, 3, or 4 days in the presence of IL-7. (C) FACS-based analysis of the cell cycle status of GFP− and GFP+ population of IL-7-treated PB T cells exposed to saturating rounds of transduction with a GFP vector and sorted for transgene expression.
To probe this parallel further, IL-7-treated T lymphocytes were repetitively exposed to a GFP-expressing lentivector until the percentage of transduced cells no longer increased. At that point, GFP+ and GFP− cells were sorted and a cell cycle analysis was performed on each subpopulation (Fig. 6C). Close to 90% of the cells expressing GFP were in G1b, whereas more than 95% of the GFP-negative cells remained in G0/G1a.
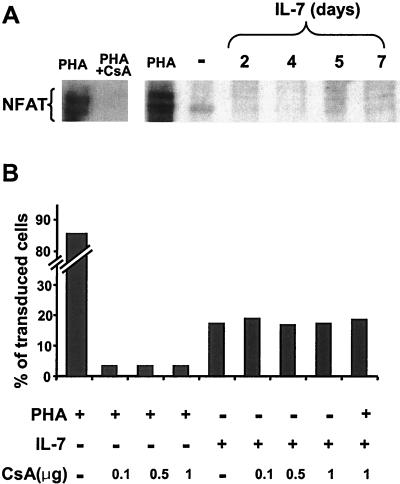
NFAT independence of IL-7-induced HIV permissiveness.
The forced constitutive expression of NFATc, a member of the family of the nuclear factor of activated T cells that is normally induced by T-cell receptor signaling (17), was previously shown to be sufficient to render T cells permissive for HIV replication in the presence of low levels of IL-2 (18). NFAT expression was thus monitored in adult resting T cells exposed to IL-7 for various lengths of time (Fig. 7A). While NFAT was readily induced in PHA-activated T lymphocytes, it remained undetectable in IL-7-treated cells. To confirm the NFAT-independence of HIV-mediated transduction of these targets, we used the drug CSA. This immunosuppressive molecule forms a complex with cyclophilin A, thereby inhibiting calcineurin, a calmodulin-dependent protein phosphatase that activates NFAT by mediating the dephosphorylation necessary for the nuclear translocation of this transcription factor (12, 22). Resting adult PB T lymphocytes were maintained in the presence of PHA or IL-7 and different concentrations of CSA for 3 days before challenge with a GFP-containing HIV-based vector, and transgene expression was examined 5 days later (Fig. 7B). Whereas CSA reduced the transduction of PHA-stimulated T cells by more than 10-fold, it had no inhibitory effect in the case of IL-7-treated T cells.
FIG. 7.
HIV-mediated transduction of IL-7-treated T cells is NFAT independent and CSA resistant. (A) Total cell extracts from highly purified resting PB T cells stimulated as indicated were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting with an anti-NFATc monoclonal antibody. PB T cells stimulated by PHA in the absence or presence of CSA (1 μg) are shown as controls (left panel). (B) Resting T cells were treated as indicated and exposed to a GFP vector 3 days later. GFP expression was assessed by FACS at day 6 posttransduction.
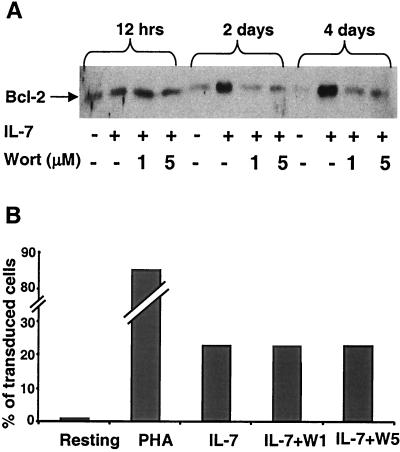
IL-7-induced HIV permissiveness is not mediated by the PI3K pathway.
In T cells, IL-7 can activate two partly distinct pathways (reviewed in reference 16). One proceeds through the Jak-STAT5 cascade, while the other uses the phosphatidylinositol 3-kinase (PI3K) as its central effector (8, 14, 21, 24, 30, 31, 41). This second pathway targets Bcl-2 and is responsible for the antiapoptotic effect of IL-7 (2, 4, 23, 39, 40). Taking advantage of the fact that wortmannin is a specific inhibitor of PI3K (27), we tested the influence of this drug on IL-7-induced survival, Bcl-2 synthesis and HIV susceptibility of T cells (Fig. 8). Wortmannin dramatically affected the antiapoptotic effect of IL-7 on PB T cells, with a 50% drop in the number of live cells after only 2 days in culture (data not shown). Shortly after purification, resting T cells contained readily detectable levels of Bcl-2, but these rapidly fell in the absence of treatment. In the presence of IL-7, Bcl-2 levels remained high and even increased over time, an effect completely blocked by wortmannin (Fig. 8A). In sharp contrast, the drug had no influence on the efficiency of HIV-mediated transduction (Fig. 8B). PI3K thus plays no role in the IL-7-mediated induction of HIV permissiveness in resting T cells.
FIG. 8.
The PI3K pathway does not mediate IL-7-induced HIV permissiveness. (A) Upregulation of Bcl-2 in IL-7-treated T lymphocytes is inhibited by wortmannin. Resting T cells were cultured in control medium or with 25 ng/ml IL-7 alone or together with 1 or 5 μM wortmannin (Wort) for indicated times. Bcl-2 expression was monitored by Western blot analysis of normalized amounts of proteins. (B) Transduction of PB T cells under indicated conditions: resting, PHA-activated, IL-7, or with IL-7 plus wortmannin at 1 or 5 μM (W1 and W5, respectively).
DISCUSSION
Although T lymphocytes represent the main target of HIV in the body, their normal state of quiescence poses an important obstacle to the virus. HIV-1 is indeed unable to perform the early postentry steps of its replication in these targets, due to blocks at several levels including reverse transcription and, most likely, nuclear transport (7, 19, 20, 43, 44). Recent reports have raised the possibility that resting T cells can be transduced with HIV-based vectors, as long as the particles are produced from packaging constructs that comprise the viral accessory genes and are pseudotyped with a foreign envelope such as VSV G (6, 9). Using highly purified resting T cells as targets and replication-defective vectors expressing a very sensitive marker, we could not confirm these claims. Instead, our data indicate that HIV-derived vectors are as inefficient as HIV itself at infecting resting T lymphocytes. It is noteworthy that, in these other studies, the background of T cells expressing prototypic activation markers was not depleted. We therefore cannot exclude that, in some partially activated T cells, transduction with an HIV-based vector can occur and in this setting depends on the presence of some accessory gene product.
Our results are in agreement with a study showing that treatment with IL-2, IL-4, IL-7, or IL-15 can allow HIV-1 infection of quiescent adult T cells (38). However, we failed to confirm the more recent claim that IL-7-treated CB lymphocytes, in spite of dividing actively, remain resistant to HIV-mediated gene transfer (10). Instead, we found that these cells are highly susceptible to transduction with a VSV G-pseudotyped HIV-derived vector as well as infection with wild-type HIV-1. This is not surprising, because IL-7-treated CB T cells can be efficiently transduced with murine leukemia virus-based vectors (11). Furthermore, CB T cells are most likely recent thymic migrants, and thymocytes can be infected by HIV-1 both in vitro and in vivo (37).
Analyzing the modalities of IL-7-induced HIV permissiveness in adult T cells yielded several types of information. First, it confirmed that in this setting both naïve and memory cells can be transduced, as previously described (38). Furthermore, it demonstrated that acquisition of susceptibility does not correlate with the expression of prototypic activation markers. Finally, based on the analysis of cells stimulated either through the T-cell receptor alone or via CD3 and CD28, it had been suggested that induction of HIV permissiveness coincides with progression of quiescent T lymphocytes to the G1b phase of the cell cycle (20). Our examination of IL-7-treated adult PB T cells supports this contention. It was previously shown that the forced expression of the transcription factor NFATc, in the presence of low levels of IL-2, can induce a highly permissive state for HIV-1 replication in primary CD4+ T cells (18). Our study does not contradict this evidence but demonstrates that NFAT activation is required for none of the steps that lead to HIV integration. Furthermore, our observation that HIV-based vectors can transduce IL-7-treated T cells in the presence of CSA indicates that HIV-1 reverse transcription, nuclear import, and integration are completely independent from signaling pathways blocked by this drug.
The vast majority of PB T lymphocytes expressed the IL-7R and responded to its ligand, as illustrated by the high rates of survival observed when these cells were cultured in the presence of this cytokine, contrasting with the rapid death of untreated cells. However, IL-7-responsive mature T lymphocytes could be separated in two distinct subsets: on the one hand, cells that stayed in G0/G1a and remained resistant to HIV-mediated gene transfer, and on the other hand, cells that progressed to G1b and became susceptible to infection. In cells that could not be transduced, reverse transcription was impaired and infection was irreversibly blocked. This closely parallels the phenotype observed in resting T lymphocytes, where the preintegration of HIV or HIV-derived vectors is highly labile (7, 43, 44; our unpublished results).
The differential response of adult T lymphocytes to IL-7 could represent quantitative or qualitative variations in the response to this cytokine. The receptors for IL-7 and four other cytokines, IL-2, IL-4, IL-9, and IL-15, have the same heteromeric structure, comprising a shared element (γc chain) (29) and one or two ligand-specific chains (21). All five γc-chain-dependent cytokines activate the Janus family tyrosine kinases Jak1 and Jak3, thereby triggering the Jak-STAT signaling cascade. Jak1 associates with the cytoplasmic tail of the lymphokine-specific component of the receptor, whereas Jak3 associates primarily with the γc chain (28). Upon IL-7 binding, Jak1 and Jak3 are activated and phosphorylate tyrosine-based docking sites on the receptor, which results in recruiting the STAT3, STAT5a, and STAT5b proteins via their SH2 domains (30, 45). These STATs are then phosphorylated, form homo- or heterodimers, and translocate to the nucleus, where they bind target sequences. IL-7 can also activate the PI3K pathway and its downstream effector protein kinase B, apparently through the recruitment of PI3K by STAT3. While the range of genes activated by IL-7 is far from fully characterized, the IL-7/PI3K/protein kinase B pathway appears to be involved in T-cell survival and proliferation, while STAT5 mediates T-cell differentiation (30). Based on the current understanding on IL-7-mediated signaling, our demonstration that wortmannin blocks IL-7-induced T-cell survival and Bcl-2 synthesis but not HIV permissiveness indicates that the latter phenomenon is independent of PI3K and suggests that it is instead likely mediated by the STAT5 pathway. Importantly, when IL-4 or IL-15 was added to IL-7, the fraction of HIV-transducible T cells did not increase. It suggests that a same factor restricts the response of circulating T cells to all three cytokines. The basis of this restriction is as yet unknown, and whether it also applies to lymphocytes residing in lymphoid organs remains to be determined.
Newly induced STAT DNA binding activity can generally be detected within minutes of cytokine addition, yet 3 to 4 days of IL-7 treatment were necessary for quiescent T lymphocytes to become maximally susceptible to HIV-mediated transduction. This strongly suggests that the establishment of virus permissiveness is a quantitatively demanding phenomenon, for instance because the buildup of significant amounts of essential substrates or a complicated degree of intracellular remodeling is required. If a crucial cellular cofactor of HIV is induced by IL-7, it is more likely to represent a master transcriptional regulator than, for instance, a chaperone interacting directly with the preintegration complex. Even though the nature of this putative regulator remains to be elucidated, our demonstration that IL-7-treated HIV-permissive cells can be easily separated from HIV-resistant cells by cell sorting opens the way to genomic- and proteomic-based comparisons of these two subsets. This will hopefully shed light on cellular parameters that are key to the completion of the immediate-early steps of HIV replication, and furthermore it will allow a differential dissection of cytokine-induced signaling pathways in T lymphocytes. Ultimately, it should also be possible to determine whether the various stimuli that can render resting T cells permissive for HIV, such as cytokine exposure, T-cell receptor stimulation, or contact with virus-loaded dendritic cells, all act through common downstream effectors or via completely distinct pathways.
Acknowledgments
We thank D. Wohlwend for help with FACS and the members of our group for helpful discussions.
This study was supported by the Swiss National Science Foundation, the Gabriella Giorgi-Cavaglieri Foundation, the Dormeur Foundation, and the National Institutes of Health (D.T.) and the INSERM (M.G.).
REFERENCES
- 1.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akashi, K., M. Kondo, U. von Freeden-Jeffry, R. Murray, and I. L. Weissman. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89:1033-1041. [DOI] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89:6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgering, B. M., and P. J. Coffer. 1995. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature 376:599-602. [DOI] [PubMed] [Google Scholar]
- 5.Chene, L., M. T. Nugeyre, E. Guillemard, N. Moulian, F. Barre-Sinoussi, and N. Israel. 1999. Thymocyte-thymic epithelial cell interaction leads to high-level replication of human immunodeficiency virus exclusively in mature CD4+ CD8− CD3+ thymocytes: a critical role for tumor necrosis factor and interleukin-7. J. Virol. 73:7533-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinnasamy, D., N. Chinnasamy, M. J. Enriquez, M. Otsu, R. A. Morgan, and F. Candotti. 2000. Lentiviral-mediated gene transfer into human lymphocytes: role of HIV-1 accessory proteins. Blood 96:1309-1316. [PubMed] [Google Scholar]
- 7.Chou, C. S., O. Ramilo, and E. S. Vitetta. 1997. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. USA 94:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran, A. E., F. M. Smart, R. J. Cowling, T. Crompton, M. J. Owen, and A. R. Venkitaraman. 1996. The interleukin-7 receptor alpha chain transmits distinct signals for proliferation and differentiation during B lymphopoiesis. EMBO J. 15:1924-1932. [PMC free article] [PubMed] [Google Scholar]
- 9.Costello, E., M. Munoz, E. Buetti, P. R. Meylan, H. Diggelmann, and M. Thali. 2000. Gene transfer into stimulated and unstimulated T lymphocytes by HIV-1-derived lentiviral vectors. Gene Ther. 7:596-604. [DOI] [PubMed] [Google Scholar]
- 10.Dardalhon, V., S. Jaleco, S. Kinet, B. Herpers, M. Steinberg, C. Ferrand, D. Froger, C. Leveau, P. Tiberghien, P. Charneau, N. Noraz, and N. Taylor. 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:9277-9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dardalhon, V., S. Jaleco, C. Rebouissou, C. Ferrand, N. Skander, L. Swainson, P. Tiberghien, H. Spits, N. Noraz, and N. Taylor. 2000. Highly efficient gene transfer in naive human T cells with a murine leukemia virus-based vector. Blood 96:885-893. [PubMed] [Google Scholar]
- 12.Flanagan, W. M., B. Corthesy, R. J. Bram, and G. R. Crabtree. 1991. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature 352:803-807. [DOI] [PubMed] [Google Scholar]
- 13.Follenzi, A., L. E. Ailles, S. Bakovic, M. Geuna, and L. Naldini. 2000. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 25:217-222. [DOI] [PubMed] [Google Scholar]
- 14.Foxwell, B. M., C. Beadling, D. Guschin, I. Kerr, and D. Cantrell. 1995. Interleukin-7 can induce the activation of Jak 1, Jak 3 and STAT 5 proteins in murine T cells. Eur. J. Immunol. 25:3041-3046. [DOI] [PubMed] [Google Scholar]
- 15.Gallay, P., T. Hope, D. Chin, and D. Trono. 1997. HIV-1 infection of nondividing cells through the recognition of integrase by the importin/karyopherin pathway. Proc. Natl. Acad. Sci. USA 94:9825-9830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofmeister, R., A. R. Khaled, N. Benbernou, E. Rajnavolgyi, K. Muegge, and S. K. Durum. 1999. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 10:41-60. [DOI] [PubMed] [Google Scholar]
- 17.Jain, J., Z. Miner, and A. Rao. 1993. Analysis of the preexisting and nuclear forms of nuclear factor of activated T cells. J. Immunol. 151:837-848. [PubMed] [Google Scholar]
- 18.Kinoshita, S., B. K. Chen, H. Kaneshima, and G. P. Nolan. 1998. Host control of HIV-1 parasitism in T cells by the nuclear factor of activated T cells. Cell 95:595-604. [DOI] [PubMed] [Google Scholar]
- 19.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korin, Y. D., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, J. X., T. S. Migone, M. Tsang, M. Friedmann, J. A. Weatherbee, L. Zhou, A. Yamauchi, E. T. Bloom, J. Mietz, S. John, and et al. 1995. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity 2:331-339. [DOI] [PubMed] [Google Scholar]
- 22.Liu, J., J. D. Farmer, Jr., W. S. Lane, J. Friedman, I. Weissman, and S. L. Schreiber. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807-815. [DOI] [PubMed] [Google Scholar]
- 23.Maraskovsky, E., L. A. O'Reilly, M. Teepe, L. M. Corcoran, J. J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89:1011-1019. [DOI] [PubMed] [Google Scholar]
- 24.Musso, T., J. A. Johnston, D. Linnekin, L. Varesio, T. K. Rowe, J. J. O'Shea, and D. W. McVicar. 1995. Regulation of JAK3 expression in human monocytes: phosphorylation in response to interleukins 2, 4, and 7. J. Exp. Med. 181:1425-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naldini, L., U. Blomer, P. Gallay, D. Ory, R. Mulligan, F. H. Gage, I. M. Verma, and D. Trono. 1996. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272:263-267. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano, L. A., R. M. Grant, S. G. Deeks, D. Schmidt, S. C. De Rosa, L. A. Herzenberg, B. G. Herndier, J. Andersson, and J. M. McCune. 2001. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat. Med. 7:73-79. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen, M., A. Svejgaard, S. Skov, P. Dobson, K. Bendtzen, C. Geisler, and N. Odum. 1996. IL-2 induces β2-integrin adhesion via a wortmannin/LY294002-sensitive, rapamycin-resistant pathway. Phosphorylation of a 125-kilodalton protein correlates with induction of adhesion, but not mitogenesis. J. Immunol. 157:5350-5358. [PubMed] [Google Scholar]
- 28.Page, T. H., F. V. Lali, N. Groome, and B. M. Foxwell. 1997. Association of the common gamma-chain with the human IL-7 receptor is modulated by T cell activation. J. Immunol. 158:5727-5735. [PubMed] [Google Scholar]
- 29.Page, T. H., J. L. Willcocks, D. A. Taylor-Fishwick, and B. M. Foxwell. 1993. Characterization of a novel high affinity human IL-7 receptor. Expression on T cells and association with IL-7 driven proliferation. J. Immunol. 151:4753-4763. [PubMed] [Google Scholar]
- 30.Pallard, C., A. P. Stegmann, T. van Kleffens, F. Smart, A. Venkitaraman, and H. Spits. 1999. Distinct roles of the phosphatidylinositol 3-kinase and STAT5 pathways in IL-7-mediated development of human thymocyte precursors. Immunity 10:525-535. [DOI] [PubMed] [Google Scholar]
- 31.Rosenthal, L. A., K. D. Winestock, and D. S. Finbloom. 1997. IL-2 and IL-7 induce heterodimerization of STAT5 isoforms in human peripheral blood T lymphoblasts. Cell. Immunol. 181:172-181. [DOI] [PubMed] [Google Scholar]
- 32.Salmon, P., V. Kindler, O. Ducrey, B. Chapuis, R. H. Zubler, and D. Trono. 2000. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood 96:3392-3398. [PubMed] [Google Scholar]
- 33.Sirven, A., F. Pflumio, V. Zennou, M. Titeux, W. Vainchenker, L. Coulombel, A. Dubart-Kupperschmitt, and P. Charneau. 2000. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood 96:4103-4110. [PubMed] [Google Scholar]
- 34.Smithgall, M. D., J. G. Wong, K. E. Critchett, and O. K. Haffar. 1996. IL-7 up-regulates HIV-1 replication in naturally infected peripheral blood mononuclear cells. J. Immunol. 156:2324-2330. [PubMed] [Google Scholar]
- 35.Spina, C. A., J. C. Guatelli, and D. D. Richman. 1995. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J. Virol. 69:2977-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 38.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vella, A., T. K. Teague, J. Ihle, J. Kappler, and P. Marrack. 1997. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J. Exp. Med. 186:325-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vella, A. T., S. Dow, T. A. Potter, J. Kappler, and P. Marrack. 1998. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 95:3810-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkitaraman, A. R., and R. J. Cowling. 1994. Interleukin-7 induces the association of phosphatidylinositol 3-kinase with the alpha chain of the interleukin-7 receptor. Eur. J. Immunol. 24:2168-2174. [DOI] [PubMed] [Google Scholar]
- 42.Weinberg, J. B., T. J. Matthews, B. R. Cullen, and M. H. Malim. 1991. Productive human immunodeficiency virus type 1 (HIV-1) infection of nonproliferating human monocytes. J. Exp. Med. 174:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 44.Zack, J. A., A. M. Haislip, P. Krogstad, and I. S. Chen. 1992. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J. Virol. 66:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng, Y. X., H. Takahashi, M. Shibata, and K. Hirokawa. 1994. JAK3 Janus kinase is involved in interleukin 7 signal pathway. FEBS Lett. 353:289-293. [DOI] [PubMed] [Google Scholar]
- 46.Zennou, V., C. Petit, D. Guetard, U. Nerhbass, L. Montagnier, and P. Charneau. 2000. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell 101:173-185. [DOI] [PubMed] [Google Scholar]
- 47.Zennou, V., C. Serguera, C. Sarkis, P. Colin, E. Perret, J. Mallet, and P. Charneau. 2001. The HIV-1 DNA flap stimulates HIV vector-mediated cell transduction in the brain. Nat. Biotechnol. 19:446-450. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 49.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]