Abstract
In a preliminary study, we observed that TGF-β1 induced both proliferation and growth arrest in prostatic stromal cells, depending on the concentration of TGF-β1 used in the culture medium. In this study, we explored possible mechanisms of this dual effect of TGF-β. Primary cultures of prostatic stromal cells, established from clinical surgical specimens and treated with low doses of TGF-β1 (0.001–0.01 ng/ml), resulted in an increase in cell proliferation. The addition of neutralizing antibody against platelet-derived growth factor (PDGF)-BB, but not anti-PDGF-AA, abrogated this stimulatory effect of TGF-β1. TGF-β1 treatment resulted in a dose-related increase in PDGF-BB production as measured by ELISA. Cells underwent growth arrest at high concentrations of TGF-β1 (1.0 and 10 ng/ml). An inhibitor of cyclin-dependent kinase (cdk), p15INK4b, was up-regulated at both transcript and protein levels in these cultures by TGF-β1 in a dose-related manner as determined by RT-PCR and Western blot analysis. The transcript, but not the protein, for another cdk inhibitor, p21Cip1, was up-regulated with treatment of TGF-β1 to these cells. Levels of other cdk inhibitors, such as p16INK4a and p27Kip1, were constitutively expressed in prostatic stromal cells and were not significantly affected by TGF-β1 treatment. Finally, the growth arrest effect of TGF-β1 was abrogated when antisense oligonucleotides to p15INH4b, but not p21Cip1, were added to the culture medium. These data indicate that the dual effect of TGF-β1 is mediated, at least, by up-regulation of PDGF-BB and p15INK4b, respectively.
Abbreviations: BPH, Benign prostatic hyperplasia; cdk, cyclin-dependent kinase; FBS, fetal bovine serum; PDGF, platelet-derived growth factor; Smad, signaling molecules identified downstream of TGFβ superfamily ligands
TGF-β IS THE prototypic member of a superfamily. TGF-β1, -β2, and -β3 have been identified in mammals (1) and they are able to mediate a wide range of cellular events (2–4). In prostatic stromal cells, all three types of mammalian TGF-β isoforms are expressed (5), with TGF-β1 being the predominant one (6). Cellular responses of prostatic stromal cells to TGF-β have been inconsistent. In most studies, TGF-β has been shown to inhibit proliferation in prostatic stromal cells (7–9). But in few reports, it has a stimulatory effect (10, 11). The above discrepancy has been resolved in our preliminary studies (12) in which TGF-β showed both stimulatory and inhibitory effects on growth of prostatic stromal cells, depending on the concentrations used in the cultures. At low concentrations TGF-β1 induced proliferation, whereas at high concentrations, it induced growth arrest. In other cell systems, the inhibitory effect of TGF-β has been linked to the induction of inhibitors to cyclin-dependent protein kinases (cdk) (13, 14). The stimulatory effect of TGF-β has been linked to the expression of platelet-derived growth factor (PDGF) (15). In the present study, we explored the possible mechanisms of these seemingly conflicting events mediated by TGF-β in human prostatic stromal cells.
Materials and Methods
Cell culture
Prostatic stromal cells were derived from 10 surgical specimens from men who were subjected to surgery for the treatment of bladder neck obstruction secondary to benign prostatic hyperplasia (BPH). The use of human surgical specimens was approved by the institutional review board, with the understanding that the identity of patients would not be revealed. Primary cultures of prostatic stromal cells were established according to the methods reported earlier (16, 17). Briefly, freshly isolated tissue specimens were mechanically and enzymatically dissociated by treatment with Dnase (Sigma, St. Louis, MO) and collagenase (Sigma). Epithelial cells were separated from stromal cells by discontinuous Percoll (Sigma) gradient centrifugation. Stromal cells were then cultured in phenol red-free RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). Cells used in this study were derived from 2nd to 15th passages.
[3H]Thymidine incorporation
Prostatic stromal cells were plated in 24-well plates at a concentration of 2 ×104 cells/ml in phenol red-free RPMI 1640 medium supplemented with 10% FBS. The medium was changed to serum free RPMI 1640 with 1% ITS (insulin, transferrin, seleneous acid; BD Biosciences, Bedford, MA) for 24 h. To test the effect of TGF-β1 on proliferation, the medium was changed to a 1% ITS or 1% ITS supplemented with various concentrations of TGF-β1 (R&D Systems, Minneapolis, MN) at 0.001, 0.01, 0.1, 1.0, or 10 ng/ml, with medium change every other day. After 5 d of incubation, [3H]thymidine (Amersham Pharmacia, Piscataway, NJ) was added at a dosage of 1.0 μCi/well for 24 h. DNA was precipitated using 10% trichloroacetic acid at 4 C for 20 min, followed by 0.4 n NaOH at 37 C overnight. [3H]Thymidine incorporation into cellular DNA was measured with a scintillation counter and was expressed as counts per minute. To determine the effect of autocrine production of PDGF (R&D Systems), cells were incubated in medium containing 0.001 ng/ml TGF-β1 with neutralizing antibody (0.5 μg/ml) against PDGF-AA or -BB to determine their effects on growth of prostatic stromal cells in culture. [3H]Thymidine incorporation assay was carried out in these cells as well.
PDGF-BB quantitation
Prostatic stromal cells were seeded at 2 × 104 cells/ml in RPMI 1640 with 10% FBS in T-25 flasks and allowed adherence for 24 h. Medium was replaced with phenol red-free RPMI 1640 supplemented with ITS+ with or without TGF-β1 (0.001, 0.1, or 10 ng/ml). After 3 d of incubation, fresh culture medium was changed according to each treatment. The conditioned medium was collected after 3 and 6 d of culture and pooled. For intracellular PDGF, cells were lysed for 20 min at 4 C, followed by centrifugation. Supernatants were then collected and stored until use. Conditioned media and cell lysates were assayed for PDGF-BB using ELISA kits from R&D Systems.
Western blot analysis
Cultures of prostatic stromal cells were treated with TGF-β1 at 0, 0.001, 0.01, 0.1, 1.0, or 10 ng/ml for 48 h. Treated cells were harvested using modified RIPA buffer [50 mm Tris-HCl (pH 7.4), 1% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, 1 mm phenylmethylsulfonyl fluoride, 1 mm NaF] and proteins denatured for 5 min in 1× sample buffer (0.5 m Tris-HCl, glycerol, 10% sodium dodecyl sulfate, bromophenol blue) at 100 C and subjected to electrophoresis. For Western blot analysis of p15INK4b, p16INK4a, p21Cip1/WAF1, and p27Kip1, transferred membranes were incubated overnight at 4 C with the following primary antibodies: antihuman p15INK4b (2 μg/ml; Upstate Biotechnology, Lake Placid, NY), antihuman p16INK4a (2 μg/ml; Upstate Biotechnology), antihuman p21Cip1/WAF1 (2.5 μg/ml; BD Biosciences), and antihuman p27Kip1 (2.5 μg/ml; BD Biosciences). Western blot analysis for glyceraldehyde-3-phosphate dehydrogenase (Chemicon, Temecula, CA) was probed using the same blots as an internal control to indicate protein loading.
RT-PCR
For RT-PCR, prostatic stromal cells were treated as described above for 24 h. Total RNA were prepared using Trizol reagent (Life Technologies, Inc., Carlsbad, CA). An aliquot of 2 μg of RNA was reverse-transcribed for 45 min at 40 C in the presence of 1 μg random hexamers (Promega, Madison, WI) in a total volume of 20 μl. PCR mixtures were prepared using 2 μl of the reverse transcriptase mixture in a total volume of 25 μl. Serial cDNA dilutions (1:1 to 1:100) were amplified with primers β-actin (sense: 5′-GGCATCGTGATGGACTCC-3′; antisense: 5′-GCTGGAAGGTGGAC-AGCG-3′) for 20 cycles using an annealing temperature of 55 C. Dilutions yielding equal amounts of product were chosen for 35-cycle amplification with the following primers: p15 primers (sense: 5′-GGGAAGAGTGTCGTTAAGTTTACG-3′; antisense: 5′-GCAGCCTTCATC-GAATTAGG-3′), p16 primers (sense: 5′-CAGACATCCCCGATTGAAAGAAC-3′; antisense: 5′-GTGCTCACTCCAGAAAACTCCAAC-3′), p21 primers (sense: 5′-CTCCAAGAGGAAGCCCTAATCC-3′; antisense: 5′-TTTGATGATGCCCCCACTCG-3′), and p27 primers (sense: 5′-TCAGACGGTTCCCCAAATGC-3′; antisense: 5′-TGCTACATCCAACGCTTTTAGAGG-3′). β-Actin (630 bp) was used as an internal control. The PCR product was visualized on a 1% agarose gel.
Effects of oligonucleotides with sequence antisense to p15 and p21
Phosphorothioate oligonucleotides were obtained from Midland Certified Reagents (Midland, TX) and were the following sequences: scramble oligo, 5′-TTGCCGCTGCTAAGTTCG-3′; P21 antisense-1, 5′-TCCGGGCCCAGCTCC-3′; P21 antisense-2, 5′-TCCCCAGCCGGTTCTGACAT-3′; P15 antisense-3, 5′-TCAGCTGGGCCAAGGGGCCGG-3′; P15 antisense-4, 5′-GCCGGCAAAGAATTCCGTTTT-3′; P21 sense, 5′-GGAGCTGGGCCCGGA-3′; and P15 sense, 5′-AGGCGCGGCCGAAGGTCCTCG-3′.
The above oligonucleotides were added into culture medium directly during the treatment of TGF-β1.
Statistical analysis
All data are of one representative experiment with mean at least three wells, unless otherwise noted. Each study was repeated with at least three experiments using different primary cultures. Differences among treatment groups were analyzed by one-way ANOVA using SigmaStat statistical package (Jandel Scientific) followed by Student’s t test by comparing the value of each treatment against the control value. P < 0.05 was considered statistically significant.
Results
TGF-β1 induces a biphasic [3H]thymidine incorporation in prostatic stromal cells
TGF-β1 added to cultures of prostatic stromal cells displayed a biphasic dose response. At low concentrations of TGF-β1 (0.001 and 0.01 ng/ml), a significant increase in [3H]thymidine incorporation was observed, whereas at high concentrations (1.0 and 10 ng/ml), a significant decrease in [3H]thymidine incorporation was observed (Fig. 1). This observation confirmed our preliminary results (12).
Fig. 1.

Effects of TGF-β1 on stromal cell growth. Cells were treated for 6 d with various concentrations of TGF-β1 (0.001–10.0 ng/ml). [3H]Thymidine incorporation was performed. Error bars represent the mean of triplicate cell counts ± sem. **, P < 0.01 vs. ITS+ control.
Effects of neutralizing antibodies to PDGF on TGF-β1 response
Previous studies have shown that PDGF plays a crucial role in stromal cell growth (18) and that TGF-β1 was able to induce expression of PDGF in cancer cells (19). Additionally, it has been shown that the PDGF promoter region contains a TGF-β/Smad (signaling molecules identified downstream of TGFβ superfamily ligands) response element (15). We sought to determine whether or not PDGF is responsible in mediating the increase in proliferation of prostate stromal cells after treatment with TGF-β1. Addition of neutralizing antibody against PDGF-AA to TGF-β1-treated cultures of prostatic stromal cells resulted in no significant change in [3H]thymidine incorporation. However, addition of PDGF-BB neutralizing antibody to TGF-β1-treated cells significantly inhibited the TGF-β1-induced [3H]thymidine incorporation (Fig. 2A). Antibody given alone did not have a significant effect on the growth of prostate stromal cells (data not shown). This observation indicated that the PDGF-BB isoform, but not PDGF-AA, played a role in TGF-β1-mediated proliferation in prostatic stromal cells.
Fig. 2.
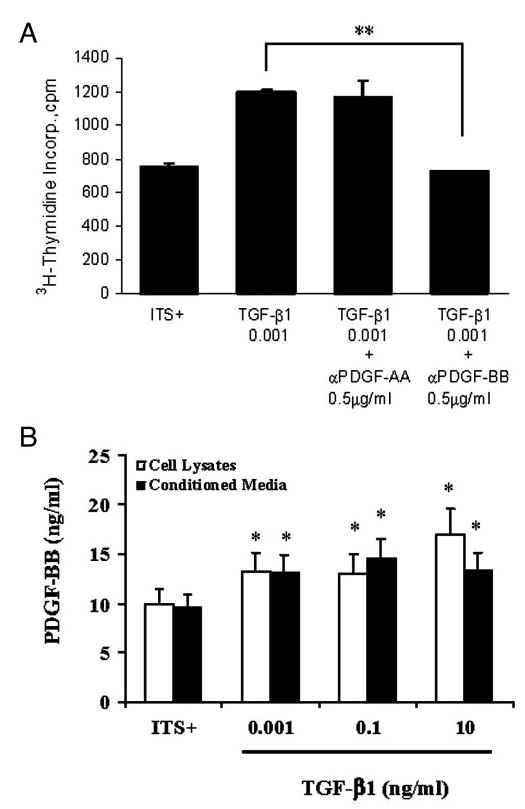
PDGF-BB mediated the TGFβ-induced proliferation. A, Effects of PDGF-AA and PDGF-BB neutralizing antibody on TGF-β1-induced growth. The addition of neutralizing antibody against PDGF-AA (0.5 μg/ml) to TGF-β1-treated stromal cultures did not have a significant effect on TGF-β1-induced cellular proliferation. The addition of neutralizing antibody against PDGF-BB (0.5 μg/ml) to TGF-β1-treated stromal culture reduced the proliferative effect of TGF-β1. **, P < 0.01 vs. TGF-β1. B, PDGF ELISA. Either conditioned media or cell lysates were used to determine whether TGF-β1 modified the expression of PDGF-BB. Cells were treated for 6 d with ITS+ (control) or TGF-β1 at 0.001, 0.01, or 10 ng/ml. Results show an increase in PDGF-BB expression in both cell lysates and conditioned media with increasing concentrations of TGF-β1. *, P < 0.05 vs. ITS+ control.
TGF-β1 induces a dose-related increase in PDGF-BB
To further elucidate the role of PDGF-BB in TGF-β1-induced proliferation in prostatic stromal cells, experiments were conducted to determine total secretion of PDGF-BB into culture media by ELISA. Results of ELISA showed that prostatic stromal cells treated with TGF-β1 had a significant dose-related increase in PDGF-BB in both secreted protein (conditioned media) and cell lysates (Fig. 2B). These results, combined with the neutralizing antibody study, strongly support the notion that PDGF-BB is the primary isoform responsible for proliferation in response to TGF-β1 treatment at low concentrations.
Increase in expression of cdk inhibitors p15 and p21 in response to TGF-β1
Despite the increase in PDGF-BB expression in response to TGF-β1 treatment at a dose-related manner, growth arrest in prostatic stromal cells occurs at higher concentrations. The regulation of cdk inhibitors by TGF-β1 has been acknowledged (14, 20). Studies have revealed TGF-β/Smad response elements on p15, p16, p21, and p27 (13, 14). We examined the expression of these cdk inhibitors in stromal cell culture after treatment with TGF-β1. Results of RT-PCR studies showed a dose-dependent increase in mRNA for p15 and p21 (Fig. 3A). RT-PCR products for p16 and p27 were constitutively expressed in prostatic stromal cells and were not changed with TGF-β1 treatment. Results of Western blot analysis of protein contents of the cdk inhibitors in TGF-β1-treated cells revealed an increase in p15 expression, whereas expression of p16, p21, and p27 remained relatively steady in these cultures at all concentrations of TGF-β1 treatment (Fig. 3B).
Fig. 3.
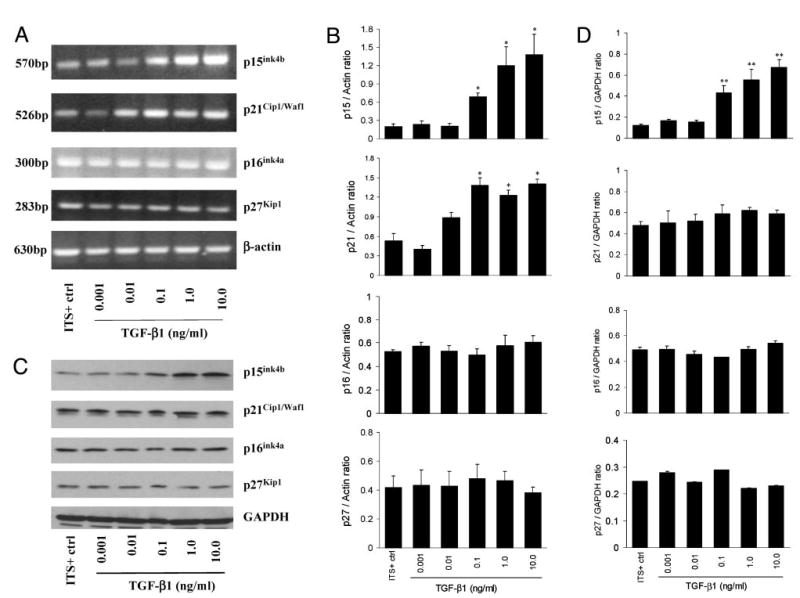
Expression of cdk inhibitors p15, p16, p21, and p27 after treatment with TGF-β1 at 0, 0.001, 0.01, 0.1, 1.0, or 10 ng/ml. A, RT-PCR analysis of cdk inhibitors mRNA expression. B, Ratios of the cdk inhibitors normalized with the intensity of Actin band. *, P < 0.05 vs. ITS+ control. Data shown are representative of three independent experiments. C, Western blot analysis of cdk inhibitors expression. D, Ratios of the cdk inhibitors normalized with the intensity of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) band. **, P < 0.01 vs. ITS+ control. Data shown are representative of three independent experiments. Results of both RT-PCR and Western blot show an increase in p15 expression with increasing TGF-β1 concentrations.
Interference of cdk inhibitors abrogated TGF-β1-mediated growth arrest in prostate stromal cells
To elucidate the role of cdk inhibitors in TGF-β1-mediated growth arrest in prostatic stromal cells, oligonucleotides with sequence antisense to p15 or p21 were used in cultures treated with 10 ng/ml of TGF-β1. We have designed two sets of antisense oligonucleotides for p15 as well as for p21. Figure 4A indicates that the addition of 2.0 μm of p15 antisense oligonucleotides significantly abrogated the growth arrest effect of TGF-β1. This effect of p15 antisense is at a dose-related manner (Fig. 4B). On the other hand, the addition of 2.0 μm of p21 antisense oligonucleotides had no significant effect on TGF-β1-mediated growth arrest in prostatic stromal cells (Fig. 4C). These results demonstrate that p15, but not p21, is responsible for the growth inhibitory effect of TGF-β1 on prostatic stromal cells.
Fig. 4.

Down-regulation of p15 abrogated the growth arrest effect of TGF-β. Oligonucleotides with sequence random, sense or antisense to p15 or p21 were used in cultures treated with 10 ng/ml of TGF-β1 for 6 d. [3H]Thymidine incorporation into cellular DNA was measured with a scintillation counter and was expressed as counts per minute (cpm). A, Addition of 2.0 μm of p15 antisense oligonucleotides (AS3, AS4) significantly abrogated the growth arrest effect of TGF-β1. **, P < 0.01 vs. ITS+ random. There is no significant change between ITS alone and random oligonucleotide. B, p15 antisense abrogated the growth arrest effect of TGF-β at a dose-related manner. **, P < 0.01. C, Addition of 2.0 μm of p21 antisense oligonucleotides (AS1, AS2) had no significant effect on TGF-β1-mediated growth arrest in prostatic stromal cells. **, P < 0.01 vs. ITS+ random.
Discussion
Results of the present study have demonstrated that, TGF-β1, at low concentrations induced proliferation in primary cultures of prostatic stromal cells, whereas at high concentrations, it induced growth arrest. The proliferative effect of TGF-β1 was mediated through the expression of PDGF, whereas the growth arrest effect was associated with the expression of a cdk inhibitor, p15.
It is now clear that both promoters of the PDGF gene (15) and the p15 gene (13, 21) contain the TGF-β/Smad response element. cdk inhibitors play a major role in cell cycle progression (22, 23). They include the p21Cip1/p27Kip1 and the p16INK4a/p15INK4b families. In many cell systems, TGF-β induces the expression of p15 and its association to cdk4, thus preventing the latter from being activated by cyclin D and also promoting the subsequent release of p27 (or p21) from cdk4 and inhibition of the cdk2-cyclin E activity (14). In the present study, TGF-β1 also induced the expression of p15 in prostatic stromal cells, but it did not change the expression of p16 or p27. Although there was some induction in p21 expression by TGF-β1, activity of p21 can be substituted by p27. Therefore, in prostatic stromal cells, p15 seems to be the rate-limiting factor in regulating cell cycle progression.
In the present study, we have demonstrated a duel role of TGF-β in prostatic stromal cells. However, the molecular mechanism of up-regulation of PDGF and p15 by TGF-β remains unknown. PDGF is a potent mitogen to prostatic stromal cells (18). The present study also demonstrated that TGF-β1 was able to induce PDGF-BB expression in a dose-related manner. Like many mitogenic growth factors, PDGF activation leads to downstream Myc activation and proliferation in target cells (24, 25). However, it is interesting to note that this elevated expression of PDGF was only mitogenic to prostatic stromal cells when low doses of TGF-β1 were used in the culture. At high doses of TGF-β1, although the expression of PDGF was further increased, proliferation in prostatic stromal cells was inhibited. It is now clear that Myc expression is inhibited by TGF-β-mediated events, resulting in p15 expression (26). On the basis of this discussion, it is possible that Myc may play an important role in TGF-β-mediated cell proliferations and growth arrest in prostatic stromal cells. Our future study will investigate the effect of TGF-β on Myc expression.
In summary, results of this study have provided insights into the possible role of TGF-β in proliferation and growth arrest of prostatic stromal cells related to BPH development. BPH is a common disorder in aging men, and it is associated with an expansion of the stromal component with advancing age. Prostatic stromal cells express all three isoforms of mammalian TGF-β (TGF-β1, -β2, and -β3) with TGF-β1 as the predominant one. We noted that TGF-β1 at low concentrations (0.001 and 0.01 ng/ml) promoted proliferation in prostatic stromal cells. At high concentrations (1.0 and 10 ng/ml), it promoted growth arrest in these cells. These observations allowed us to postulate that TGF-β may be an important mediator in growth of human prostate. Because the human prostate is able to grow late in life, it is likely that a small amount of TGF-β is being activated within the prostatic stroma, leading to BPH. Such a concept will be the topic of our future investigation.
Acknowledgments
This work was supported in part by NIH Grants DK43541 and DK47561, and by a Doxazosin Investigators and Consultants Educational Exchange research grant from the Pfizer Pharmaceuticals Group and Grant 30271297 from the Chinese National Science Foundation.
Footnotes
W.Z. and I.P. contributed equally to this work.
Current address for K.I.: Institute for Science Education and Communication, Columbia College Chicago, 600 South Michigan Avenue, Chicago, Illinois 60605.
References
- 1.Derynck R, Jarret JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Geoddel DV. Human transforming growth factor-β complementary DNA sequences and expression in normal and transformed cells. Nature. 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- 2.Moses HL, Yang EY, Pietenpol JA. TGF-β stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990;63:245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 3.Robert AB, Sporn MB 1990 The transforming growth factor-βs. In: Sporn MB, Roberts AB, eds. Peptide growth factors and their receptors, part I. Vol 95. Berlin: Springer-Verlag; 419–472
- 4.Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- 5.Itoh N, Patel U, Cupp AS, Skinner MK. Developmental and hormonal regulation of transforming growth factor-β1 (TGF-β1), -2, and -3 gene expression in isolated prostatic epithelial and stromal cells: epidermal growth factor and TGF-β interactions. Endocrinology. 1998;139:1378–1388. doi: 10.1210/endo.139.3.5787. [DOI] [PubMed] [Google Scholar]
- 6.Timme TL, Truong LD, Merz VW, Krebs T, Kadmon D, Flanders KC, Park SH, Thompson TC. Mesenchymal-epithelial interactions and transforming growth factor-β expression during mouse prostate morphogenesis. Endocrinology. 1994;134:1039–1045. doi: 10.1210/endo.134.3.8119140. [DOI] [PubMed] [Google Scholar]
- 7.Cohen P, Nunn SE, Peehl DM. Transforming growth factor-β induces growth inhibition and IGF-binding protein-3 production in prostatic stromal cells: abnormalities in cells cultured from benign prostatic hyperplasia tissues. J Endocrinol. 2000;164:215–223. doi: 10.1677/joe.0.1640215. [DOI] [PubMed] [Google Scholar]
- 8.Klingler HC, Bretland AJ, Reid SV, Chapple CR, Eaton CL. Regulation of prostatic stromal cell growth by transforming growth factor β (TGFβ) Prostate. 1999;41:110–120. doi: 10.1002/(sici)1097-0045(19991001)41:2<110::aid-pros5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Niu Y, Xu Y, Zhang J, Bai J, Yang H, Ma T. Proliferation and differentiation of prostatic stromal cells. BJU Int. 2001;87:386–393. doi: 10.1046/j.1464-410x.2001.00103.x. [DOI] [PubMed] [Google Scholar]
- 10.Story MT, Hopp KA, Meier DA. Regulation of basic fibroblast growth factor expression by transforming growth factor β in cultured human prostate stromal cells. Prostate. 1996;28:219–226. doi: 10.1002/(SICI)1097-0045(199604)28:4<219::AID-PROS2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 11.Bretland AJ, Reid SV, Chapple CR, Eaton CL. Role of endogenous transforming growth factor β (TGFβ)1 in prostatic stromal cells. Prostate. 2001;48:297–304. doi: 10.1002/pros.1110. [DOI] [PubMed] [Google Scholar]
- 12.Park I, Kim S, Lee C, Ilio K, Transforming growth factor-β1-induced proliferation of prostate stromal cells in culture is mediated by platelet-derived growth factor. Proc Fall Meeting of the Society for Basic Urologic Research, Sanibel Harbor, FL, 2000, p 15 (Abstract 62)
- 13.Li JM, Nichols MA, Chandrasekharan S, Xiong Y, Wang XF. Transforming growth factor-β activates the promoter of cyclin-dependent kinase inhibitor p15INK4B through an SP-1 consensus site. J Biol Chem. 1995;270:26750–26753. doi: 10.1074/jbc.270.45.26750. [DOI] [PubMed] [Google Scholar]
- 14.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/cip and ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-β. Gene Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 15.Taylor LM, Khachigian LM. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-β involves transactivation by Smads. J Biol Chem. 2000;275:16709–16716. doi: 10.1074/jbc.275.22.16709. [DOI] [PubMed] [Google Scholar]
- 16.Kassen A, Sutkowski DM, Ahn HJ, Sensibar JA, Kozlowski JM, Lee C. Stromal cells of human prostate: initial isolation and characterization. Prostate. 1996;28:89–97. doi: 10.1002/(SICI)1097-0045(199602)28:2<89::AID-PROS3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.Sensibar JA, Pruden SJ, Kasjanski RZ, Rademaker A, Lee C, Grayhack JT, Kozlowski JM. Differential growth rates in stromal cultures of the human prostate derived from varying patient ages. Prostate. 1999;38:110–117. doi: 10.1002/(sici)1097-0045(19990201)38:2<110::aid-pros4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Peehl DM, Sellers RG. Basic FGF, EGF, and PDGF modify TGF-β-induction of smooth muscle cell phenotype in human prostatic stromal cells. Prostate. 1998;35:125–134. doi: 10.1002/(sici)1097-0045(19980501)35:2<125::aid-pros6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Sintich SM, Lamm MLG, Sensibar JA, Lee C. Transforming growth factor-β1 induced proliferation of the prostate cancer cell line, TSU-Pr1: the role of platelet-derived growth factor. Endocrinology. 1999;140:3411–3415. doi: 10.1210/endo.140.8.6921. [DOI] [PubMed] [Google Scholar]
- 20.Reynisdottir I, Massague J. The subcellular locations of p15INK4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- 21.Hannon GJ, Beach D. p15INK4b is a potential effector of cell cycle arrest mediated by TGF-β. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 22.Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 23.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 24.Facchini IM, Penn IZ. The molecular role of Myc in growth and transformation: recent discoveries lead to new insights. FASEB J. 1998;12:633–651. [PubMed] [Google Scholar]
- 25.Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1–11. doi: 10.1128/mcb.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, Massague J. TGFβ influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]


