Abstract
Studies have found an association between glycemic status and indices of health-related quality of life in people with diabetes mellitus and comorbid depression. No study to date has examined the relative strength of influences of glycemic status and health-related quality of life on depression in people with diabetes mellitus, nor have important moderators in this relationship been examined. This study examined the relative strength of correlations between glycemic status and health-related quality of life and depressive symptoms and the degree to which those correlations were moderated by sociodemographic variables in 146 people with type 2 diabetes. Depressive symptoms were measured with the Centers for Epidemiological Studies—Depression (CES-D) scale. Health-related quality of life was measured with the SF-36 Health Survey. Hemoglobin A1c (HbA1c) was used as a measure of glycemic status and body mass index and waist–hip ratio were measured. Results indicated that SF-36 scores accounted for a greater proportion of the variance in CES-D scores. The association between CES-D and SF-36 scores was moderated by HbA1c, sex, education, marital status, and social support. The implications and limitations of these results were discussed in the context of past studies.
Keywords: diabetes mellitus, depression, quality of life, glycemic status
Diabetes mellitus is a chronic metabolic disease that involves insulin secretion abnormalities, resulting in hyperglycemia or elevated blood glucose levels (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2001). In the United States, diabetes mellitus is the seventh leading cause of death (U.S. Department of Health and Human Services, 2000). It is estimated that 10.5 million people have a diagnosis of diabetes mellitus, of which 80% have type 2 diabetes (Harris, 1998), and another 5.4 million people are estimated to have been undiagnosed with diabetes mellitus (American Diabetes Association, 1998).
Hyperglycemia in people with diabetes mellitus is associated with microvascular (e.g., kidney and eye disease) and macrovascular (e.g., stroke and ischemic heart disease) complications, which can have additional medical sequelae such as amputation, physical disability, and blindness (Klein and Klein, 1998). It is estimated that 50–75% of people with diabetes mellitus develop serious long-term complications (Strowig and Raskin, 1992). Approximately 12% of all health care expenditures in the United States ($86 billion) can be attributed to diabetes (Herman and Eastman, 1998) of which nearly half can be accounted for by diabetes-related complications alone (Clark, 1998). However, many diabetes-related complications may be prevented, or can be delayed, with proper control of blood glucose levels.
DEPRESSIVE SYMPTOMS AND DIABETES MELLITUS
An elevated prevalence of depression has been significantly associated with diabetes mellitus. The mean prevalence of depression in people with diabetes mellitus has been reported to be as high as 31.7% (Anderson et al., 2001). An increased prevalence of depression in people with diabetes mellitus (26.1–29.8%) has been observed compared to first-degree relatives (9.5%; Popkin et al., 1988), the general population (16%; Gavard et al., 1993), and people with other chronic medical illnesses (15.2%; Grandinetti et al., 2000).
Depressive symptoms in people with diabetes mellitus are of concern because of their association with poor diabetes self-management (i.e., diet modification, physical activity, insulin injections) and an increased risk for diabetes-related complications (Black, 1999; De Groot et al., 2001). Furthermore, comorbid depression in people with diabetes mellitus is associated with functional disability, low work productivity, and low health service use (Black, 1999; Black and Markides, 1998; Ciechanowski et al., 2000). As a result, increased attention in recent years has been given to understanding the relationship between depressive symptoms and diabetes mellitus (see Lustman et al., 2000; Talbot and Nouwen, 2000).
Despite the growing interest in the relationship between depressive symptoms and diabetes mellitus, the causal mechanism underlying the association between the two has yet to be elucidated (Talbot and Nouwen, 2000). However, two primary explanations for their association have been postulated: (1) Depressive symptoms are associated with biochemical changes (i.e., hyperglycemia) due to diabetes mellitus and (2) depressive symptoms are related to psychosocial hardships (i.e., burden of illness on quality of life) associated with the illness (Jacobson, 1993; Lustman et al., 1992). Given that depressive symptoms in people with diabetes mellitus are often addressed by behavioral (e.g., cognitive–behavioral therapy; Lustman et al., 1998) and/or medical (e.g., antidepressants; Goodnick, 2001) interventions, our understanding of the relative influence of important biological and psychosocial variables and their sociodemographic moderators has direct implications for the design and effectiveness of treatment in this population.
Depressive Symptoms and Glycemic Status
Several studies have reported a significant association between depressive symptoms and glycemic status. A community-based study among Native Hawaiians, with and without diabetes mellitus, found a significant association (odds ratio = 3.2) between prevalence of depression and elevated hemoglobin A1c (HbA1c) levels (≥7%), after controlling for the effects of age, sex, education, social support, and body mass index (BMI; Grandinetti et al., 2000). Other studies have also found a significant association between depressive symptoms and glycemic status among people with type 1 and type 2 diabetes, after controlling for such variables as number of complications, smoking (Haire-Joshu et al., 1994), age, sex, education, type of diabetes, and perceived health status (Von Dras and Lichty, 1990). Lloyd et al. 2000 investigated the effects of sex on the relationship between depressive symptoms and HbA1c and found that the prevalence of moderate to severe depression was significantly associated with elevated HbA1c (>9%) in men but not in women.
Lustman et al. 2000 conducted a meta-analytical review of studies that reported statistically significant and nonsignificant findings on the relationship between depressive symptoms and glycemic status. They reported small (0.11) to moderate (0.19) effect sizes for the relationship between depressive symptoms and glycemic status among 21 studies that included people with type 1 and type 2 diabetes. A similar mean effect size (0.16) for the relationship between depressive symptoms and glycemic status was reported among seven studies that included only people with type 2 diabetes. The findings of this meta-analysis support a significant low-moderate association between depressive symptoms and glycemic status.
Depressive Symptoms and Health-Related, Quality of Life
The degree to which diabetes mellitus affects health-related quality of life (e.g., physical, social, and occupational functioning, and role obligations) has been found to be affected by severity of depressive symptoms (e.g., Talbot and Nouwen, 2000). Connell et al. 1994 found that physical functioning, perceived threats of diabetes on daily life activities, and perceived availability of general social support were significantly associated with depressive symptoms in people with type 1 and type 2 diabetes. Talbot et al. 1999 reported similar findings where perceived intrusion of illness on work, social, and recreational activities was significantly associated with depressive symptoms in people with type 2 diabetes. Other studies among people with type 2 diabetes have also reported a significant association between depressive symptoms and other indices of health-related quality of life, such as degree of difficulty in leisure, work, and family functioning (Mayou et al., 1990).
Further support for the impact of diabetes mellitus on health-related quality of life can be extrapolated from studies that compared people who were previously diagnosed with diabetes and, therefore, knew of their diagnosis (previously diagnosed) and people who were recently diagnosed with diabetes and, therefore, were not aware of having the illness at the time of the study (newly diagnosed). A population-based study reported the prevalence of depression to be 3.7 times higher among people previously diagnosed with diabetes than among people newly diagnosed (Palinkas et al., 1991). Another study of people with type 2 diabetes reported similar findings where the people previously diagnosed with diabetes reported a higher prevalence of depression (25%) than did people newly diagnosed (11.5%) and those with no diabetes diagnosis (11.7%; Rajala et al., 1997). These studies suggest that knowledge of having a diagnosis of diabetes may be associated with depressive symptoms.
Rationale and Goals of the Present Study
No study to date has compared the relative strengths of association between important biological and psychosocial variables and their possible interaction on depressive symptoms in persons with diabetes mellitus. Furthermore, many of the past studies did not examine sociodemographic variables that may serve to moderate the relationship between depressive symptoms and diabetes mellitus, such as age, sex, education, marital status, and social support. Although studies statistically controlled (e.g., usually through analysis of covariance) for these sociodemographic variables, they did not examine their interaction effects (moderator variables) on the relationship between depressive symptoms and diabetes. The strength of the relationship between depressive symptoms and diabetes may vary as a function of specific sociodemographic variables. The relative importance of biological and psychosocial variables as well as variables that moderate their impact has direct clinical implications for the treatment of depressive symptoms in people with diabetes mellitus.
Second, many studies did not take into account possible differences between people with type 1 and type 2 diabetes. The relationship between depressive symptoms and glycemic status may differ between types of diabetes because the severity of insulin deficiencies and the response to glucose stimulation differ between these two types (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2001). Therefore, the magnitude of correlation between depressive symptoms and glycemic status may differ across types of diabetes mellitus.
Third, many of the studies did not control for other biological variables that may have influenced depressive symptoms in people with diabetes, such as obesity and central adiposity. Studies have reported a significant statistical association between both obesity, measured by body mass index (BMI), and central adiposity, measured by waist–hip ratio (WHR), and depressive symptoms in people with diabetes (Lloyd et al., 1996; Viinamaki et al., 1995; Wing, et al., 1990).
Finally, this study sought to examine the relationship between depressive symptoms and diabetes among a diverse and unique Asian and Pacific Island population (e.g., Native Hawaiians, Filipino Americans, and Japanese Americans). These are ethnic groups that have been underrepresented in health behavior research, but who are at an increased risk for type 2 diabetes compared to the general population (Grandinetti et al., 1998; Ryan et al., 2000).
Therefore, the goals of this study were (1) to examine the proportion of variance in depressive symptoms accounted for by glycemic status while controlling for the effects of obesity and central adiposity; (2) to examine the proportion of variance in depressive symptoms accounted for by health-related quality if life while controlling for the effect of knowledge of diabetes diagnosis; (3) to examine the proportion of variance in depressive symptoms accounted for by both glycemic status and health-related quality of life as well as their interaction; and (4) to examine the moderating effects of age, sex, education, marital status, and social support on the association between depressive symptoms and type 2 diabetes.
METHODS
Participants
Cross-sectional data from the Native Hawaiian Health Research (NHHR) Project were used for this study. The NHHR project is an ongoing multiethnic, epidemiological study of diabetes and cardiovascular risk factors in North Kohala, Hawai‘i. Of the 1220 participants in the NHHR database at the time of this study, 146 (67 males and 79 females) were identified as having diabetes mellitus according to World Health Organization (WHO) criteria (fasting blood glucose ≥125 mg/dL or 2-h postchallenge blood glucose ≥200 mg/dL; Puavilai et al., 1999). All 146 participants were further identified as having type 2 diabetes on the basis of c-peptide traces in their fasting blood samples (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, 2001).
The 146 participants were from various ethnic groups, which included Hawaiian/part-Hawaiian (40.4%), Filipino American (17.1%), Japanese American (14.4%), Caucasian (6.8%), and people of mixed ancestry (21.2%). The age of the participants ranged from 26 to 89 with a mean age of 59.8 years (SD = 12.5). The mean educational attainment level was 11.79 (SD = 2.98). The marital status distribution was as follow: 12.3% never married, 64.4% were married, and 23.4% were separated, divorced, or widowed. Sixty-eight of the participants (46.6%) were previously diagnosed with diabetes prior to participation in the NHHR project (referred from here on as previously diagnosed diabetes) and 78 (53.4%) were diagnosed with diabetes mellitus by the NHHR project (referred from here on as newly diagnosed diabetes). The mean HbA1c value was 7.33 (SD = 1.8). The mean BMI was 30.7 (SD = 7.1) and the mean WHR was 1.0 (SD = 0.31).
ASSESSMENT INSTRUMENTS
Personal Information
The following data were collected using the Personal History Form designed by NHHR: address and phone number, gender, marital status, education level, work history, annual household income, and ethnic ancestry and identification. Ethnic ancestry information was based on participants’ self-report and, in addition to specific ethnic ancestries, percentage of blood quantum (i.e., less than 25%, 25–49%, 50–74%, 75–99%, and 100%) for each ethnic ancestry was asked. The Personal History Form was interviewer-administered.
Glycemic Status
Blood samples were collected from all participants after an overnight fasting of 10–14 h. Participants not taking insulin or oral diabetic medication underwent a 2-h, 75-g Oral Glucose Tolerance Test (OGTT; World Health Organization and Expert Committee on Diabetes, 1985). Fasting and 2-h post-OGTT blood samples were drawn from each participant, except those taking insulin or oral diabetic medication (only fasting blood samples were collected from these participants). Plasma glucose levels were assayed in the NHHR laboratory by using the glucose oxidase method on a YSI autoanalyzer. Because fasting and 2-h post-OGTT plasma glucose are subjected to daily fluctuations and may vary across ethnic groups (Gelding et al., 1995), hemoglobin A1c (HbA1c) was used as a measure of glycemic status, which was assayed by affinity chromatography using a minicolumn (BioRad, Hercule, CA.).
Anthropometrics
Weight, height, and waist and hip circumferences were obtained from each participant, using standardized protocols. Weight was measured using kilograms, height using meters, and waist and hip circumferences using centimeters (Lohman et al., 1988). The average of three measures for each was used for the final measurement. BMI was assessed by m/kg2 and a BMI ≥30 was used as a measure of obesity (Najjar and Rowland, 1987). WHR was measured by dividing the hip measurement from the waist measurement and a WHR ≥0.9 and 0.8 for women and men, respectively, was used as a measure of central adiposity (Bray, 1992).
Center for Epidemiological Studies—Depression Scale (CES-D)
Depressive symptoms were measured using the 20-item CES-D, which was designed for use among the general population (Radloff, 1977). It measures the frequency with which participants have experienced a specific symptom of depression within the preceding week, using a 4-point rating scale that ranged from 0 (“rarely to none of the time”) to 3 (“most or all of the time”). Scores ranged from 0 to 60, where higher scores indicated greater frequency of depressive symptoms (Radloff, 1977). Cronbach’s alpha coefficients have ranged from 0.63 to 0.93 with a test–retest coefficient of 0.61 (3-month lag) in past research (Devins et al., 1988). Measures from the CES-D have been found to have strong criterion validity, when compared to measures based on structured diagnostic interviews (Beekman et al., 1997; Somervell et al., 1993). Its validity has also been established across various ethnic groups (Devins et al., 1988; Hertzog et al., 1990).
SF-36 Health Survey (SF-36)
Health-related quality of life was measured using the SF-36 Health Survey (SF-36; Ware et al., 1993), which was designed to measure eight health domains on the basis of the participants’ perceived burden of their illness. Factor analysis of the SF-S6 has consistently reported a two-factor solution: physical and mental (Hays and Stewart, 1990; McHorney et al., 1993). To avoid item contamination between the SF-36 and the CES-D, only the following subscales that loaded on the physical dimension from factor analysis were used: (1) Physical Functioning, (2) Role Limitations due to Physical Health Problems, (3) Bodily Pain, (4) General Health, and (5) Social Functioning. The Physical Functioning subscale measured limitations in behavioral performance of everyday physical activity. The Role Limitations due to Physical Health Problems subscale measured the extent of disability in everyday activities due to physical problems. The Bodily Pain subscale measured severity of bodily pain and resulting limitations in activities. The Social Functioning subscale measured limitations in social activities due to physical and emotional problems. The General Health subscale measured the participant’s perceived overall health and the degree to which he or she believed it will get worse. In this study, a composite score of the SF-36 physical dimensions subscales was calculated, using Z score transformation (score range of 0–100), and used as a measure of participants’ overall health-related quality of life (higher scores indicated better health-related quality of life). Cronbach’s alpha coefficients for the SF-36 among patients with diabetes have ranged from 0.43 (Bodily Pain) to 0.90 (Physical Functioning; Nerenz et al., 1992). The convergent validity of the SF-36 subscales has been demonstrated among people with various medical conditions and severity, including type 2 diabetes (Ware et al., 1993). It has been compared to the Diabetes Care Profile (Fitzgerald et al., 1996), a diabetes-specific measure of quality of life, and found to be a comparable measure of health-related quality of life in people with diabetes (Anderson et al., 1997).
Lubben Social Network Scale (LSNS)
Social support was measured using a 6-item subscale of the 10-item LSNS (Lubben, 1988). The six items were designed to measure social support received from family and friends. Using a 5-point rating scale, participants’ were asked to rate the availability of assistance from family and friends for various needs, ranging from 1 (“definitely true”) to 5 (“definitely false”). The possible range of scores was from 6 to 30, where lower scores indicated greater social support. The LSNS has been found to be significantly correlated with measures of other construct, such as depression, life satisfaction, and other measures of social support (Newsom and Schulz, 1996). The entire LSNS was not examined because the NHHR project was only interested in the subscale that measured social support from family and friends. The other four items of the LSNS was not included because they measured social support received from confident relationships and the items were focused on whether or not others turned to or relied on the respondent for support and assistance.
PROCEDURE
Native Hawaiian participants who had participated in a previous NHHR study were contacted via telephone, mail, or a home visit for possible reparticipation with NHHR (see Grandinetti et al., 1998). A participant was identified as Native Hawaiian if he or she had ancestors residing in the islands of Hawai‘i prior to 1778. All other participants (both Native Hawaiian and non-Native Hawaiian) were solicited via telephone using a cross-reference directory, local public television announcements, flyers posted at community centers and stores, and presentations given to community organizations. Interested persons were asked to call the NHHR clinic to receive more information about the study and to make an appointment. Eligibility criteria for participation were as follows: (1) 18 years of age and older, (2) resident of North Kohala, and (3), if female, not pregnant. For people who responded to the various solicitations, eligibility was determined over the phone. If the prospective participant was determined to be eligible and indicated a willingness to participate, an appointment was made at that time. Each participant received a $20 gift certificate to a local grocery store for their participation. Participants were told to fast (no eating or drinking, with the exception of water) for 10–14 h prior to the appointment, and that the appointment would take approximately 2–3 h and involve two blood draws, anthropometric measurement, an electrocardiogram (ECG), pulmonary testing, and a battery of health behavior and psychosocial questionnaires. A day before the appointment, each participant was given a reminder call or visit (for those without phones) and reminded to fast. All appointments were made for the early morning hours (7–10) to make fasting easier for participants.
The clinical examination was then done by a licensed nurse according to standardized protocols. It consisted of fasting and post-OGTT blood draws, vital signs, and anthropometric measurements, ECG testing, urine sampling, and pulmonary testing. During the clinical examinations but prior to the glucose challenge, participants were asked if they had a diagnosis of diabetes and/or used diabetes medication and whether or not they had fasted the night before and for how long. Participants not taking insulin or oral diabetic medication underwent a 2-h, 75-g oral glucose tolerance test and, after the participant interview, the second blood draw was taken.
Following the clinical examination but before the second blood draw, interviews were done by a trained NHHR staff member and consisted of a battery of sociodemographic and health behavior questionnaires. They included a detailed diet and a physical activity questionnaire, an alternative medicine use questionnaire, and a medical history questionnaire. Upon completion of the interview, participants completed the CES-D, SF-36, and LSNS alone. After completion of all questionnaires, the interviewers checked the questionnaires for missing data while participants were waiting for second blood draw. Refreshments were offered to participants after second blood draw and any questions the participants may have had were addressed following the interview.
Within a few weeks of the clinical examinations, participants were mailed a summary of their clinical examination results with an explanation and possible medical conditions to follow up with a physician. Participants’ clinical results were also sent to specified primary health care providers for those who gave signed consent to do so. For Native Hawaiian participants, clinical results were also sent to a Native Hawaiian health care agency (Hui Mālama Ola Nā ‘Ōiwi) for those who gave signed consent to do so.
DATA REDUCTION
Five participants did not complete the CES-D, SF-36, and LSNS, and were removed from analyses. The resulting sample size was 141 people. Of these, two participants did not respond to one item on the CES-D. Therefore, these missing data were replaced by taking the average score across completed items in the same scale. Sociodemographic variables were interval-coded for regression analyses, with the exception of marital status, which was dummy-coded. The separated, divorced, and widowed marital statuses were aggregated because of small sample size, and is referred from here on as “disrupted marital status.” Participants that scored >8 on the LSNS were categorized as having “low social support” and those that scored ≤8 were categorized as having “high social support.” These cut scores for the social support levels were determined on the basis of median split. Participants that responded “yes” to the question of their knowledge of a diabetes diagnosis were categorized as having “previously diagnosed diabetes” (dummy code = 1) and those that responded “no” or “don’t know” but met WHO criteria for diabetes diagnosis were categorized as having “newly diagnosed diabetes” (dummy code = 0). HbA1c values were also categorized for interaction analysis into “low HbA1c level” (HbA1c < 7%), “moderate HbA1c level” (HbA1c = 7–9%), and “high HbA1c level” (HbA1c > 9.0%; see Lloyd et al., 2000). All analyses were done using SPSS Statistical Software for Windows, release 7.5.1 (SPSS, Inc., 1996).
RESULTS
Internal Consistency of CES-D, SF-36, and LSNS
Internal consistency of Cronbach’s alpha was .80 for the CES-D and .70 for the LSNS. Because the SF-36 subscales measured various aspects of health-related quality of life, which were aggregated in this study into a single measure, each subscale was considered an item for internal consistency analysis. The internal consistency coefficient was .74 for the SF-36 composite measure.
Descriptive Statistics and Intercorrelations
A summary of descriptive statistics for each variable is presented in Table I. One-way analysis of variance (ANOVA) was used to examine between-group differences on the CES-D, SF-36, and LSNS mean scores. Analyses were conducted to examine differences between means within each categorical group using Tukey’s β. Significant differences between CES-D mean scores were found for the categorical groups of education (F(3, 137) = 3.28, p < .05), marital status F(2, 138) = 9.33, p < .001), and social support F(1, 139) = 4.90, p < .05. No significant between-group differences were found for SF-36 and LSNS mean scores across all categorical groups. The Pearson product–moment intercorrelations among measures are presented in Table II.
Table I.
Participants’ Characteristics and Descriptive Statistics of CES-D, SF-36, and LSNS Across Groups
| CES-D scores
|
SF-36 Scores
|
LSNS Scores
|
||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | n | % | M | SD | M | SD | M | SD |
| Total | 146 | 9.5 | 7.3 | 73.6 | 19.2 | 9.0 | 3.5 | |
| Sex | ||||||||
| Male | 67 | 45.9 | 9.1 | 7.0 | 73.0 | 19.3 | 9.4 | 3.5 |
| Female | 79 | 54.1 | 9.9 | 7.5 | 74.2 | 19.3 | 8.7 | 3.4 |
| Age group | ||||||||
| 29–39 | 5 | 3.4 | 6.8 | 4.7 | 73.7 | 15.5 | 10.0 | 4.2 |
| 40–49 | 23 | 15.8 | 9.4 | 5.4 | 78.2 | 11.8 | 9.3 | 3.4 |
| 50–59 | 41 | 28.1 | 9.8 | 7.5 | 78.0 | 17.0 | 9.3 | 3.9 |
| 60–69 | 37 | 25.3 | 9.2 | 6.8 | 70.9 | 21.7 | 7.9 | 2.8 |
| 70 and over | 40 | 27.4 | 10.0 | 8.8 | 69.2 | 22.0 | 9.6 | 3.4 |
| Education group | ||||||||
| Non-high school graduate | 39 | 26.7 | 12.1a | 9.4 | 68.5 | 21.5 | 9.2 | 3.9 |
| High school graduate | 70 | 47.9 | 8.0b | 5.7 | 76.4 | 18.1 | 8.7 | 3.2 |
| Some college/tech training | 23 | 15.8 | 10.7c | 7.4 | 73.7 | 18.2 | 9.0 | 3.4 |
| College graduate | 10 | 6.8 | 7.7b | 4.5 | 74.8 | 18.1 | 11.0 | 3.6 |
| Ethnicity | ||||||||
| Hawaiian/part–Hawaiian | 59 | 40.4 | 10.6 | 6.9 | 76.2 | 15.5 | 8.6 | 3.5 |
| Filipino American | 25 | 17.1 | 10.8 | 10.0 | 65.8 | 23.3 | 9.9 | 3.7 |
| Japanese American | 21 | 14.4 | 6.8 | 6.4 | 78.4 | 20.5 | 8.6 | 2.5 |
| Caucasian | 10 | 6.8 | 6.5 | 4.4 | 77.6 | 24.4 | 7.5 | 2.3 |
| Mixed ethnicity | 31 | 21.2 | 9.3 | 6.1 | 70.3 | 18.0 | 10.2 | 3.8 |
| Marital status | ||||||||
| Never married | 18 | 12.3 | 7.2a | 5.2 | 75.8 | 19.0 | 9.1 | 4.0 |
| Currently married | 94 | 64.4 | 8.3a | 6.4 | 74.8 | 18.2 | 9.0 | 3.4 |
| Disrupted marital status | 34 | 23.4 | 14.0b | 8.7 | 69.4 | 21.8 | 9.2 | 3.4 |
| Diabetes diagnosis history | ||||||||
| Knowledge of diabetes | 68 | 46.6 | 9.5 | 8.0 | 74.2 | 19.3 | 9.2 | 3.4 |
| No knowledge of diabetes | 78 | 53.4 | 9.5 | 6.6 | 73.0 | 19.3 | 8.9 | 3.6 |
| Social support | ||||||||
| High | 91 | 64.5 | 8.5a | 7.1 | 75.4 | 19.3 | 6.9a | 1.2 |
| Low | 50 | 35.5 | 11.3b | 7.3 | 70.5 | 18.8 | 13.0b | 2.8 |
| HbA1c (%) | ||||||||
| Low HbA1c <7% | 71 | 48.6 | 8.6 | 6.6 | 73.8 | 19.7 | 9.4 | 3.5 |
| Moderate HbA1c 7–9% | 46 | 31.5 | 10.3 | 7.7 | 71.9 | 18.9 | 9.0 | 3.8 |
| High HbA1c >9.0% | 29 | 19.9 | 9.5 | 8.1 | 76.4 | 19.1 | 8.3 | 2.6 |
| BMI | ||||||||
| ≥30 | 75 | 51.4 | 10.6 | 6.8 | 76.4 | 22.0 | 9.0 | 3.5 |
| <30 | 63 | 43.2 | 8.6 | 7.6 | 71.0 | 15.6 | 9.3 | 3.3 |
| WHR | ||||||||
| Women ≥0.9 | 54 | 70.1 | 10.3 | 7.8 | 72.6 | 20.3 | 9.1 | 3.7 |
| <0.9 | 23 | 29.9 | 9.0 | 1.4 | 77.9 | 16.8 | 8.0 | 2.6 |
| Men ≥0.8 | 62 | 96.9 | 9.3 | 7.1 | 72.9 | 19.4 | 9.4 | 3.4 |
| <0.8 | 2 | 3.1 | 4.0 | 0.0 | 77.2 | 17.3 | 12.0 | 8.5 |
Note. Means with different subscripts differ significantly at p < .05 by Tukey’s β significant difference comparison. N = 141 for all ANOVA analysis due to missing data, except for BMI and WHR. N = 134 for BMI and WHR was N = 77 for women and N = 64 for men. CES-D = Center for Epidemiological Studies—Depression Scale; SF-36 = SF-36 Health Survey; and LSNS = Lubben Social Network Scale.
Table II.
Intercorrelations Among Biological, Psychosocial, and Sociodemographic Variables
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. CES-D | 1.0 | −0.40** | 0.19* | 0.09 | 0.08 | −0.06 | 0.06 | −0.18* | 0.05 | −0.12 | −0.22** | −0.001 |
| 2. SF-36 | 1.0 | −0.07 | 0.09 | 0.08 | 0.02 | −0.22** | 0.17* | 0.03 | 0.04 | 0.08 | 0.03 | |
| 3. LSNS | 1.0 | −0.10 | −0.02 | 0.01 | −0.01 | 0.03 | −0.10 | 0.01 | −0.02 | −0.06 | ||
| 4. HbA1c | 1.0 | 0.14 | −0.04 | −0.30** | 0.05 | −0.01 | 0.15 | −0.12 | 0.38** | |||
| 5. BMI | 1.0 | −0.004 | −0.46** | 0.08 | 0.04 | 0.06 | −0.01 | 0.11 | ||||
| 6. WHR | 1.0 | −0.04 | 0.02 | −0.17* | −0.02 | −0.07 | 0.13 | |||||
| 7. Age | 1.0 | −0.36** | −0.02 | −0.21* | −0.05 | −0.15 | ||||||
| 8. Education | 1.0 | −0.04 | −0.08 | 0.26** | −0.01 | |||||||
| 9. Sex | 1.0 | −0.03 | −0.14 | 0.06 | ||||||||
| 10. Never married | 1.0 | — | −0.02 | |||||||||
| 11. Married | 1.0 | 0.04 | ||||||||||
| 12. Diabetes history | 1.0 |
Note. Age and education were included as continuous variables for intercorrelation analyses. Point-biserial correlation coefficients are reported for the dummy coded marital statuses of never married and married, with disrupted marital status as the comparison group.
p < .05,
p < .01.
The Relationship Between HbA1c Values and CES-D Scores
A hierarchical regression analysis was conducted to examine the degree to which variance in CES-D scores could be accounted for by HbA1c and indices of obesity above and beyond that accounted for by indices of obesity alone. A regression model that included CES-D scores predicted by HbA1c values, BMI, and WHR (Model 1) were compared to a regression model with HbA1c values removed (Model 2). No statistically significant difference in R2s (R diff2 = −.01, p > .05) was found between Model 1 (R2 = .02, F(3, 136) = .72, p > .05) and Model 2 [R2 = .01, F(2, 137) = .70, p > .05). The inclusion of HbA1c values in the regression model did not significantly increase the variance accounted for in CES-D scores.
The Relationship Between SF-36 Scores and CES-D Scores
A hierarchical regression analysis was conducted to examine the degree to which variance in CES-D scores could be accounted for by SF-36 scores and knowledge of diabetes diagnosis above and beyond that accounted for by knowledge of diabetes diagnosis alone. A regression model that included CES-D scores predicted by SF-36 scores and knowledge of diabetes diagnosis (Model 1) were compared to a regression model with SF-36 scores removed (Model 2). A significant difference in R2s (R diff2 = −.16, p < .001) was found between Model 1 (R2 = .16, F(2, 138) = 13.01, p < .001) and Model 2 (R2 = .00, F(1, 139) = .00, p > .05). The inclusion of SF-36 scores in the regression model significantly increased the variance accounted for in CES-D scores.
The Relative Influence of HbA1c Levels and SF-36 Scores on CES-D Scores
A hierarchical regression analysis was conducted to examine the relative contribution of HbA1c levels and SF-36 scores in accounting for variance in CES-D scores. A regression model that included CES-D scores predicted by HbA1c levels and SF-36 scores (Model 1) were compared to a regression model with HbA1c removed (Model 2). No statistically significant difference in R2s (R diff2 = −.02, p > .05) was found between Model 1 (R2 = .18, F(2, 138) = 14.63, p > .05] and Model 2 (R2 = .16, F(1, 139) = 26.17, p > .05). The inclusion of HbA1c in the regression model did not significantly increase the variance accounted for in CES-D scores.
The Interaction Between HbA1c Levels and SF-36 Scores on CES-D Scores
A hierarchical regression analysis was conducted to examine an interaction between HbA1c categorical levels and SF-36 scores in accounting for variance in CES-D scores. A regression model that included HbA1c levels, SF-36 scores, and their interaction term as predictors of CES-D scores (Model 1) were compared to a regression model with the interaction term removed (Model 2). The interaction is graphed in Fig. 1. A statistically significant difference in R2s (R diff2 = −.04, p < .01) was reported between Model 1 (R2 = .22, F(3, 137) = 12.56, p < .001) and Model 2 (R2 = .18, F(2, 138) = 14.86, p < .001). The inclusion of the interaction term of Hba1c levels and SF-36 scores significantly increased the variance accounted for in CES-D scores. The strength of the negative correlation between CES-D and SF-36 scores varied as a function of HbA1c levels.
Fig. 1.
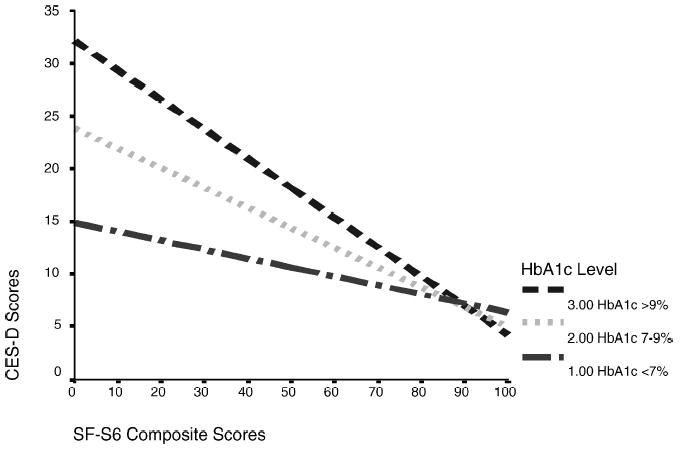
The relationship between SF-36 scores and CES-D scores across HbA1c levels.
Examination of Sociodemographic Moderating Variables
Because SF-36 scores were a significantly stronger predictor of CES-D scores in the previous analyses than was HbA1c, SF-36 scores were used to examine sociodemographic variables. Two-way interaction terms between SF-36 scores and each sociodemographic variable were examined separately to determine their independent contribution in accounting for variance in CES-D scores. Model 1 of each analysis consisted of SF-36 scores and a sociodemographic variable and their interaction variable and Model 2 of each analysis had the interaction variable removed. As a result, sex, education, marital status, and social support were found to moderate the relationship between SF-36 scores and CES-D scores. Age was not found to be an important moderator in this relationship. The results of the significant moderators are summarized in Table III. The strength of the correlation between SF-36 scores and CES-D scores varied as a function of sex, education level, marital status, and social support level.
Table III.
Summary of Hierarchical Regression Analysis of CES-D Scores Predicted by SF-36 Scores and Each Moderating Variable (N = 140)
| Variable | B | SE B | β | R2 | F | Rdiff2 | Fcomp |
|---|---|---|---|---|---|---|---|
| Sex moderator | |||||||
| Model 1 | 0.19 | 10.67*** | |||||
| SF-36 scores | 4.11 | 0.10 | 0.11 | ||||
| Sex (male = 1; female = 2) | 10.09 | 4.45 | 0.69 | ||||
| 2-way interaction | −0.12 | 0.06 | −0.84 | ||||
| Model 2 | 0.16 | 13.41*** | |||||
| SF-36 scores | −0.15 | 0.03 | −0.40 | ||||
| Sex | 0.95 | 1.13 | 0.07 | ||||
| Comparison of Model 1 and Model 2 | −0.03 | 4.51* | |||||
| Education moderator | |||||||
| Model 1 | 0.20 | 11.18*** | |||||
| SF-36 scores | −0.30 | 0.07 | −0.78 | ||||
| Education | −6.42 | 2.58 | −0.75 | ||||
| 2-way interaction | 7.83 | 0.03 | 0.82 | ||||
| Model 2 | 0.17 | 13.77*** | |||||
| SF-36 scores | −0.15 | 0.03 | −0.39 | ||||
| Education | −0.76 | 0.67 | −0.09 | ||||
| Comparison of Model 1 and Model 2 | −0.03 | 5.17* | |||||
| Marital status moderatora | |||||||
| Model 1 | 0.30 | 11.45*** | |||||
| SF-36 scores | −0.25 | 0.05 | −0.66 | ||||
| Never married | −26.25 | 7.34 | −1.18 | ||||
| Married | −14.81 | 4.58 | −0.98 | ||||
| Never married × SF-36 Scores | 0.28 | 0.10 | 0.98 | ||||
| Married × SF-36 Scores | 0.14 | 0.06 | 0.75 | ||||
| Model 2 | 0.25 | 14.99*** | |||||
| SF-36 scores | −0.14 | 0.03 | −0.36 | ||||
| Never married | −5.91 | 1.91 | −0.27 | ||||
| Married | −4.93 | 1.31 | −0.33 | ||||
| Comparison of Model 1 and Model 2 | −0.05 | 4.86** | |||||
| Social support moderator | |||||||
| Model 1 | 0.20 | 11.58*** | |||||
| SF-36 scores | 6.63 | 0.11 | 0.18 | ||||
| Social support (low = 1; high = 2) | 7.06 | 4.58 | 0.47 | ||||
| 2-way Interaction term | −0.13 | 0.06 | −0.88 | ||||
| Model 2 | 0.18 | 14.87*** | |||||
| SF-36 scores | −0.14 | 0.03 | −0.38 | ||||
| Social support | −2.10 | 1.18 | −0.14 | ||||
| Comparison of Model 1 and Model 2 | −0.02 | 4.28* | |||||
Note. HbA1c = hemoglobin A1c; SF-36 = SF-36 Health Survey and CES-D = Center for Epidemiological Studies—Depression Scale.
Marital Statuses were dummy coded (0 or 1), using disrupted marital status as the comparison group.
p < .05,
p < .01,
p < .001.
DISCUSSION
This study examined the degree to which biological and psychosocial factors influence the relationship between depressive symptoms and type 2 diabetes among a multiethnic, community-based population. It was the first to examine the degree to which depressive symptoms among people with type 2 diabetes could be accounted for by the interaction of glycemic status and indices of health-related quality of life, and the degree to which this relationship was moderated by sociodemographic variables. The methodological advantages of this study were the inclusion of people from various ethnic groups and only those with type 2 diabetes. First, the sample in this study included unique ethnic groups (e.g., Native Hawaiians, Filipino Americans, Japanese Americans, and those of mixed ancestries) who have been under-represented in health behavior research, but who are at an increased risk for type 2 diabetes compared to the general population (Grandinetti et al., 1998; Ryan et al., 2000). Although the ability to generalize these findings across other populations in the United States is limited, the ethnic representation in this study was comparable to the prevalence of type 2 diabetes by ethnicity in the State of Hawai‘i (Papa Ola Lokahi, 1992). Second, the inclusion of only people with type 2 diabetes reduced possible confounds due to differences in severity of insulin deficiencies and response to glucose stimulation between type 1 and type 2 diabetes.
The first goal of this study was to examine the proportion of variance in depressive symptoms accounted for by glycemic status while controlling for obesity indices. The results indicated that glycemic status did not account for a significant proportion of the variance in CES-D scores, above that accounted for by BMI and WHR. Furthermore, neither BMI nor WHR accounted for a significant proportion of the variance in CES-D scores as well. The nonsignificant correlation observed in this study between depressive symptoms and glycemic status was consistent with the results of several past studies (e.g., Gary et al., 2000; Padgett, 1993; Pibernik-Okanovic et al., 1993; Viiinamaki et al., 1995). However, many other studies have reported a significant correlation between depressive symptoms and glycemic status as presented earlier (e.g., Grandinetti et al., 2000; Haire-Joshu et al., 1994; Van der Does et al., 1996; Von Dras and Litchy, 1990). In addition, the non-significant correlation observed in this study between depressive symptoms and BMI and WHR was inconsistent with past studies, which reported a significant association between depressive symptoms and indices of obesity in people with diabetes (e.g., Lloyd et al., 1996; Viinamaki et al., 1995; Wing et al., 1990).
Some methodological issues may explain the nonsignificant correlations observed between depressive symptoms and the biological variables in this study and those of other studies. First, this study included a community-based population of people with diabetes mellitus whereas many other studies included a patient population (e.g., participants recruited from a hospital or health clinic; e.g., Lloyd et al., 2000; Padgett, 1993). There may be differences between the two populations on such variables as glycemic status, comorbidity with other medical illnesses, duration of diabetes, and the presence of diabetes-related complications. Second, other studies included both people with type 1 and people with type 2 diabetes. As noted earlier, these diabetes types differ in the severity of insulin deficiencies and in response to glucose stimulation. Third, there was very little variation in BMI and WHR in the participants of this study. For example, half (51.4%) of the participants were considered obese (BMI ≥ 30) and nearly all (96.9%) of the men and nearly three-fourths (70.1%) of the women had central adiposity (WHR ≥ 9 for women; ≥ 8 for men). Therefore, the correlations between depressive symptoms and BMI and WHR may have been attenuated because of the small variability in BMI and WHR among this study’s participants.
The second goal of this study was to examine the proportion of variance in depressive symptoms accounted for by health-related quality of life while controlling for knowledge of diabetes diagnosis. The results indicated that SF-36 scores accounted for a significant proportion of the variance in CES-D scores, above that accounted for by knowledge of diabetes diagnosis. The observed significant correlation between depressive symptoms and health-related quality of life in this study was consistent with the results of past studies (e.g., Connell et al., 1990, 1994; Mayou et al., 1990; Talbot et al., 1999). Specifically, studies have reported that restrictions in daily physical activities, an inability to fulfill role obligations, and a disruption to social relationships and activities were associated with depressive symptoms in people with diabetes (e.g., Connell et al., 1992; Talbot and Nouwen, 2000). However, the nonsignificant correlation between depressive symptoms and knowledge of diabetes diagnosis observed in this study was inconsistent with past studies, which reported a significantly higher prevalence of depressive symptoms among people previously diagnosed with diabetes compared to people newly diagnosed with the disorder (Palinkas et al., 1991; Rajala et al., 1997).
The third goal of this study was to examine the proportion of variance in depressive symptoms accounted for by a combination of glycemic status and health-related quality of life. The results indicated that SF-36 scores accounted for a significant proportion of the variance in CES-D scores compared to HbA1c values. HbA1c values did not significantly increase the proportion of variance accounted for in CES-D scores above that accounted for by SF-36 scores. These results were somewhat consistent with the results of Connell et al.’s study where indices of quality of life accounted for a greater proportion of the variance in depressive symptoms than glycated hemoglobin, 31 and 13% respectively (Connell et al., 1990).
Another goal of this study was to examine the proportion of variance accounted for in depressive symptoms by the interaction between glycemic status and health-related quality of life. As the results indicated, a significant interaction between HbA1c levels and SF-36 scores in accounting for variance in CES-D scores was observed. These findings suggested that the strength of the relationship between depressive symptoms and health-related quality of life differed across glycemic levels. The strength of the relationship was stronger for people with elevated glycemic levels than for people with lower glycemic levels. Although glycemic status was not significantly correlated with depressive symptoms in this study, it significantly moderated the strength of the relationship between depressive symptoms and health-related quality of life, which may explain the disparate findings among past studies that examined the relationship between depressive symptoms and glycemic status.
Finally, the fourth goal of this study was to examine sex, age, education, marital status, and social support as sociodemographic moderators of the association between depressive symptoms and type 2 diabetes. The results indicated that the strength of the relationship between depressive symptoms and health-related quality of life varied as a function of sex, education, marital status, and social support. The strength of the relationship between depressive symptoms and health-related quality of life was stronger for females than for males; stronger for people who were not high school graduates than for those with a high school degree or higher; stronger for people who were either separated, divorced, or widowed than for married and never-married people; and stronger for people with higher levels of social support than for those with low levels of social support.
The findings of marital status and social support as moderators of the association between depressive symptoms and type 2 diabetes are consistent with the results of past studies. For example, Connell et al.’s study reported an association between marital status and depressive symptoms in people with diabetes mellitus, which they attributed to differences in the availability of social support across marital statuses (Connell et al., 1994). The present study explored this possible explanation by using a one-way ANOVA to examine the association between marital status and social support in this sample, but no significant statistical association was observed. Studies have also reported associations between depressive symptoms and social support among people with diabetes mellitus as those observed in this study (e.g., Connell et al., 1994; Littlefield et al., 1990; Talbot and Nouwen, 2000).
In summary, health-related quality of life had the greatest magnitude of effect on depressive symptoms in people with type 2 diabetes compared to glycemic status and knowledge of diabetes diagnosis. Furthermore, the relationship between depressive symptoms and health-related quality of life was directly influenced by glycemic status, sex, education, marital status, and social support. The strength of the relationship between depressive symptoms and health-related quality of life was stronger for people with elevated HbA1c values, females, people with low educational attainment, people with a disrupted marital status, and people with low levels of social support.
The overall findings of this study have several important implications for research, assessment, and treatment of depression in people with type 2 diabetes. First, more studies examining specific facets of health-related quality of life (i.e., restrictions on physical and social activities and role obligations) and their moderators (i.e., sex and social support) are warranted, given the findings of this study. This study aggregated the physical subscales of the SF-36 for an overall measure of health-related quality of life. However, specific facets of this construct may have had a greater magnitude of effect on depressive symptoms than others. Second, assessment of depressive symptoms in people with diabetes often involves the identification and specification of important modifiable variables that are implicated in the cause or maintenance of the depressive symptoms. Therefore, the findings of this study can provide clinicians with focal points for their assessments. For example, assessing a client’s perceived physical and social limitations resulting from the illness may provide useful data for understanding his or her depression. Finally, the findings of this study support therapeutic approaches that focus on facets of health-related quality of life in addressing depressive symptoms in people with type 2 diabetes. Such approaches may focus on client’s perceived burden of diabetes on daily functioning, client’s ability to elicit needed social support from family and friends, and time management skills needed to monitor blood glucose levels, for diet modification, and for scheduling of activities.
Acknowledgments
The authors thank the staff of the Native Hawaiian Health Research (NHHR) Project for use of NHHR data. The authors also thank Jo Ann Mor for her technical assistance in the merging of data sets and Elaine Heiby, PhD, for her valuable comments and suggestions to the preparation of the manuscript. Most of all, the authors thank the North Kohala community on the island of Hawai‘i for allowing the NHHR project into their community and for recognizing the importance of health research in improving the overall well-being of their community and that of others. The NHHR was a project of the Pacific Biomedical Research Center, University of Hawai‘i, and was supported in part by awards from the Research Centers in Minority Institutions Program of The National Center for Research Resources, National Institutes of Health, to the University of Hawai‘i at Manoa, Grant Nos. RR03061 and P20 RR/AI 110901.
References
- American Diabetes Association. Economic consequences of diabetes mellitus in the U.S. in 1997. Diabetes Care. 1998;21:296–309. doi: 10.2337/diacare.21.2.296. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Fitzgerald JT, Wisdom K, Davis WK, Hiss RG. A comparison of global versus disease-specific quality-of-life measures in patients with NIDDM. Diabetes Care. 1997;20:299–305. doi: 10.2337/diacare.20.3.299. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Beekman ATF, Deeg DJH, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Centers for Epidemiological Studies Depression Scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- Black SA. Increased health burden associated with comorbid depression in older diabetic Mexican-Americans: Results from the Hispanic established population for the epidemiologic study of the elderly survey. Diabetes Care. 1999;22:56–64. doi: 10.2337/diacare.22.1.56. [DOI] [PubMed] [Google Scholar]
- Black SA, Markides KS. Depressive symptoms and mortality in older Mexican Americans. Ann Epidemiol. 1998;9:45–52. doi: 10.1016/s1047-2797(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Bray, G. (1992). An approach to the classification and evaluation of obesity. In Bjorntorp P., and Brodoff, B. (Eds.), Obesity, Lippincott, Philadelphia, pp. 294–308.
- Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: Impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–8. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- Clark CM. The burden of chronic hyperglycemia. Diabetes Care. 1998;21(Suppl 3):C32–C34. doi: 10.2337/diacare.21.3.c32. [DOI] [PubMed] [Google Scholar]
- Connell CM, Davis WK, Gallant MP, Sharpe PA. Impact of social support, social cognitive variables, and perceived threat on depression among adults with diabetes. Health Psychol. 1994;13:263–273. doi: 10.1037//0278-6133.13.3.263. [DOI] [PubMed] [Google Scholar]
- Connell CM, Fisher EB, Jr, Houston CA. Relationships among social support, diabetes outcome, and morale for older men and women. J Aging Health. 1992;4:77–100. [Google Scholar]
- Connell CM, Storandt M, Lichty W. Impact of health belief and diabetes-specific psychosocial context variables on self-care behavior, metabolic control, and depression of older adults with diabetes. Behav Health Aging. 1990;1:183–196. [Google Scholar]
- De Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: A meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- Devins GM, Orme CM, Costello CG, Binik YM, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiological Studies Depression (CES-D) scale. Psychol Health. 1988;2:139–156. [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2001;24(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald JT, Davis WK, Connell CM, Hess GE, Funnell MM, Hiss RG. Development and validation of the Diabetes Care Profile. Eval Health Prof. 1996;19:208–230. doi: 10.1177/016327879601900205. [DOI] [PubMed] [Google Scholar]
- Gary TL, Crum RM, Cooper-Patrick L, Ford D, Brancati FL. Depressive symptoms and metabolic control in African-Americans with type 2 diabetes. Diabetes Care. 2000;23:23–29. doi: 10.2337/diacare.23.1.23. [DOI] [PubMed] [Google Scholar]
- Gavard JA, Lustman PJ, Clouse RE. Prevalence of depression in adults with diabetes: An epidemiological evaluation. Diabetes Care. 1993;16:1167–1178. doi: 10.2337/diacare.16.8.1167. [DOI] [PubMed] [Google Scholar]
- Gelding SV, Andres C, Niththyananthan R, Gray IP, Mather H, Johnston DG. Increased secretion of 32,33 split proinsulin after intravenous glucose in glucose-tolerant first-degree relatives of patients with non-insulin dependent diabetes of European, but not Asian, origin. Clin Endocrinol. 1995;42:255–264. doi: 10.1111/j.1365-2265.1995.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ. Use of antidepressants in treatment of comorbid diabetes mellitus and depression as well as in diabetic neuropathy. Ann Clin Psychiatry. 2001;13:31–41. doi: 10.1023/a:1009012815127. [DOI] [PubMed] [Google Scholar]
- Grandinetti A, Chang HK, Mau MK, Curb JD, Kinney EK, Sagum R, Arakaki RF. Prevalence of glucose intolerance among Native Hawaiians in two rural communities. Diabetes Care. 1998;21:549–554. doi: 10.2337/diacare.21.4.549. [DOI] [PubMed] [Google Scholar]
- Grandinetti A, Kaholokula JK, Crabbe KM, Kenui CK, Chen R, Chang HK. Relationship between depressive symptoms and diabetes among Native Hawaiians. Psychoneuroendocrinology. 2000;25:239–246. doi: 10.1016/s0306-4530(99)00047-5. [DOI] [PubMed] [Google Scholar]
- Hairu-Joshu D, Heady S, Thomas L, Schechtman K, Fisher EB., Jr Depressive symptomatology and smoking among persons with diabetes. Res Nurs Health. 1994;17:273–282. doi: 10.1002/nur.4770170406. [DOI] [PubMed] [Google Scholar]
- Harris MI. Diabetes in America: Epidemiology and scope of the problem. Diabetes Care. 1998;21(Suppl 3):C11–C14. doi: 10.2337/diacare.21.3.c11. [DOI] [PubMed] [Google Scholar]
- Haynes, S. N., Nelson, K. G., Thacher, I., and Kaholokula, J. K. (2001). Outpatient behavioral assessment and treatment target selection. In Hersen M., and Prozelius, L. K. (Eds.), Diagnosis, Conceptualization, and Treatment Planning for Adults: A Textbook, Erlbaum, Hillsdale, NJ, pp. 35–70.
- Hays RD, Stewart AL. The structure of self-reported health in chronic disease patients. J Consult Clin Psychol. 1990;2:22–30. [Google Scholar]
- Herman WH, Eastman RC. The effects of treatment on the direct costs of diabetes. Diabetes Care. 1998;21(Suppl 3):C19–C24. doi: 10.2337/diacare.21.3.c19. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Alistine JV, Usala PD, Hultsch DF, Dixon R. Measurement properties of the center for epidemiological studies depression scale (CES-D) in older populations. Psychol Assess. 1990;2:64–72. [Google Scholar]
- Jacobson AM. Depression and diabetes. Diabetes Care. 1993;16(12):1621–1623. doi: 10.2337/diacare.16.12.1621. [DOI] [PubMed] [Google Scholar]
- Jones-Webb R, Snowden LR. Symptoms of depression among blacks and whites. Am J Public Health. 1993;83:240–244. doi: 10.2105/ajph.83.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BEK. Relation of glycemic control to diabetic complications and health outcomes. Diabetes Care. 1998;21(Suppl 3):C39–C43. doi: 10.2337/diacare.21.3.c39. [DOI] [PubMed] [Google Scholar]
- Littlefield CH, Rodin GM, Murray MA, Craven JL. Influence of functional impairment and social support on depressive symptoms in persons with diabetes. Health Psychol. 1990;9:737–749. doi: 10.1037//0278-6133.9.6.737. [DOI] [PubMed] [Google Scholar]
- Lloyd CE, Dyer PH, Barnett AH. Prevalence of symptoms of depression and anxiety in a diabetic clinic population. Diabet Med. 2000;17:198–202. doi: 10.1046/j.1464-5491.2000.00260.x. [DOI] [PubMed] [Google Scholar]
- Lloyd CE, Wing RR, Orchard TJ. Waist to hip ratio and psychosocial variables in adults with insulin-dependent diabetes mellitus: The Pittsburgh Epidemiology of Diabetes Complications study. Metabolism. 1996;45:268–272. doi: 10.1016/s0026-0495(96)90065-7. [DOI] [PubMed] [Google Scholar]
- Lohman, T., Roche, A. F., and Martorell, R. (1988). Anthropometric Standardization Reference Manual, Human Kinetics Books, Champaign, IL, pp. 3–8.
- Lubben JE. Assessing social networks among elderly populations. J Fam Community Health. 1988;11:42–52. [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type 2 diabetes mellitus: A randomized, controlled trial. Ann Intern Med. 1998;129:613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Gavard JA, Clouse RE. Depression in adults with diabetes. Diabetes Care. 1992;15:1631–1639. doi: 10.2337/diacare.15.11.1631. [DOI] [PubMed] [Google Scholar]
- Mayou R, Bryant B, Turner R. Quality of life in non-insulin-dependent diabetes and a comparison with insulin-dependent diabetes. J Psychosom Res. 1990;34(1):1–11. doi: 10.1016/0022-3999(90)90002-l. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Raczek AE. The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- Najjar, M., and Rowland, M. (1987). Anthropometric Reference Data and Prevalence of Overweight, U.S. 1976–1980, Department of Health and Human Services Hyattsville, MD (Vital and Health Statisitcs, Ser. 11, no. 238). [PubMed]
- Nerenz DR, Repasky DP, Whitehouse FW, Kahkonen DM. Ongoing assessment of health status in patients with diabetes mellitus. Med Care. 1992;30(Suppl):MS112–MS124. doi: 10.1097/00005650-199205001-00010. [DOI] [PubMed] [Google Scholar]
- Newsom JT, Schulz R. Social support as a mediator in the relation between functional status and quality of life in older adults. Psychol Aging. 1996;11:34–44. doi: 10.1037/0882-7974.11.1.34. [DOI] [PubMed] [Google Scholar]
- Padgett DK. Sociodemographic and disease-related correlates of depressive morbidity among diabetic patients in Zagreb, Croatia. J Nerv Ment Dis. 1993;181:123–129. doi: 10.1097/00005053-199302000-00008. [DOI] [PubMed] [Google Scholar]
- Palinkas LA, Barrett-Connor E, Wingard DL. Type 2 diabetes and depressive symptoms in older adults: A population-based study. Diabet Med. 1991;8:532–539. doi: 10.1111/j.1464-5491.1991.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Papa Ola Lokahi (1992). Native Hawaiian Health Data Book Papa Ola Lokahi, Honolulu, HI.
- Pibernik-Okanovic M, Roglic G, Prasek M, Metelko Z. War-induced stress and metabolic control in type 2 diabetic patients. Psychol Med. 1993;23:645–651. doi: 10.1017/s0033291700025423. [DOI] [PubMed] [Google Scholar]
- Popkin MK, Callies AL, Lentz RD, Colon EA, Sutherland DE. Prevalence of major depression, simple phobia, and other psychiatric disorders in patients with longstanding type 1 diabetes mellitus. Arch Gen Psychiatry. 1988;45:64–68. doi: 10.1001/archpsyc.1988.01800250078010. [DOI] [PubMed] [Google Scholar]
- Puavilai G, Chanprasertyotin S, Sriphrapradaeng A. Diagnostic criteria for diabetes mellitus and other categories of glucose intolerance: 1997 criteria by the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (ADA), 1998 WHO consultation criteria, and 1985 WHO criteria (World Health Organization) Diabetes Res Clin Pract. 1999;44:21–26. doi: 10.1016/s0168-8227(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Rajala U, Keinaemen-Kuikaanniemi S, Kivelae SL. Non-insulin-dependent diabetes mellitus and depression in a middle-aged Finnish population. Soc Psychiatry Psychiatr Epidemiol. 1997;32:363–367. doi: 10.1007/BF00805442. [DOI] [PubMed] [Google Scholar]
- Ryan C, Shaw R, Pliam M, Zapolanski AJ, Murphy M, Valle HV, Myler R. Coronary heart disease in Filipino and Filipino-American patients: Prevalence of risk variables and outcomes of treatment. J Invasive Cardiol. 2000;12:134–139. [PubMed] [Google Scholar]
- Somervell PD, Beals J, Kinzie JD, Boehnlein J, Leung P, Mansonet SM. Criterion validity of the Center for Epidemiologic Studies Depression Scale in a population sample from an American Indian village. Psychiatr Res. 1993;47:255–266. doi: 10.1016/0165-1781(93)90083-s. [DOI] [PubMed] [Google Scholar]
- Strowig S, Raskin P. Glycemic control and diabetic complications. Diabetes Care. 1992;15:1126–1140. doi: 10.2337/diacare.15.9.1126. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults. Diabetes Care. 2000;23:1556–1561. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A, Gingras J, Belanger A, Audet J. Relations of diabetes intrusiveness and personal control to symptoms of depression among adults with diabetes. Health Psychol. 1999;18:537–542. doi: 10.1037//0278-6133.18.5.537. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2000). HHS Target Efforts on Diabetes (HHS Fact Sheet, October 13, 2000). U.S. Government Printing Office Washington, DC.
- Van der Does FEE, De Neeling JND, Snoek FJ, Kostense PJ, Grootenhuis PA, Bouter LM, Heine RJ. Symptoms and well-being in relationship to glycemic control in type 2 diabetes. Diabetes Care. 1996;19:204–210. doi: 10.2337/diacare.19.3.204. [DOI] [PubMed] [Google Scholar]
- Viinamaki H, Niskanen L, Uusitupa M. Mental well-being in people with non-insulin-dependent diabetes. Acta Psychiatr Scand. 1995;92:392–397. doi: 10.1111/j.1600-0447.1995.tb09602.x. [DOI] [PubMed] [Google Scholar]
- Von Dras D, Lichty W. Correlates of depression in diabetic adults. Behav Health Aging. 1990;1:79–84. [Google Scholar]
- Ware, J. E., Snow, K. K., Kosinski, M., and Gandek, B. (1993). SF-36 Health Survey: Manual and Interpretation Guide, The Health Institute, New England Medical Center, Boston.
- Wing RR, Marcus MD, Blair EH, Epstein LH, Burton LR. Depressive symptomatology in obese adults with type 2 diabetes. Diabetes Care. 1990;13:170–172. doi: 10.2337/diacare.13.2.170. [DOI] [PubMed] [Google Scholar]
- World Health Organization and Expert Committee on Diabetes (1985). Report of WHO Study Group, (Technical Report Series, no. 727, 1–113). [PubMed]


