Abstract
Two clusters of necrotizing fasciitis (NF) due to group A streptococcus (GAS) were identified on the Hawaiian islands of Kauai and Maui during 1997 and 2002, respectively. The emm gene sequence types and the pulsed-field gel electrophoresis patterns were determined for 6 isolates recovered from patients with NF and for 116 isolates recovered from patients with temporally associated community-acquired GAS infection. No predominant emm type was identified, and the emm types of 64 (52.5%) of the isolates were considered to be uncommon in the continental United States. These findings suggest that unusual emm types might be responsible for invasive GAS infections in patients from Hawaii.
During the past several years, there have been several reports of sporadic cases of necrotizing fasciitis (NF) due to group A streptococcus (GAS) [1–4]. Secondary cases of NF are rare but have been reported to occur among family members of patients with NF, among health care personnel caring for patients with NF, and among meat handlers [5, 6]. Community clusters or outbreaks of NF due to GAS are unusual, and epidemiologic data on such clusters or outbreaks are very limited. Observation of 2 clusters of cases of NF that occurred in 2 distinct communities in Hawaii within a 6-year period prompted us to study the emm gene sequence types and PFGE patterns of GAS isolates recovered from patients with NF and from patients with temporally associated community-acquired bacteremia or severe soft-tissue infection [7, 8].
Patients and methods
A convenience sample of 122 GAS isolates was collected in Hawaii after 4 cases of NF were identified on the island of Kauai in 1997 and after 3 fatal cases of NF were identified on the island of Maui in 2002. Isolates recovered from sterile body sites and from sites of severe skin infection due to GAS were collected within 12 months after the identification of each of the 2 clusters. After the cluster of cases was identified in 1997 (i.e., the “Kauai cluster”), isolates were recovered only from patients on Kauai. After the cluster of cases was identified in 2002 (i.e., the “Maui cluster”), isolates were recovered from patients on the islands of Maui, Oahu, Kauai, and Hawaii. Isolates recovered from nonresidents of the state of Hawaii were excluded.
Staff of the State of Hawaii Department of Health (Honolulu) obtained limited demographic and epidemiologic data from patients, including a history of any contact with individuals who had a previous GAS infection. Additional clinical information was further ascertained after the Maui cluster was identified. “Invasive GAS infection” was defined by the presence of a clinically compatible illness and by recovery of GAS isolates from a normally sterile body site (e.g., blood). “NF” was defined by the presence of invasive disease with widespread necrosis of the fascia and subcutaneous tissue and, also, by the growth of GAS from deep tissue and/or blood. “Severe GAS skin infection” was defined by the presence of deep, extensive cellulitis in the involved tissues, which resulted in patient hospitalization and in intravenous administration of antibiotic treatment [1].
GAS isolates were analyzed by emm gene sequence typing and by PFGE. Typing of the emm gene sequences was done according to PCR protocols described elsewhere [9, 10]. All isolates were studied by PFGE performed after digestion with the restriction endonuclease SmaI [11]. DNA fingerprints were compared using Molecular Analyst Fingerprinting Plus with Data Sharing Tools software, version 1.6 (Bio-Rad Laboratories).
Data on the frequencies of emm types among GAS isolates recovered from sterile body sites at surveillance locations in the United States in 1995–2001 were obtained from the Centers for Disease Control and Prevention (CDC) surveillance program [9]. An emm type was considered to be common if it was among the 28 emm types most frequently reported through the CDC surveillance program [9]. The frequency of occurrence of emm types identified among isolates from Hawaii was compared with the frequency of occurrence of emm types identified among isolates from the CDC surveillance program. The identified emm types were compared with the emm types included in a candidate multivalent GAS vaccine [9, 12].
Results
A total of 122 GAS isolates recovered from patients in Hawaii were analyzed; 21 isolates were analyzed after the identification of the Kauai cluster in 1997, and 101 isolates were analyzed after the identification of the Maui cluster in 2002 (table 1). The statewide sample collected after the identification of the Maui cluster included 42 isolates from Maui, 30 from Hawaii, 28 from Oahu, and 1 from Kauai.
Table 1.
Emm types identified among isolates recovered from patients with necrotizing fasciitis (NF), bacteremia, and severe cellulitis due to group A streptococcus.
|
emm Type(s) identified,a according to isolate site or patient diagnosis
|
||||
|---|---|---|---|---|
| Case cluster (year of identification) and island of isolate origin | NF (n = 6) | Blood (n = 42) | Skin infection (n = 74) | Total no. of emm type(s) identified (n = 122) |
| Kauai cluster (1997), Kauai
Maui cluster (2002) |
1, 99, and 103 | 11 | 11 (9), 56 (2), and 89 (6) | 21 |
| Maui | 74 and 102 | 22 (2), 58 (5), 74, 77, and 85 (2) | 58 (11), 67 (2), 74 (7), 81, 85 (2), 102, 103, 106, 109, and 117 (2) | 42 |
| Oahu | 1 (2), 9, 22, 28 (2), 49 (6), 56, 58, 69/65 (3), 70, 74, 78, 81 (2), 85, 92 (3), and 97 | 1 | 28 | |
| Kauai | 75 | 1 | ||
| Hawaii | 12 | 33 and 69/65 | 1, 58 (5), 69/65 (14), 73, 74 (2), 75, 81, and 92 (2) | 30 |
Nos. in parentheses are percentages that denote the frequency of occurrence of the emm type among isolates. The most prevalent emm types (i.e., emm types identified in ≥5 patients) are shown in boldface type.
Fifteen patients had NF diagnosed during the study period, and GAS isolates were available from 6 of these 15 patients, including 1 patient from Maui who had a fatal infection. Two of these 6 patients for whom isolates were available had positive blood culture results. Another 42 GAS isolates (34% of all 122 isolates) were obtained from patients with GAS bacteremia, and 74 isolates (61% of all 122 isolates) were recovered from dermal sites and/or wounds of patients with severe skin infections. Four (27%) of the 15 patients with NF died, and these deaths were attributed to NF and streptococcal toxic shock syndrome.
Overall, 28 different emm types were identified among the 122 isolates (table 1). The most common emm type—emm type 58—was identified in 22 (18%) of the 122 isolates; the next most common emm type—emm type 69/65—was identified in 18 (15%) of the isolates (table 1).
Among the 21 isolates from the Kauai cluster, 6 different emm types (emm types 1, 11, 56, 89, 99, and 103) were identified. After identification of the Maui cluster in 2002, a total of 26 different emm types were identified statewide. The distribution of the emm types varied according to the island from which the isolates were collected. Emm type 58 (identified in 16 isolates) and emm type 74 (identified in 9 isolates) were commonly noted among isolates recovered from patients on the island of Maui. Emm type 65/69 (identified in 15 isolates) and emm type 58 (identified in 5 isolates) were also commonly identified among isolates recovered from patients on the island of Hawaii. The emm type 49 (identified in 6 isolates) appeared to be common among isolates recovered from patients on the island of Oahu (table 1). The most common emm type identified among the isolates recovered in 1997 (i.e., emm type 11) was not identified among the isolates recovered in 2002.
Six different emm types were identified among the 6 isolates recovered from patients with NF (table 1). The emm types 22, 49, 58, 69/65, 85, and 92 were identified in ≥3 patients with bacteremia.
When the emm types of isolates recovered from patients with NF and blood cultures were compared with the emm types of isolates recovered from patients with skin infection, emm type 74 was identified among all groups of isolates recovered from all patients on Maui, and all 9 isolates with that type had PFGE patterns that were indistinguishable (table 1). Although emm type 102 was identified in an isolate recovered from 1 patient with NF and in an isolate recovered from 1 patient with a skin infection, these 2 isolates of emm type 102 had different emm gene sequence subtypes (figure 1). Among isolates recovered from patients on the island of Hawaii, emm type 69/65 was identified by blood and skin cultures. Isolates that were of the same emm sequence type and subgroup had identical patterns on PFGE analysis, regardless of the island from which they were collected; examples of such isolates are shown in figure 1.
Figure 1.
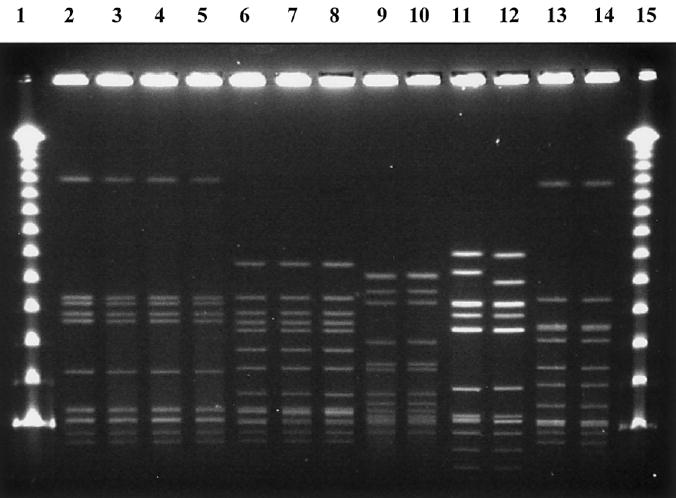
PFGE patterns of the group A streptococcus isolates recovered from a cluster of cases of necrotizing fasciitis that occurred on the island of Maui, Hawaii, in 2002. Lanes 1 and 15, the bacteriophage λ ladder; lanes 2–5, emm type 74; lanes 6–8, emm type 58; lanes 9 and 10, emm type 85; lane 11, emm type 102.3 (identified in a blood culture from a patient with necrotizing fasciitis); lane 12, emm type 102.2 (identified in an isolate from a patient with a GAS skin infection); and lanes 13 and 14, emm type 22.
In the present study, 64 isolates (52.5%) had emm types that were considered to be uncommon in the continental United States, compared with data on emm types from the CDC. The only available isolate recovered from a patient with a fatal NF infection was of emm type 74, and this emm type was not reported from sites in the continental United States during CDC surveillance studies (table 2). In addition to emm type 74, emm types 67, 70, 97, 99, 106, and 109 were not reported among isolates examined at the ABC (Active Bacterial Core) surveillance sites. Furthermore, the emm types of 90 (74%) of the 122 isolates did not match the emm types in the candidate vaccine, and the emm types of 32 isolates (67%) recovered from patients with NF and blood cultures and from patients with GAS bacteremia were types that were not represented in the candidate vaccine.
Table 2.
The frequency of occurrence of emm types among 48 isolates obtained from patients in Hawaii who had bacteremia and/or necrotizing fasciitis, compared with the reported frequency of emm types among isolates recovered from sterile body sites of individuals in the continental United States.
| Frequency of occurrencea of emm types among isolates
|
||
|---|---|---|
| emm Type | From Hawaii, % of isolates (no. of isolates) | From the continental United States,b % of isolates |
| 1 | 6.2 (3) | 20 |
| 22 | 6.2 (3) | 2.5 |
| 49 | 12.5 (6) | 0.5 |
| 58 | 12.5 (6) | 1.3 |
| 69/65 | 8.3 (4) | 1.3 |
| 74 | 6.2 (3) | 0 |
| 85 | 6.2 (3) | 0.03 |
| 92 | 6.2 (3) | 1.5 |
Frequency of occurrence was determined only for those emm types that were identified among ≥3 isolates.
Data are from [9].
Demographic information, including sex and age, was available for 122 patients. Eighty-four patients (69%) were male. The mean patient age was 44 years. Detailed clinical information was available for only 38 of the 42 patients from Maui. Of these patients, 11 had diabetes mellitus and 10 were heavy alcohol consumers. Preexisting chronic medical conditions were not reported for 11 patients. Three patients had a history of injection drug use, and 1 of these patients was also a heavy alcohol consumer. Four patients were homeless; 2 of these 4 patients had diabetes mellitus, and 1 was also a heavy alcohol consumer. The mean age of patients with invasive disease and NF in this subset of patients from Maui (65.8 years) was higher than the mean age of the whole study sample. Of 13 patients who had bacteremia and NF, 6 had chronic conditions, including diabetes mellitus (4 patients), injection drug use (1 patient), and heavy alcohol consumption (1 patient), and 4 patients had chronic health conditions, including high blood pressure (1 patient), renal failure requiring dialysis (1 patient), hepatitis B virus and hepatitis C virus infections (1 patient), and depression (1 patient).
Discussion
The epidemiology of GAS infections in Hawaii is different from that in the continental United States, in that pharyngitis and skin infections due to GAS do not appear to be seasonal in this subtropical climate [8]. Skin infections are common, as are complications associated with GAS infections, such as acute rheumatic fever and poststreptococcal glomerulonephritis. More interestingly, the invasive GAS infections in Hawaii may manifest as clusters of cases of community-associated NF caused by a wide array of isolates with unusual emm types [7, 8].
Epidemiologic data have not been available for any clusters of cases of community-associated NF. The available clinical data for the patients in the present study revealed risk factors for invasive GAS infection (e.g., diabetes mellitus and injection drug use) that were similar to risk factors reported elsewhere [13] for sporadic infections. In the present study, many of the GAS emm types identified among isolates were uncommon (i.e., a substantial proportion of isolates [52.5%] had unusual emm types, such as emm types 9, 49, 56, 69/65, 70, 74, 78, 81, 85, 97, 99, 102, and 103). In addition, analysis of the NF clusters did not reveal any common shared emm types, and different frequencies of occurrence of emm types were noted on each Hawaiian island after the identification of the Maui cluster in 2002.
Temporal and geographic changes in the most prevalent GAS strains in different populations have been documented elsewhere [14, 15]. Our observation of diverse emm types among isolates recovered from patients on different islands of Hawaii in 1997 and 2002 may be representative of these epidemiologic phenomena, but it does not explain the prevalence of unusual emm types. In some population-based studies, certain emm/M types, such as M1 and M3, were commonly identified in isolates recovered from patients with NF and invasive GAS infection [2, 3]. In the small sample in the present study, no M3 isolates were identified, and M1 was not common.
Although the number of isolates was limited, certain emm types, such as emm types 74, 69/65, and 102, appear to be associated with both skin infections and invasive GAS infections. The higher prevalence of unusual emm types in cultures obtained from sterile and nonsterile body sites also raised the question about the effectiveness of a candidate 26-valent group A streptococcal vaccine [12]. These findings suggested that unusual emm types may be responsible for the invasive GAS infections on different Hawaiian islands over a period of 6 years.
Acknowledgments
We thank Dana Tamashiro and Precilia Calimlim for performing PFGE analysis of the gels.
Financial support. Grants from the American Heart Association, National Institutes of Health, Centers of Biomedical Research Association (grant P20RR018727-02), and Research Centers in Minority Institutions (grant P20RR11091-06).
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Kaul R, McGeer A, Low DE, Green K, Schwartz B. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario Group A Streptococcal Study. Am J Med. 1997;103:18–24. doi: 10.1016/s0002-9343(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien K, Beall B, Barrett NL, et al. Epidemiology of invasive group A streptococcus disease in the United States, 1995–1999. Clin Infect Dis. 2002;35:268–76. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 3.Sharkawy A, Low DE, Saginur R, et al. Severe group A streptococcal soft-tissue infections in Ontario: 1992–1996. Clin Infect Dis. 2002;34:454–60. doi: 10.1086/338466. [DOI] [PubMed] [Google Scholar]
- 4.Stevens DL. Invasive streptococcal infections. J Infect Chemother. 2001;7:69–80. doi: 10.1007/s101560100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.File TM. Necrotizing soft tissue infections. Curr Infect Dis Rep. 2003;5:407–15. doi: 10.1007/s11908-003-0021-y. [DOI] [PubMed] [Google Scholar]
- 6.Fraser CA, Ball LC, Morris CA, Noah ND. Serological characterization of group-A streptococci associated with skin sepsis in meat handlers. J Hyg (Lond) 1977;78:283–96. doi: 10.1017/s0022172400056175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nekomoto TS, Manea SJ, Kanenaka RY, Burr RK, Effler PV. Characterization of group A streptococci isolates from hospitalized case patients and asymptomatic school children, Kauai, Hawaii, 1997. In: Program and abstracts of the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy (Chicago). San Francisco: American Society for Microbiology, 1999:657.
- 8.Erdem G, Abe L, Kanenaka RY, et al. Characterization of a community cluster of group A streptococcal invasive disease in Maui, Hawaii. Pediatr Infect Dis J. 2004;23:677–9. doi: 10.1097/01.inf.0000130956.47691.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Streptococcus pyogenes emm sequence database. Available at: http://www.cdc.gov/ncidod/biotech/strep/strepindex.htm Accessed 28 April 2005.
- 10.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–8. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiPersio JR, File TM, Stevens DL, Gardner WG, Petropoulos G, Dinsa K. Spread of serious disease-producing M3 clones of group A streptococcus among family members and health care workers. Clin Infect Dis. 1996;22:490–5. doi: 10.1093/clinids/22.3.490. [DOI] [PubMed] [Google Scholar]
- 12.Hu MC, Walls MA, Stroop SD, Reddish MA, Beall B, Dale JB. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect Immun. 2002;70:2171–7. doi: 10.1128/IAI.70.4.2171-2177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Factor SH. Invasive group A streptococcal disease: risk factors for adults. Emerg Infect Dis. 2003;9:970–7. doi: 10.3201/eid0908.020745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwartz B, Facklam R, Breiman R. Changing epidemiology of group A streptococcal infection in the USA. Lancet. 1990;336:1167–71. doi: 10.1016/0140-6736(90)92777-f. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Wotton JT, Johnson DR. Dynamic epidemiology of group A streptococcal serotypes associated with pharyngitis. Lancet. 2001;358:1334–7. doi: 10.1016/S0140-6736(01)06415-7. [DOI] [PubMed] [Google Scholar]


