Abstract
The efficient release of many enveloped viruses from cells involves the coalescence of viral components at sites of budding on the plasma membrane of infected cells. This coalescence is believed to require interactions between the cytoplasmic tails of surface glycoproteins and the matrix (M) protein. For the paramyxovirus simian virus 5 (SV5), the cytoplasmic tail of the hemagglutinin-neuraminidase (HN) protein has been shown previously to be important for normal virus budding. To investigate a role for the cytoplasmic tail of the fusion (F) protein in virus assembly and budding, we generated a series of F cytoplasmic tail-truncated recombinant viruses. Analysis of these viruses in tissue culture indicated that the cytoplasmic tail of the F protein was dispensable for normal virus replication and budding. To investigate further the requirements for assembly and budding of SV5, we generated two double-mutant recombinant viruses that lack 8 amino acids of the predicted 17-amino-acid HN protein cytoplasmic tail in combination with truncation of either 10 or 18 amino acids from the predicted 20-amino-acid F protein cytoplasmic tail. Both of the double mutant recombinant viruses displayed a replication defect in tissue culture and a budding defect, the extent of which was dependant on the length of the remaining F cytoplasmic tail. Taken together, this work and our earlier data on virus-like particle formation (A. P. Schmitt, G. P. Leser, D. L. Waning, and R. A. Lamb, J. Virol. 76:3953-3964, 2002) suggest a redundant role for the cytoplasmic tails of the HN and F proteins in virus assembly and budding.
Many enveloped viruses assemble and bud from the plasma membranes of virus-infected cells. Assembly involves the coalescence of both soluble viral proteins and viral integral membrane glycoproteins at sites of budding. Assembly in many cases also requires the segregation of cellular proteins away from sites of budding such that released virions are comprised mainly of viral components. The subsequent step of budding requires vesicularization of the plasma membrane with fission occurring to pinch off progeny virions.
The details of the roles of viral components in assembly and budding vary greatly among enveloped viruses. For alphaviruses it has been well established that an interaction between the cytoplasmic tail of the transmembrane spike glycoprotein and the internal nucleocapsid structure is critical for normal virus budding (58, 64). In contrast, retrovirus budding does not require expression of the envelope glycoprotein and budding of virus-like particles (VLPs) is very efficient in cells expressing only the retroviral core Gag polyprotein (9, 17).
For the enveloped negative-strand RNA viruses, the viral components thought to be critical for normal virus assembly and budding include (i) the nucleocapsid (RNP), which contains the nucleocapsid protein (NP)-encapsidated genome RNA and the associated RNA-dependent RNA transcriptase complex, (ii) the matrix protein (M), which forms a layer underlying the plasma membrane, and (iii) the integral membrane glycoprotein(s), which are expressed at the cell surface.
For the rhabdovirus rabies virus, deletion of either the M protein or the cytoplasmic tail of the surface glycoprotein (G) in recombinant virus leads to a severe budding defect (36, 37). For the rhabdovirus vesicular stomatitis virus (VSV), although the natural cytoplasmic tail of the G protein enables efficient budding, the presence of a nonspecific cytoplasmic tail sequence suffices for budding of recombinant virus and increases the budding efficiency of virus lacking the G protein cytoplasmic tail (54, 63). Studies with the orthomyxovirus influenza virus, have shown that truncation of the cytoplasmic tail of either the neuraminidase (NA) or the hemagglutinin (HA) glycoprotein individually does not have a great effect on budding of recombinant virus but that deletion of both the HA and NA cytoplasmic tails together leads to greatly altered virion morphology. These data suggest a redundant role for the cytoplasmic tails of HA and NA in the assembly process (29).
For the paramyxovirus, Sendai virus, it has been reported that the M protein binds independently to the cytoplasmic tails of the surface glycoproteins, hemagglutinin-neuraminidase (HN) and fusion (F) (1, 51). In studies with recombinant Sendai virus harboring deletions of either the cytoplasmic tail of the HN or the F protein, it was observed that HN was poorly incorporated into virions and that HN and F, respectively, were required for normal virus budding (13, 59). Studies on another paramyxovirus, measles virus, indicated that truncation of the cytoplasmic tail of the hemagglutinin (H) protein or the F protein led to defects in incorporation of the respective glycoproteins into virions and an increase in nonspecific cellular protein incorporation (7). In addition, recombinant measles virus lacking the M protein was severely defective in assembly. Furthermore, it was observed that M protein expression was required for colocalization of glycoproteins with the viral RNP (6). One caveat that has to be added concerning interpretation of experiments with measles virus assembly and budding is that, even for wild-type (wt) measles virus, budding is a very inefficient process (39). For the paramyxovirus simian virus 5 (SV5), it has been shown that recombinant viruses harboring deletions in the HN cytoplasmic tail are budding defective. The few virions released also have a deficiency in incorporation of the HN cytoplasmic tail-deleted protein, and there is poor segregation of cellular and viral proteins during assembly (52).
SV5, like other paramyxoviruses, consists of a core of genomic nonsegmented negative-strand RNA that is encapsidated by the NP protein to form the RNP. Virions assemble at the plasma membrane and the RNP is enveloped by plasma membrane containing the viral integral membrane proteins HN, F and small hydrophobic (SH) protein (reviewed in reference 31). The HN protein mediates virus cell attachment to sialic acid residues on surface proteins of target cells. HN also has neuraminidase (receptor destroying) activity that is thought to remove sialic acid from the viral glycoproteins such that assembled virions at the surface of infected cells are efficiently released. The F protein mediates viral entry into cells by causing fusion of the virus envelope with the target cell plasma membrane. The third integral membrane protein, the 44-amino-acid residue SH protein, probably does not play a role in virus assembly since this protein is dispensable for normal virus replication in tissue culture cells (23). However, recent work has shown that one role of the SH protein is to block apoptosis in virus-infected cells (24).
Here we report properties of recombinant SV5 (rSV5) that lacks the cytoplasmic tail of the F protein and double-mutant rSV5 that lacks the cytoplasmic tails of both the HN and F proteins. Analysis of the replication and budding of these viruses shows that the F protein cytoplasmic tail is dispensable for efficient virus replication and budding in tissue culture cells in the presence of a wt HN protein. However, truncation of the cytoplasmic tails of both the HN and the F proteins causes a severe budding defect, suggesting a redundant role for the HN and F protein cytoplasmic tails in virus assembly.
MATERIALS AND METHODS
Cells and viruses.
MDBK, CV-1, 293T, and BSR T7/5 (4) cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. BHK-21F cells were maintained in DMEM supplemented with 10% fetal bovine serum and 10% tryptose phosphate broth. rSV5 was propagated in MDBK cells, and titers were determined based on the 50% tissue culture infectious doses (TCID50) in BHK-21F cells by using eight replicates in 96-well plates and then monitoring the presence or absence of syncytium formation. Endpoint dilutions were calculated by the method of Reed and Muench (47). Plaque purification was done on BHK-21F cells in 6-cm dishes (44).
Recombinant plasmid vectors and oligonucleotide-directed mutagenesis.
Mutations in the cytoplasmic tails of HN and F were generated by oligonucleotide-directed mutagenesis with the SV5 infectious cDNA clone, pBH276 (25) as the template for PCR. Amplified sequences were then cloned into either the expression vector pGEM2 or into the SV5 infectious cDNA clone to generate the series pGEM2 FΔ2 to FΔ20 and the series pBH276 FΔ2 to FΔ20. The nucleotide sequence of each PCR amplified product was confirmed by automated sequencing by using an ABI Prism 310 genetic analyzer (Applied Biosystems, Inc., Foster City, Calif.). The DNA fragment encoding the SV5 L protein was subcloned from pBH276 into the eukaryotic expression vector pCAGGS (42) to generate pCAGGS-L. pCAGGS-NP and pCAGGS-P which express the SV5 NP and P proteins were those described previously (34, 53).
Recovery of mutant rSV5 by reverse genetics.
BSR T7/5 cells in 3.5-cm-diameter dishes (∼60 to 90% confluent) were transfected with the SV5 genomic cDNA clones containing the HN and F cytoplasmic tail-truncations, together with the support plasmids pCAGGS-NP, pCAGGS-L, and pCAGGS-P, by using Lipofectamine-Plus reagents according to the manufacturer's recommendations (Invitrogen, San Diego, Calif.). The plasmid amounts were as follows: 1.0 μg of SV5 genome plasmid, 100 ng of pCAGGS-NP, 20 ng of pCAGGS-P, and 500 ng of pCAGGS-L. Cells were incubated with the transfection mix for 12 to 16 h at 37°C. Cells were then washed once with phosphate-buffered saline (PBS), and the medium was replaced with DMEM supplemented with 2% fetal bovine serum. At 2 to 4 days posttransfection (p.t.) the media were assayed for the presence of rSV5 by monitoring syncytium formation on BHK-21F cells. The supernatant fluid from positive dishes was then used for plaque purification (44).
RNA isolation and RT-PCR.
Viral RNA was isolated directly from high-titer virus stocks by using a QIAamp viral RNA extraction kit (Qiagen, Valencia, Calif.) according to the manufacturer's recommendations. A total of 500 ng of viral RNA was used in a 20-μl reverse transcriptase (RT) reaction containing a 1 μM concentration of oligonucleotide primer, which annealed to viral RNA minus strand appropriate for either HN or F, and Moloney murine leukemia virus RT (New England Biolabs, Inc., Beverly, Mass.). Then, 5 μl of the RT reaction was used as a template during PCR amplification with a 1 μM concentration of each oligonucleotide primer specific for HN and F products. PCR products were agarose gel purified and analyzed by automated nucleotide sequencing. The nucleotide sequences of the primers used in the RT-PCR will be provided upon request.
Expression of mutant F proteins.
Expression of wt and cytoplasmic tail-truncated F proteins from cDNA was performed by using the recombinant vaccinia virus-T7 RNA polymerase transient-expression system (14). CV-1 cells in 3.5-cm dishes (∼90% confluent) were infected with the recombinant vaccinia virus vTF7.3 at a multiplicity of infection (MOI) of 10 PFU/cell. At 1 h postinfection (p.i.) cells were transfected with pGEM2 plasmids encoding cytoplasmic tail-truncated F proteins (pGEM2 FΔ2 to FΔ20) by using liposomes made in our laboratory (49). At 4 to 6 h p.t., the medium was replaced with DMEM supplemented with 2% fetal bovine serum, and the cultures incubated an additional 18 h at 33°C. For expression of wt and cytoplasmic tail-truncated F proteins from rSV5 virus, CV-1 cells in 3.5-cm dishes (∼90% confluent) were infected at an MOI of 0.1 TCID50 unit/cell. After 1 h the medium was replaced with DMEM supplemented with 2% fetal bovine serum, and the cultures were incubated for 14 to 16 h at 37°C before subsequent analysis.
Flow cytometry.
CV-1 cells expressing wt and cytoplasmic tail-truncated F proteins were chilled on ice and washed twice with PBS containing 0.02% sodium azide (PBS+azide). Cells were then incubated for 30 min with the F-specific monoclonal antibody (MAb) F1a (46). Cells were washed five times with PBS+azide and treated for 30 min with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG) secondary antibody. Cells were then washed five times with PBS+azide and twice with PBS deficient in calcium and magnesium. Cells were removed from dishes by treatment with PBS containing 50 mM EDTA and fixed in suspension by the addition of methanol-free formaldehyde to a final concentration of 1%. Fluorescence intensity of 10,000 cells was measured by using a FACSCalibur flow cytometer (Beckton Dickinson, San Jose, Calif.).
Immunoprecipitation of polypeptides.
CV-1 cells were infected with wt or mutant rSV5 at an MOI of 1.0 TCID50 unit/cell. At 18 h p.i., cells were labeled with 100 μCi of [35S]-Promix (Amersham Pharmacia Biotech, Piscataway, N.J.)/ml in 1.5 ml of DMEM lacking cysteine and methionine for 2 h. Cells were then lysed in radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris pH 7.4; 1% deoxycholate; 1% Triton X-100; 0.1% sodium dodecyl sulfate [SDS]) containing 0.15 M NaCl, 50 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitors (44). Cell lysates were clarified by centrifugation at 132,000 × g for 15 min in a Beckman TLA 100.2 rotor. SV5 F protein was immunoprecipitated from lysates with either a combination of rabbit sera raised against synthetic peptides corresponding to three different regions of F2 (α-F2) and synthetic peptide corresponding to amino acids 388 to 402 within F1 (F1-244) (11, 26) or rabbit serum raised against recombinant vaccinia virus expressing SV5 F (α-vacF) (45). Immunoprecipitation of M, NP, and HN from lysates of virus-infected cells was done with MAbs M-h, NPa, and HN1b (46). Immune complexes were then adsorbed to protein A-Sepharose beads for 30 min at 4°C. The beads were then washed three times with RIPA plus 0.3 M NaCl, twice with RIPA plus 0.15 M NaCl, and once with 50 mM Tris (pH 7.4)-0.25 mM EDTA-0.15 M NaCl. Samples were then resuspended in SDS-polyacrylamide gel electrophoresis (PAGE) sample loading buffer (10% SDS, 1 M Tris [pH 6.8]) containing 2.5% dithiothreitol (wt/vol), boiled, and fractionated by SDS-PAGE on 10 or 15% gels (44). Quantification of radiolabeled protein bands was done by using a Fuji BioImager 1000 and ImageQuant v3.3 software (Fuji Medical System, Stamford, Conn.).
Virus growth curves.
MDBK cells in 0.8-cm-diameter wells were infected in duplicate with wt or mutant rSV5 at an MOI of 5.0 TCID50/cell. After incubation for 1.5 h, the inocula were removed and the cells were washed three times with PBS. Cultures were grown in 0.5 ml of DMEM supplemented with 2% fetal bovine serum for the following time points: 0, 6, 12, 18, 24, 36, 48, 72, and 96 h p.i. at which point the medium was collected and the titer was determined based on the TCID50.
Cytoplasmic content mixing fusion assays.
The cytoplasmic content mixing assay was based on the cytoplasmic activation of chloramphenicol acetyltransferase (CAT) as described previously (23) with the following modifications. BSR T7/5 cells (4) in 6-cm dishes were infected with either wt rSV5 or rSV5 harboring F proteins containing cytoplasmic tail deletions at an MOI of 7.0 TCID50/cell in triplicate. Concomitantly, BHK-21F cells in 6-cm dishes were transfected with 2 μg of pBH82 (23), which encodes CAT under the control of the T7 RNA polymerase promoter, by using Lipofectamine-Plus reagents. At 16 h p.i. or p.t., the BHK-21F cells were removed from dishes and resuspended in DMEM supplemented with 2% fetal bovine serum and overlaid onto the BSR T7/5 cells. After coculture for 8 h, cell lysates were prepared by resuspending the cells in 200 μl of 0.25 M Tris (pH 7.9) and disruption by three cycles of freezing and thawing. CAT activity was measured by taking 50 μl of cleared lysate and mixing it with 200 μl of 1.25 mM chloramphenicol and 0.1 M Tris (pH 7.9) plus 0.1 μCi of [14C]butyryl coenzyme A (NEN Life Science Products, Inc., Boston, Mass.)/ml. Detection of butyrylated 14C-labeled chloramphenicol produced by the activity of CAT was performed by liquid scintillation counting for 1 min after 0, 30, 60, 90, 120, and 150 min of incubation. The numerical slope of the rate curve of CAT activity was used for determination of the extent of cytoplasmic content mixing.
Fluorescence microscopy.
CV-1 cells grown on glass coverslips were infected with wt or mutant rSV5 at an MOI of 0.1 TCID50/cell. At 16 h p.i., monolayers were fixed with 1% methanol-free formaldehyde for 15 min and blocked with 1% bovine serum albumin in PBS for 30 min. For M protein staining, solutions contained 0.1% saponin (Sigma-Aldrich Co., St. Louis, Mo.) to permeabilize the cells. For surface staining of HN or F protein, cells were left unpermeabilized. Cells were incubated for 30 min separately with MAb F1a, HN1b, or M-h. Cells were washed five times in PBS and incubated for 30 min with FITC-conjugated goat anti-mouse IgG secondary antibody. Cells were washed five times with PBS and fixed again in 1% methanol-free formaldehyde for 15 min. Samples were analyzed with a LSM 410 confocal microscope (Zeiss, Inc., Thornwood, N.Y.)
Purification of virions and Western blotting.
MDBK cells in 10-cm dishes (∼100% confluent) were infected in duplicate with wt or mutant rSV5 at an MOI of 1.0 TCID50 per cell and incubated in DMEM supplemented with 2% fetal bovine serum. At 24 h p.i., the medium was replaced with 6 ml of DMEM containing one-tenth the normal amount of methionine and cysteine and 25 μCi of [35S]-Promix/ml and then incubated for 6 h at 37°C. Media from duplicate 10 cm dishes were harvested by pelleting the virus through a 35% sucrose cushion (35% sucrose [wt/vol] in NTE [0.1 M NaCl, 0.01 M Tris [pH 7.4], 0.001 M EDTA]) by centrifugation at 165,000 × g for 2 h in a Beckman 70 Ti rotor at 4°C. Virus pellets were resuspended in 100 μl of SDS-PAGE sample loading buffer containing 2.5% dithiothreitol (wt/vol), and polypeptides were analyzed by SDS-PAGE on a 15% gel.
Nonradiolabeled wt and mutant rSV5 were grown and purified by procedures similar to those used for production of 35S-labeled virions. Virion polypeptides were fractionated by SDS-PAGE on a 15% gel and then wet transferred to an Immobilon-P PVDF membrane (Millipore, Bedford, Mass.) by using a Bio-Rad Trans-Blot transfer cell (Bio-Rad Laboratories, Hercules, Calif.) for 1 h at 50 V. The membrane was blocked for 1 h in PBS-T (PBS containing 0.3% polyoxyethylene-sorbitan monolaurate [Tween 20]) and 5% dry milk (wt/vol). The F protein was immunoblotted by using a combination of synthetic peptide rabbit sera, α-F2 and F1-244, and the M protein was immunoblotted with rabbit serum raised against purified M protein (Dudet-αM) (56). Alkaline phosphatase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.) and a chemiluminescent substrate were added, and chemiluminescence was detected and imaged by using a Storm 860 system (Molecular Dynamics, Sunnyvale, Calif.).
Budding assay.
293T cells in 6-cm dishes (∼80% confluent) were infected with wt or mutant rSV5 at an MOI of 1.0 TCID50/cell. At 24 h p.i., the medium was replaced with 1.5 ml of DMEM containing one-tenth the normal amount of methionine and cysteine and 50 μCi of [35S]-Promix/ml. At 48 h p.i., the medium was collected and centrifuged at 5,000 × g for 5 min to remove cell debris and overlaid onto a 35% sucrose cushion in NTE and centrifuged at 186,000 × g for 2 h in a Beckman type 70.1 Ti rotor at 4°C. The pellet was resuspended in 1 ml of RIPA containing 50 mM iodoacetamide, 1 mM PMSF, and protease inhibitors and then immunoprecipitated with rabbit sera α-F2 and F1-244 and with MAbs HN1b, M-h, and NPa. In some cases, the material that pelleted through the 35% sucrose cushion was resuspended in 0.5 ml of NTE and combined with 1.3 ml of 80% sucrose in NTE for flotation. Additional layers containing 50% sucrose (1.8 ml) and 10% sucrose (0.6 ml) were applied to the top of the sample, followed by centrifugation at 186,000 × g overnight in a Beckman SW41 Ti rotor at 4°C. The top 2.1 ml were collected as a single fraction from the gradient, and the floated proteins were immunoprecipitated. To analyze the intracellular proteins, cells were scraped from dishes, pelleted by centrifugation (14,000 rpm for 5 min), resuspended in Dounce buffer (25 mM NaCl, 25 mM HEPES [pH 7.3], 1 mM PMSF), and placed on ice for 10 to 30 min. Cells were then disrupted by Dounce homogenization on ice (30 strokes). Nuclei and cell debris were removed by centrifugation at 8,000 rpm for 2 min. Proteins were immunoprecipitated with rabbit sera α-F2 and F1-244 and with MAbs HN1b, M-h, and NPa. To calculate the budding efficiency, protein bands were quantified by using a Fuji BioImager 1000, and the fraction of M protein in the media as a percentage of the total was determined as follows: counts in medium/(counts in lysate + counts in medium).
Electron microscopy.
Electron microscopy was performed essentially as described previously (29). Diluted virions were allowed to adsorb onto Parlodion-coated copper grids for 30 s. Excess solution was wicked off, and grids were negatively stained with 2% phosphotungstic acid (pH 6.6). A thin layer of carbon was evaporated onto the grids prior to examination in a JEOL JEM-100CX II electron microscope.
RESULTS
Truncation of the F protein cytoplasmic tail does not affect its cell surface transport.
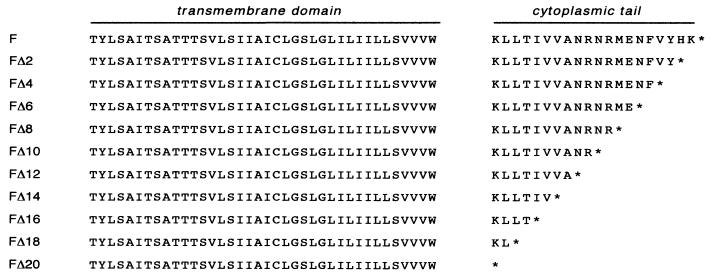
To investigate a possible role for the cytoplasmic tail of the paramyxovirus F protein in virus assembly and budding, a series of sequential two-amino-acid stepwise truncation mutants (Fig. 1) was constructed that encompasses the entire predicted F protein cytoplasmic tail (43). Truncation by 2n residues was considered important, since recombinant viruses harboring these mutations would maintain the genome length as a multiple of six nucleotides. Adherence to this so-called “rule of six” has been shown to be important for the efficient replication of paramyxoviruses, including SV5 (5, 40).
FIG. 1.
Schematic diagram of the amino acid sequences of SV5 F proteins with truncated cytoplasmic tails. The cytoplasmic tail of the F protein is predicted to comprise the C-terminal 20 amino acids of the protein. The indicated nested set of F proteins containing progressive C-terminal deletions FΔ2 to FΔ20 was generated by site-directed mutagenesis of the F cDNA by using a four-primer PCR strategy.
A possible problem with generating F protein cytoplasmic tail truncations is that the engineered changes may cause protein misfolding, which in turn would affect deleteriously cell surface expression levels and subsequent virus assembly steps. Thus, the extent of cell surface expression of each F protein cytoplasmic tail truncation mutant was determined by flow cytometry. Each of the F protein mutants was transiently expressed from cDNA by using the recombinant vaccinia virus T7 expression system (14). For the F cytoplasmic tail-truncated proteins FΔ2-FΔ18, the surface expression of F (percent positive cells, 53.44 to 67.14; relative mean fluorescence intensity, 0.89 to 1.10) was found to be very similar to wt F (percent positive cells, 58.12; relative mean fluorescence intensity, 1.00). However, truncation of the entire cytoplasmic tail of the F protein (FΔ20) led to a defect in surface expression (percent positive cells, 48.28; relative mean fluorescence intensity, 0.60) (Table 1). Thus, for the F protein cytoplasmic tail truncations Δ2 to Δ18, there was little effect of the truncations on surface expression compared to wt F protein.
TABLE 1.
Surface expression of wt F protein and cytoplasmic tail-truncated F proteinsa
| Protein expressed | Surface expression efficiency from:
|
|||
|---|---|---|---|---|
| Transfected DNAb
|
Recombinant virusc
|
|||
| % Positive cells | Relative MFI | % Positive cells | Relative MFI | |
| F | 58.12 | 1.00 | 99.12 | 1.00 |
| FΔ2 | 56.04 | 0.95 | 98.96 | 0.89 |
| FΔ4 | 61.91 | 1.10 | 97.48 | 1.07 |
| FΔ6 | 61.32 | 0.89 | 88.27 | 0.85 |
| FΔ8 | 63.57 | 1.00 | 94.49 | 1.14 |
| FΔ10 | 59.45 | 1.02 | 97.08 | 0.91 |
| FΔ12 | 67.14 | 1.02 | 97.98 | 0.92 |
| FΔ14 | 58.62 | 0.97 | 98.55 | 0.86 |
| FΔ16 | 53.44 | 0.92 | 98.65 | 0.95 |
| FΔ18 | 60.62 | 0.98 | 98.43 | 0.87 |
| FΔ20 | 48.28 | 0.60 | 81.62 | 0.22 |
Surface expression efficiency was determined by flow cytometry. CV-1 cells were bound with MAb F1a and an FITC-conjugated secondary antibody. MFI, mean fluorescence intensity.
Expression of F protein by using the vaccinia virus T7 expression system.
Expression of F protein from rSV5-infected cells.
Generation of recombinant SV5 harboring F protein cytoplasmic tail truncations by reverse genetics.
To study the role of the F protein cytoplasmic tail in paramyxovirus assembly and budding, recombinant viruses harboring F protein cytoplasmic tail truncations were obtained from infectious cDNAs of the SV5 genome by a modification of the rescue scheme previously established for SV5 (25). Rather than infecting cells with vaccinia virus expressing bacteriophage T7 RNA polymerase, the cell line BSR T7/5 (4), which stably expresses T7 RNA polymerase, was used. The wt and mutant infectious cDNA plasmids were cotransfected, along with expression plasmids encoding the viral proteins NP, P, and L, into BSR T7/5 cells such that SV5 plus-strand antigenome RNA and mRNAs for NP, P, and L were transcribed. It was found that each of the mutant infectious cDNA constructs encoding cytoplasmic tail-truncated F proteins (FΔ2 to FΔ20) gave rise to recombinant virus (rSV5 FΔ2 to rSV5 FΔ20) on the first attempt. Rescued viruses were plaque purified on BHK-21F cells, and virus stocks ranging from 2.5 × 106 to 6.3 × 107 TCID50/ml were generated within two passages on MDBK cells (virus titers were determined based on the TCID50 because some of the F cytoplasmic tail truncations caused the viral plaques to be fuzzy and difficult to count [see below]). Confirmation of the engineered mutations in each of the recovered rSV5 stocks was obtained by nucleotide sequencing of RT-PCR products derived from vRNA (data not shown).
Surface expression of the wt F and F cytoplasmic tail-truncated proteins expressed in rSV5-infected cells was examined by flow cytometry. Similar to the data obtained from cells transiently expressing the altered F proteins, each of the cytoplasmic tail-truncated F proteins FΔ2 to FΔ18 expressed from recombinant viruses were found to be present at the cell surface at similar levels to wt F protein (relative mean fluorescence intensity, 0.85 to 1.14). The only exception was protein FΔ20 which, when expressed in rSV5 FΔ20-infected cells, was present at the cell surface at low levels compared to wt F protein (relative mean fluorescence intensity, 0.22) (Table 1).
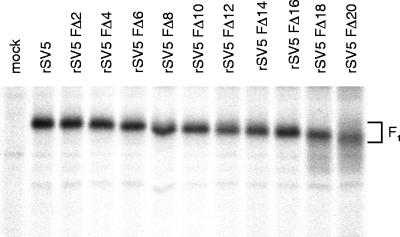
Expression of the SV5 cytoplasmic tail-truncated F proteins from recombinant viruses was also examined biochemically. CV-1 cells were infected with wt and F cytoplasmic tail-truncated rSV5 and were pulse-labeled with [35S]-Promix at 16 h p.i. Cells were lysed, and the F proteins were immunoprecipitated and analyzed by SDS-PAGE. As shown in Fig. 2, successive deletion of the F protein cytoplasmic tail resulted in increasing gel mobility of the F1 subunit. The diffuse migration pattern of the FΔ18 and FΔ20 proteins is presumably due to an altered carbohydrate modification. We observed previously that for a transiently expressed F protein lacking 19 residues of the cytoplasmic tail (FΔ19) that FΔ19 was modified by the addition of polylactosaminoglycan (2).
FIG. 2.
Expression of SV5 F protein from cells infected with rSV5 harboring F protein cytoplasmic tail-truncations. CV-1 cells were infected with wt rSV5 and rSV5 FΔ2 to rSV5 FΔ20 and, at 18 h p.i., cells were radiolabeled with [35S]-Promix for 2 h. Cells were lysed in RIPA buffer, and F proteins were immunoprecipitated and analyzed by SDS-PAGE on a 10% gel as described in Materials and Methods. The F1 polypeptide chain of the F cytoplasmic tail-truncated proteins exhibits a progressively faster electrophoretic mobility consistent with successive removal of residues from the F protein cytoplasmic tail.
Replication of rSV5 harboring F protein cytoplasmic tail truncations in cultured cells.
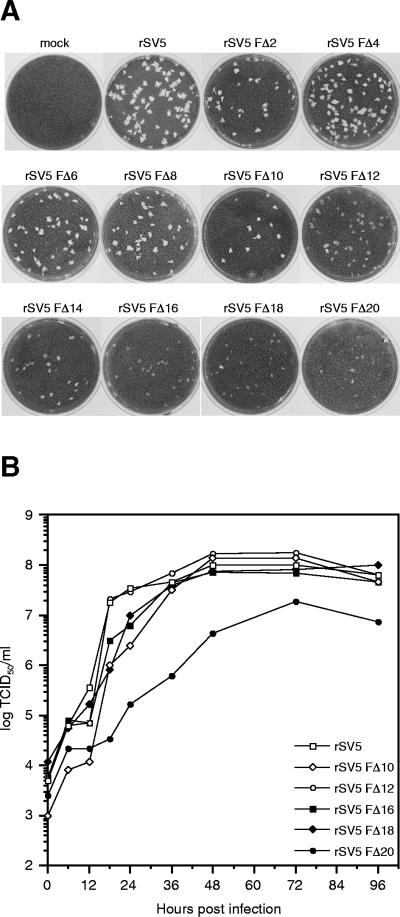
The recovered viruses rSV5 FΔ2 to rSV5 FΔ20 exhibited a plaque morphology on BHK-21F cells that depended on the extent of truncation of the F protein cytoplasmic tail. Truncation of up to 10 residues from the cytoplasmic tail of the F protein (rSV5 FΔ2 to rSV5 FΔ10) did not affect plaque morphology. However, further truncation led to a gradient of plaque morphologies, which ranged from slightly opaque for rSV5 FΔ12 to very tiny opaque (fuzzy) plaques for rSV5 FΔ20 (Fig. 3A).
FIG. 3.
Plaque formation and growth kinetics of rSV5 harboring F protein cytoplasmic tail truncations. (A) Plaques formed by rSV5 harboring F protein cytoplasmic tail truncations after 5 days in BHK-21F cells. (B) Growth curves of rSV5 F cytoplasmic tail-truncated viruses. MDBK cells were infected with the indicated viruses at an MOI of 5.0 TCID50/cell, and the culture media was harvested at the indicted times p.i. Due to the fuzzy morphology of plaques formed by rSV5 FΔ12 to rSV5 FΔ20, all virus titers were determined by endpoint dilution (i.e., the TCID50) and calculated as described in Materials and Methods. Titers at each time point are the average of duplicate experiments.
To study the growth rate of the recovered viruses containing the F protein cytoplasmic tail-truncations, MDBK cells were infected with wt or mutant rSV5 at an MOI of 5.0 TCID50/cell. At various times p.i. up to 96 h, the culture medium was harvested and virus titers were determined based on the TCID50 (eight replicates per dilution; cytopathic effect after 5 days was monitored by light microscopy). As shown in Fig. 3B, all of the recovered viruses grew at very similar rates as wt rSV5 except for rSV5 FΔ20, which grew at a slower rate and reached a lower maximum titer. Thus, the presence of just two amino acids in the cytoplasmic tail of the F protein in mutant recombinant virus (rSV5 FΔ18) permitted replication to occur at a similar rate and similar maximum titer as for wt rSV5, indicating that the vast majority of the F protein cytoplasmic tail is dispensable for normal replication in tissue culture.
Truncation of the F protein cytoplasmic tail does not affect cell-cell fusion in recombinant virus-infected cells.
Previous studies on the fusion characteristics of a cytoplasmic tail-truncated F protein containing only a single charged residue at the transmembrane-cytoplasmic tail domain boundary (FΔ19) showed that the FΔ19 protein exhibited a defect in the expansion of viral fusion pores (12). Thus, one possibility for the altered plaque morphologies for mutant recombinant viruses having severely truncated F protein cytoplasmic tails (Fig. 3A) was that these viruses could be defective in F-mediated fusion in infected cells. To investigate the fusion capacity of the cytoplasmic tail-truncated F proteins in the context of a viral infection, fusion studies were performed with cells infected with either wt or F protein cytoplasmic tail-truncated recombinant viruses. In this assay BSR T7/5 cells were infected with wt or mutant rSV5 at an MOI of 7.0 TCID50/cell. Concomitantly, BHK-21F cells were transfected with plasmid pBH82, which encodes CAT under the control of the T7 RNA polymerase promoter (23). At 14 h p.i. or p.t., the BHK-21F cells were removed from the plates and overlaid onto the BSR T7/5 cells. Mixing of the cell cytoplasms after viral fusion pore formation resulted in transcription of the CAT gene by T7 RNA polymerase. After 8 h of cocultivation, CAT activity in cell lysates was assayed by measuring butyrylated 14C-labeled chloramphenicol production by liquid scintillation counting. It was found that truncation of the cytoplasmic tail of the F protein did not significantly affect the ability of the FΔ2 to FΔ18 proteins to mediate cell-cell fusion, as measured by content mixing, in virus-infected cells (Table 2). For the most severe cytoplasmic tail-truncated mutant protein tested (FΔ18), there was less than a 1.3-fold drop in the relative CAT activity in rSV5 FΔ18-infected cells compared to wt rSV5-infected cells (Table 2). Similar results were obtained when CAT activity was measured after 4 h of cocultivation (data not shown). It was not possible to test accurately the fusion capacity of the FΔ20 protein in rSV5 FΔ20-infected cells as the level of surface expression of FΔ20 protein was always less than expression of wt F protein (data not shown), which would lead to an under-representation of the true amount of virus-mediated fusion. These data extend our earlier experiments using transient expression of F proteins with altered cytoplasmic tails (2) by showing that the extent of cell-cell fusion in virus-infected cells is not affected by truncations to the cytoplasmic tail of the F protein.
TABLE 2.
Fusion activity of rSV5 harboring F protein cytoplasmic tail truncationsa
| Virus | Avg relative CAT activity ± SD |
|---|---|
| Mock | 10 ± 9 |
| rSV5 | 4,775 ± 83 |
| rSV5 FΔ2 | 4,651 ± 69 |
| rSV5 FΔ8 | 4,459 ± 220 |
| rSV5 Fd10 | 4,388 ± 108 |
| rSV5 FΔ12 | 3,841 ± 191 |
| rSV5 FΔ14 | 4,116 ± 66 |
| rSV5 FΔ16 | 3,591 ± 171 |
| rSV5 FΔ18 | 3,660 ± 102 |
BSR T7/5 cells were infected with wt rSV5 or the indicated rSV5 F cytoplasmic tail-truncated viruses. In parallel, BHK-21F cells were transfected with the plasmid pBH82 (23), which encodes CAT under the control of the T7 RNA polymerase promoter. At 16 h p.i. or p.t., the BHK-21F cells were trypsinized and overlaid onto the BSR T7/5 cells. At 24 h p.i. or p.t., CAT activity was measured as described in Materials and Methods. Each CAT assay was performed in triplicate.
Truncation of the F protein cytoplasmic tail does not disrupt subcellular localization of F or M in virus-infected cells.
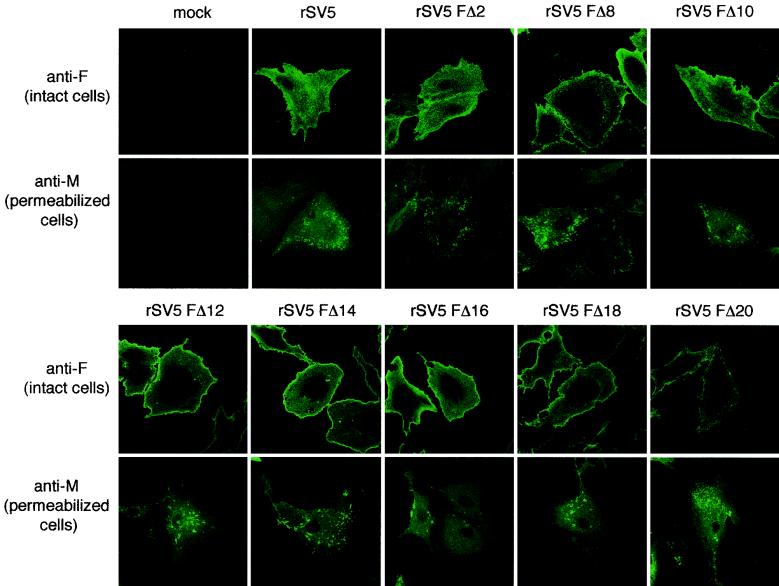
A critical step in virus assembly is thought to be the coalescence of viral components at sites on the plasma membrane that are enriched in viral proteins and from where virus buds. It has been shown previously that HN and M proteins colocalize in brightly staining punctate bodies that are thought to be budding virions in wt rSV5-infected cells (52). Furthermore, when cells were infected with a mutant virus that harbors an HN protein lacking most of its cytoplasmic tail (rSV5 HNΔ2-9), the cells showed a visible redistribution of the HN and M proteins away from this punctate staining pattern (52) which correlates with the finding that rSV5 HNΔ2-9 buds poorly. To investigate the surface distribution of the F cytoplasmic tail-truncated proteins and the subcellular distribution of the M protein in mutant rSV5-infected cells, cells were stained with an F-specific MAb (Fla) at 18 h p.i. In parallel, mutant rSV5-infected cells, which were saponin permeabilized, were stained with an M-specific MAb. It was observed (Fig. 4) that wt F protein and the F cytoplasmic tail-truncated proteins all had a similar staining pattern, and were distributed evenly over the surface of virus-infected cells. In contrast, distribution of the M protein showed the characteristic punctate staining pattern in the virus-infected cell (Fig. 4). Thus, unlike deletion of the HN protein cytoplasmic tail, which caused a redistribution of the HN staining pattern, deletion of the F protein cytoplasmic tail did not alter the localization of the F proteins in virus-infected cells.
FIG. 4.
Subcellular localization of F and M proteins in cells infected with rSV5 harboring F protein cytoplasmic tail truncations. CV-1 cells on glass coverslips were infected with the indicated viruses. At 16 h p.i., cells were fixed with formaldehyde (and for M protein staining, the cells were permeabilized with 0.1% saponin) and bound with MAbs specific for SV5 F (Fla) or M (M-h) and then with FITC-conjugated goat anti-mouse secondary antibody. Fluorescence was examined with a Zeiss LSM 410 confocal microscope.
Protein composition of released virions is unaffected by truncation of the F protein cytoplasmic tail.
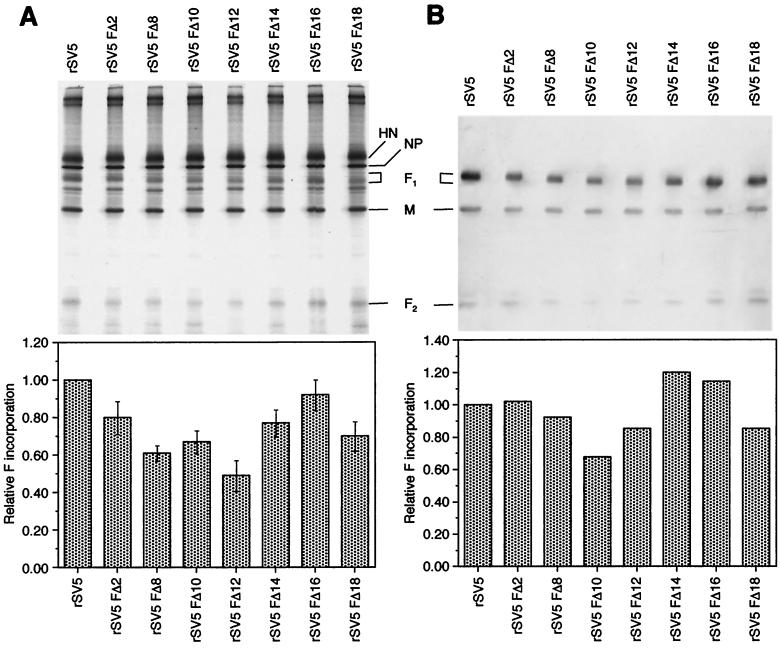
To investigate whether the F protein cytoplasmic tail is required for incorporation of the F protein into virions or for the segregation of viral proteins away from cellular proteins during assembly, the polypeptide composition of purified wt and F protein cytoplasmic tail-truncated recombinant virions grown in MDBK cells was examined. Both 35S-metabolically labeled virions and nonlabeled virions were purified by sedimentation through a 35% sucrose cushion, and the polypeptides were analyzed by SDS-PAGE. For nonradiolabeled virions, the polypeptides F1, F2, and M were detected by immunoblot analysis. As shown in Fig. 5, the F protein cytoplasmic tail-truncated recombinant virions had a very similar polypeptide profile as wt rSV5 virions. The ratio of the viral proteins M, NP, and HN incorporated in purified F protein cytoplasmic tail-truncated virions was similar to that of wt rSV5. In addition, none of the purified mutant virion preparations showed a particular increase in nonspecific incorporation of cellular proteins compared to wt rSV5 virions. Quantitation of F1 from radiolabeled virions indicated up to a twofold reduction (maximum for rSV5 FΔ12) in incorporation of cytoplasmic tail-truncated F protein into recombinant virions (Fig. 5A). Quantitation of the F1 band as detected by immunoblotting indicated up to a 1.3-fold reduction (maximum for rSV5 FΔ12) in incorporation of cytoplasmic tail-truncated F protein compared to wt rSV5 (Fig. 5B). The numerical values for incorporation of F protein into virions between the two methods of detection of polypeptides was quite similar and, furthermore, the overall trends observed by both methods, were very similar. Quantitation of the F protein was done by normalizing the amount of M protein present in each sample.
FIG. 5.
Polypeptide composition of rSV5 harboring F protein cytoplasmic tail truncations. rSV5 and representative rSV5 F cytoplasmic tail-truncated viruses were grown in MDBK cells. For total viral polypeptide composition, cells were metabolically labeled with [35S]-Promix. Virions were purified through a 35% sucrose cushion. (A) Radiolabeled virions were fractionated by SDS-PAGE, and polypeptide radioactivity was quantified on a Fuji BioImager 1000. Incorporation of F protein into virions was calculated by quantification of the F1 band. The histogram indicates the average data from three independent experiments. (B) Nonradiolabeled virions were fractionated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and probed with antibodies specific for F1, F2, and M, followed by the addition of alkaline phosphatase-conjugated secondary antibodies. Chemiluminescence was imaged on a STORM 860 system.
Truncation of the F protein cytoplasmic tail does not affect budding of recombinant virus.
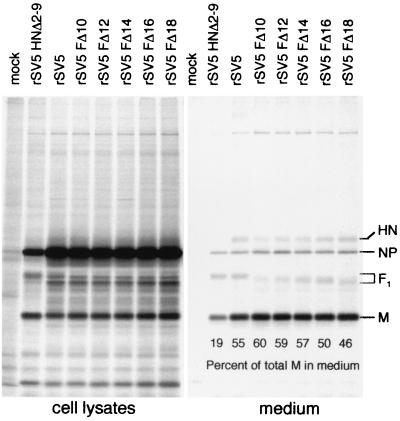
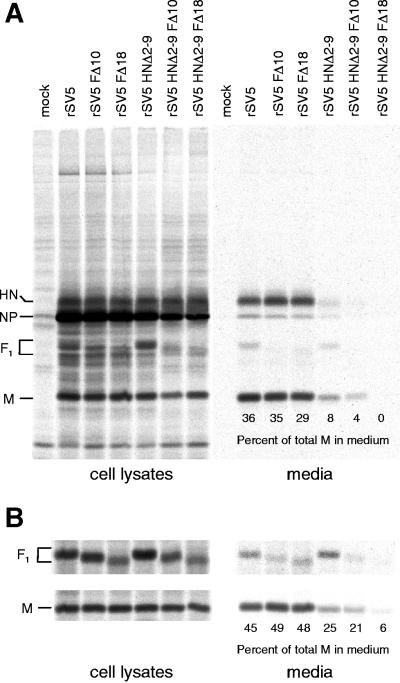
rSV5 which lacks most of the cytoplasmic tail of the HN protein (rSV5 HNΔ2-9) is defective for budding (52). To investigate a role of the F protein cytoplasmic tail in directing virus budding, virion release from infected cells was assessed by performing a budding assay. 293T cells were infected with either wt or mutant rSV5 and, at 24 h p.i., cells were metabolically labeled with [35S]-Promix for 24 h. Both the cells and the media were harvested, and virions in the media were pelleted through a 35% sucrose cushion. Viral proteins from both the cell lysates and medium fractions were immunoprecipitated with specific antisera, and polypeptides were then analyzed by SDS-PAGE. Budding efficiency was assessed by measuring the amount of M protein released into the medium as a percentage of the total M protein in the cell lysate plus the medium (53). As shown in Fig. 6, 55% of the total amount of M protein expressed in wt rSV5-infected cells was released into the medium. In contrast, for rSV5 HNΔ2-9 only 19% of the total expressed M protein was released into the medium. For cells infected with the F protein cytoplasmic tail-truncated recombinant viruses (rSV5 FΔ10 to rSV5 FΔ18), the release of M protein (46 to 60%) into the medium was similar to release of M protein (55%) from wt rSV5-infected cells (Fig. 6). Thus, in the context of a recombinant virus the cytoplasmic tail of the F protein is dispensable for normal virus budding. The ratio of M to NP protein observed in the cell lysate of rSV5 HNΔ2-9-infected cells in this experiment is not typical compared to other similar experiments that also showed a decrease in viral protein release. The example of the data shown was chosen because in one gel it shows clearly the F protein incorporation into all of the released virions.
FIG. 6.
Analysis of budding of rSV5 harboring F cytoplasmic tail truncations. 293T cells were infected with wt rSV5, rSV5 HNΔ2-9 (which contains an HN cytoplasmic tail truncation), and selected examples (rSV5 FΔ10 to rSV5 FΔ20) of the F protein cytoplasmic tail-truncated viruses. At 24 h p.i., cells were radiolabeled with [35S]-Promix as described in Materials and Methods. Culture media and the cell lysates were harvested separately, and virions in the media were pelleted through a 35% sucrose cushion. Viral polypeptides (HN, F, NP, and M) were immunoprecipitated from both cell lysates and media as described in Materials and Methods, and polypeptides were analyzed by SDS-PAGE. The amount of M protein released into the medium was quantified and was used as a measure of viral budding competence. The percentage of total M protein in the medium was calculated from the total amount of M protein present in the cell lysate plus the medium.
Generation of recombinant viruses with cytoplasmic tail-truncated HN and F proteins.
Studies on the requirements for the formation of SV5 VLPs, suggests a redundant role for the cytoplasmic tails of the HN and F proteins (53). Since the recombinant viruses harboring the F protein cytoplasmic tail truncations contained a wt HN protein, we constructed infectious cDNA clones of SV5 that encoded cytoplasmic tail truncations in both the HN and the F proteins, and we recovered both viruses on the first attempt (rSV5 HNΔ2-9 FΔ10 and rSV5 HNΔ2-9 FΔ18). Confirmation of the engineered mutations in both of the recovered rSV5 stocks was obtained by nucleotide sequencing of RT-PCR products derived from vRNA (data not shown).
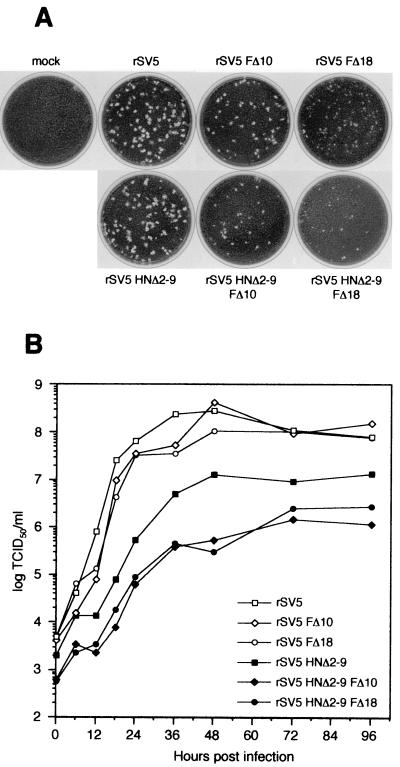
The plaque morphology of each double mutant rSV5 in BHK-21F cells was examined (Fig. 7A). rSV5 HNΔ2-9 plaques were slightly smaller than wt rSV5 plaques (52); rSV5 HNΔ2-9 FΔ10 plaques were smaller still and resembled the size of rSV5 FΔ10 plaques, and rSV5 HNΔ2-9 FΔ18 plaques were small and cloudy and resembled those formed by SV5 FΔ18 (cf. Fig. 3A and 7A).
FIG. 7.
Plaque formation and growth kinetics of rSV5 harboring HN and F protein double cytoplasmic tail truncations. (A) Plaques formed by the double mutants rSV5 HNΔ2-9 FΔ10 and rSV5 HNΔ2-9 FΔ18 and related single-mutant recombinant viruses after 5 days in BHK-21F cells. (B) Growth curves of rSV5 harboring HN and F double cytoplasmic tail truncations. MDBK cells were infected with the indicated viruses at an MOI of 5.0 TCID50/cell, and the culture media was harvested at the indicated times p.i. Virus titers were determined by endpoint dilution (i.e., the TCID50) and calculated as described in Materials and Methods. The titers at each time point are the average of duplicate experiments.
To analyze further the growth properties of the double mutant rSV5, the growth of these viruses in MDBK cells between 0 to 96 h p.i. was determined by TCID50 analysis in BHK-21F cells. Whereas truncations in the cytoplasmic tail of the F protein alone had no effect on virus replication (cf. Fig. 3B and 7B) for rSV5 HNΔ2-9, the growth rate was slower and the maximum titer reached was lower, as found previously (52). The rSV5 HNΔ2-9 FΔ10 and rSV5 HNΔ2-9 FΔ18 double-mutant viruses exhibited a defect in virus replication that was greater than that seen for rSV5 HNΔ2-9 (Fig. 7B). These data are consistent with the notion of a redundant role for the cytoplasmic tails of the HN and F proteins in the virus life cycle since truncation of both HN and F protein cytoplasmic tails led to a replication defect that was more severe than deletion of either the HN or F protein cytoplasmic tail alone.
Recombinant viruses with cytoplasmic tail-truncated HN and F proteins exhibit an altered subcellular localization of HN and M proteins in virus-infected cells.
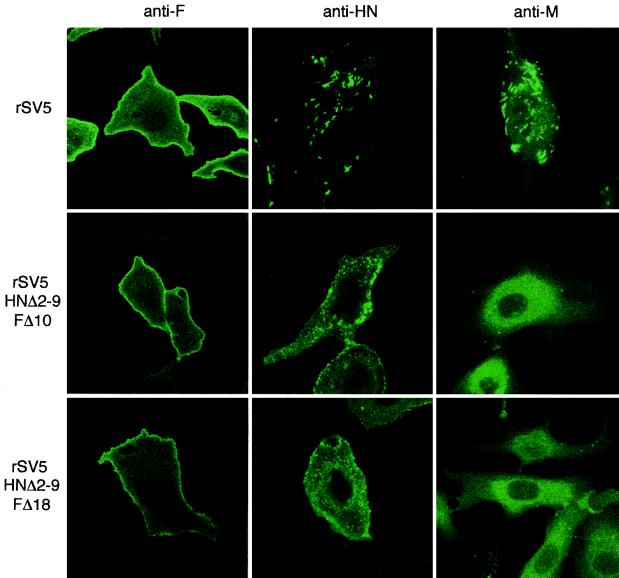
To examine the subcellular localization of the HN, F and M proteins in the double mutant rSV5-infected cells, CV-1 cells on glass coverslips at 18 h p.i. were stained with either F-specific or HN-specific MAbs or were saponin-permeabilized and stained with M-specific MAb. As shown in Fig. 8, cells infected with the double-mutant rSV5 harboring the HN and F protein cytoplasmic tail truncations displayed a similar redistribution of the HN and M proteins that was seen for rSV5 HNΔ2-9 (52). In wt rSV5-infected cells, the HN and M proteins localize into punctate bodies that exhibit bright fluorescence compared to a more evenly distributed localization of F protein across the cell surface. Infection of cells with the double mutant rSV5 led to a redistribution of both HN and M proteins away from these punctate bodies (Fig. 8).
FIG. 8.
Localization of HN, F, and M in cells infected with rSV5 harboring HN and F double cytoplasmic tail truncations. CV-1 cells on glass coverslips were infected with rSV5, rSV5 HNΔ2-9 FΔ10, and rSV5 HNΔ2-9 FΔ18. At 16 h p.i., the surface localization of HN and F proteins was detected by binding HN and F specific MAbs (HN1b and F1a) to intact cells. Intracellular localization of M protein was detected by binding of M-h MAb to saponin-permeabilized cells. The cells were then stained with a FITC-conjugated goat anti-mouse secondary antibody. Fluorescence was detected by using a Zeiss LSM 410 confocal microscope.
Recombinant viruses with cytoplasmic tail-truncated HN and F proteins exhibit a budding defect.
To analyze the efficiency of budding of the double mutant rSV5, a budding assay was performed. The efficiency of budding of rSV5 HNΔ2-9 FΔ10 and rSV5 HNΔ2-9 FΔ18 was compared to that of rSV5, rSV5 HNΔ2-9, rSV5 FΔ10, and rSV5 FΔ18. 293T cells were infected with either wt or single- or double-mutant rSV5. The polypeptide composition of viruses released into the media was analyzed after pelleting of the virions through a 35% sucrose cushion or, by using a more rigorous method, the virions were further purified by flotation on a discontinuous sucrose gradient. For rSV5 HNΔ2-9, which was known to be budding defective, the more rigorous purification scheme showed that only 8% of total M protein was released into the medium (Fig. 9A). However, by using the less-rigorous purification scheme of virions released into the medium, 25% of the total M protein was released from rSV5 HNΔ2-9-infected cells (Fig. 9B). Given that for wt rSV5 the more rigorous method indicated 36% release of M protein compared to a 45% release of M protein for the less-rigorous method, it seems likely that in measuring M protein release, some M protein is released from virus-infected cells that is not associated with virions. Hence, inclusion of a flotation gradient to look at membrane-associated proteins is important for examining viruses or VLPs that assemble inefficiently. The rSV5 FΔ10 and rSV5 FΔ18 viruses showed a budding efficiency that was very similar to wt rSV5 (Fig. 9; see also Fig. 6). In contrast, the budding efficiency of the double-mutant viruses rSV5 HNΔ2-9 FΔ10 and rSV5 HNΔ2-9 FΔ18 was more severely defective than rSV5 HNΔ2-9 (Fig. 9).
FIG. 9.
Budding efficiency of rSV5 harboring cytoplasmic tail truncations in both the HN and F proteins. (A) 293T cells were infected with wt rSV5 or rSV5 harboring single or double cytoplasmic tail truncations to the HN and F proteins as indicated. At 24 h p.i., cells were radiolabeled for 16 h with [35S]-Promix, followed by collection of both cell lysates and culture media. Culture media was clarified by low-speed centrifugation, centrifuged through 35% sucrose cushions, and separated on sucrose flotation gradients. Proteins in the top 2.1 ml of the flotation gradient were analyzed by immunoprecipitation. Cells were disrupted by Dounce homogenization and centrifuged at a low speed to remove nuclei and debris. SV5 proteins were immunoprecipitated from the samples, and polypeptides were analyzed by SDS-PAGE on 10% gels as described in Materials and Methods. (B) Analysis of polypeptides in cell lysates and media in an experiment similar to that described in panel A except that the flotation gradient was not performed. Proteins in the media were analyzed directly after pelleting through a 35% sucrose cushion. In panels A and B, the budding efficiency was quantified as the percentage of M protein detected in the medium compared to the total M protein in the cell lysate plus the medium. In panel A, the time of phosphorimaging was longer for the medium samples than for the cell lysates, but quantitation of M protein was done on similarly exposed gels.
Athough HNΔ2-9 FΔ18 does produce some viable virus, it has a titer 2 logs lower than that of the wt virus. Thus, if budding is the sole defect for the reduction in infectivity, only 1% of the wt rSV5 amount of viral protein would be released from cells. Given the limits of detection, it would be difficult to detect this small amount.
Morphology of recombinant viruses with cytoplasmic tail-truncated HN and F proteins.
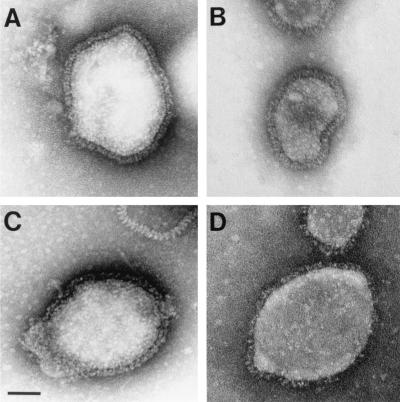
To analyze the morphology of released double-mutant rSV5 virions, we performed electron microscopy of negatively stained virions. We compared preparations of wt rSV5 to each of the single mutant viruses (rSV5 HNΔ2-9 and rSV5 FΔ18) and to the more severe double-mutant virus (rSV5 HNΔ2-9 FΔ18). Our results show that the single mutant rSV5 and wt rSV5 had very similar virion morphologies (Fig. 10A to C). Analysis of the double-mutant rSV5 shows virions of approximately the same size as wt rSV5. However, we observed a decrease in the number of visible spikes for many of the double-mutant virions analyzed (Fig. 10D). This finding is consistent with the observation that purified double-mutant rSV5 showed a decrease in glycoprotein incorporation as demonstrated by SDS-PAGE (data not shown).
FIG. 10.
Morphology of rSV5 harboring cytoplasmic tail truncations in both the HN and F proteins. The virions wt rSV5 (A), rSV5 HNΔ2-9 (B), rSV5 FΔ18 (C), and rSV5 HNΔ2-9 FΔ18 (D) were grown in MDBK cells and purified on sucrose density gradients. Virions were adsorbed onto copper grids and negatively stained with phosphotungstic acid and examined by electron microscopy as described in Materials and Methods by using a JEOL JEM-100CX II electron microscope. Bar, 50 nm.
DISCUSSION
The budding of enveloped viruses at the plasma membrane of virus-infected cells involves specific interactions of viral proteins, and most likely cellular proteins, to form the nascent enveloped viral particle and to cause fission of the plasma membrane to release the budded particle. For human immunodeficiency virus type 1 (HIV-1), protein interactions have been shown, in a functional assay, between the virally encoded Gag polyprotein and the cellular protein Tsg101. Furthermore, this protein, along with Vps4, has been found to be essential for normal budding of HIV-1 virions (10, 16, 35, 62). These proteins in yeast are involved in vacuolar protein sorting, and in mammalian cells they are thought to be involved in multivesicular body formation.
For retroviruses, the interactions that lead to virus budding do not require participation of envelope components, since the expression of soluble Gag polyprotein alone in cells results in efficient budding of VLPs (9, 17). Similarly, for many of the negative-strand RNA viruses envelope components do not appear to be strictly required for budding to occur. Recombinant rabies virus (36) and VSV (55) that completely lack viral glycoprotein have been obtained and can bud from cells. Consistent with this, VLP budding has been detected upon expression of matrix proteins in the absence of any other viral proteins for several negative-strand RNA viruses, including VSV (22, 30, 33), Ebola virus (21, 27, 61), influenza virus (19, 32, 41), Sendai virus (60), and human parainfluenza virus type 1 (8). However, some caution is required in the interpretation of these data because, as shown here for SV5, the methods used to purify released virions (Fig. 9) and VLPs (53) away from other viral proteins released into the medium by nonbudding processes influences the data and its interpretation. Mechanistically, the VSV and Ebola virus matrix proteins may mediate budding in a manner similar to the HIV-1 Gag polyprotein as the VSV and Ebola matrix proteins both contain protein motifs known to interact with Tsg101 (20, 21, 35).
Although envelope components are not strictly required for the budding of negative-strand RNA viruses, it is clear that glycoproteins are required in many cases to make budding efficient. For example, although budding of recombinant rabies virus and VSV occurs in the absence of glycoprotein, it is ca. 20- to 30-fold less efficient than the budding of wt virus (36, 55). Similarly, deletion of the cytoplasmic tails of influenza virus or Sendai virus glycoproteins results in inefficient budding of recombinant viruses (13, 29). For VSV, efficient budding has been found to depend on a 12- to 16-amino-acid budding domain in the membrane-proximal stem of the G protein (48), as well as on the presence of a nonspecific cytoplasmic tail sequence (54). Thus, although the ca. 10 to 20 molecules of Env (18) that are incorporated into each HIV-1 virion appear to be dispensable for efficient budding, the ca. 400 trimers of G (50) that are incorporated into each VSV virion appear to be quite important for efficient budding to occur.
It was shown recently for SV5 that efficient VLP budding occurs upon coexpression of the M protein with NP and either viral glycoprotein HN or F (53). Budding decreased more than 25-fold when neither of the SV5 glycoproteins were expressed. Similarly, efficient budding of Ebola VLPs was recently shown to require coexpression of the matrix protein with the glycoprotein, GP (3). For SV5, the HN and F glycoproteins were found to be completely interchangeable for VLP budding, with similar and highly efficient budding occurring regardless of whether the glycoprotein being expressed was HN, F, or both HN and F together. A role for the HN and F protein cytoplasmic tails in VLP formation was also investigated and deletion analysis indicated that neither of these proteins could function normally for budding in the absence of its cytoplasmic tail (53). These results suggest that the HN and F proteins have redundant functions in the budding of SV5.
To study the role of the SV5 HN protein cytoplasmic tail in a virus infection, reverse genetic procedures were used to generate recombinant SV5 harboring HN protein cytoplasmic tail truncation (rSV5 HNΔ2-9). It was found that deletion of the cytoplasmic tail of the HN protein led to poor virus budding. Furthermore, deletion of the HN protein cytoplasmic tail caused a disruption in bud site formation (52). These results are in seeming contrast with the SV5 VLP assays that showed that the HN protein is not required for VLP budding provided that the F protein is present. An investigation of this conundrum showed that, whereas VLP budding occurred efficiently in the presence of the F protein without the HN protein, budding was poor in the presence of the F protein, together with the mutant HNΔ2-9 protein, indicating that deletion of the HN protein cytoplasmic tail had a negative effect on budding in the VLP assay and thus explained the poor budding of rSV5 HNΔ2-9 (53). A very similar set of observations has been reported previously for Sendai virus. A temperature-sensitive mutant was characterized that efficiently buds particles lacking HN protein at the restrictive temperature (57), and a recombinant Sendai virus harboring HN protein lacking a specific incorporation signal in its cytoplasmic tail was able to efficiently bud particles with greatly reduced amount of HN protein. However, a more severe truncation to the HN protein cytoplasmic tail inhibited virus budding (13). Based on these findings, it was proposed that the absence of the HN protein is not necessarily detrimental to virus budding but that severe truncation to the HN protein cytoplasmic tail leads to severely defective virus budding.
To study the role of the SV5 F protein cytoplasmic tail in virus-infected cells, we generated truncation mutants in the F protein cytoplasmic tail in the context of a wt HN protein. Recovery of rSV5 harboring each of the cytoplasmic tail truncations FΔ2 to FΔ20 was successful, unlike the rescue of recombinant viruses containing the complete nested set of HN protein cytoplasmic tail truncations (52). This difference is probably due to the modifications made to the SV5 reverse genetics rescue scheme used in this study, which allows for more efficient recovery of recombinant SV5.
Each of the recombinant viruses rSV5 FΔ2 to rSV5 FΔ18 displayed replication kinetics in cell culture similar to wt rSV5. Only rSV5 FΔ20 displayed a slower initial rate of replication and a lower maximum titer. This observation correlates with the observations that the FΔ20 protein exhibited reduced levels of surface expression compared to wt F protein when transiently expressed from cDNA and when expressed in virus-infected cells (Table 1). Each of the recombinant viruses rSV5 FΔ16 to rSV5 FΔ20 showed a reduced plaque size, but only for rSV5 FΔ20 did the reduced plaque size correlate with altered replication kinetics (Fig. 3A and B). However, the reduced plaque size did correlate with slightly lower levels of cytoplasmic content mixing in fusion assays of rSV5 FΔ16 to rSV5 FΔ18 (Table 2), although caution has to be exerted in correlating plaque morphology with any growth characteristic of a virus or with any cell-cell fusion assay. We did not feel it was meaningful to measure the fusion activity of rSV5 FΔ20 because this mutant exhibited reduced cell surface expression levels.
The lack of a major phenotype with the F protein cytoplasmic tail-truncated viruses was consistent with the observation that the subcellular distribution of the F and M proteins was not altered in any of the mutant rSV5-infected cells and suggest that virus bud sites are not disrupted (Fig. 4). Consistent with the notion that the F protein cytoplasmic tail-truncated viruses do not affect the normal assembly of viral components at bud sites, it was found that that these viruses were not impaired in budding. These data are in contrast with the observations made for the subcellular distribution of the HN and M proteins in cells infected with rSV5 HNΔ2-9, which showed that the HN and M proteins had moved away from a punctate staining pattern (indicative of bud sites and budding virions). This altered staining pattern of the HN and M proteins correlated with a budding defect in virus-infected cells (52). However, the lack of a phenotype for viruses harboring the F protein cytoplasmic tail truncations correlates with the data from VLP experiments that indicate that the presence of a wt HN protein cytoplasmic tail is sufficient to drive efficient budding (53).
To investigate further the contribution of the F protein cytoplasmic tail in virus assembly and budding, double cytoplasmic tail-truncated recombinant viruses were generated that lacked the cytoplasmic tails of both the HN and F proteins. These double-mutant rSV5 viruses exhibited a severe replication defect in cell culture (Fig. 7B). In addition, the double mutant rSV5 displayed a redistribution of both the HN and M proteins in virus-infected cells (Fig. 8) analogous to what was observed in rSV5 HNΔ2-9-infected cells (52). Furthermore, the double mutant rSV5 were more severely defective in virus budding than rSV5 HNΔ2-9 (Fig. 9). The data obtained also confirm the notion that the cytoplasmic tail-truncated HN protein is inhibitory toward budding, perhaps due to forming an inappropriate conformation that blocks the normal F protein cytoplasmic tail from functioning in virus assembly or by preventing the M protein from accumulating at bud sites (53).
The data described here are also consistent with those obtained in SV5 VLP experiments and support the notion of a redundant role for the cytoplasmic tails of the HN and F proteins in the assembly and budding of SV5. A redundant role for the cytoplasmic tails of the HN and F proteins of paramyxoviruses is analogous to the situation found for influenza A virus in which truncation of the cytoplasmic tails of both the neuraminidase (NA) and hemagglutinin (HA) proteins led to severe budding defects whereas truncation of either the NA or HA proteins alone led to much less severe assembly defects in recombinant virus-infected cells (15, 28, 29, 38). However, unlike the recombinant influenza viruses harboring double cytoplasmic tail truncations in the HA and NA proteins, which showed greatly extended virion lengths and diameters (29), the rSV5 harboring double cytoplasmic tail truncations in the HN and F proteins showed a morphology similar to that of wt rSV5.
Acknowledgments
We thank Klaus Conzelmann for making available the BSR T7/5 cells.
This work was supported in part by research grant AI-23173 from the National Institute of Allergy and Infectious Disease. D.L.W. was supported in part by a Gramm Travel Fellowship Award from the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. A.P.S. is an Associate and R.A.L. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Ali, A., and D. P. Nayak. 2000. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276:289-303. [DOI] [PubMed] [Google Scholar]
- 2.Bagai, S., and R. A. Lamb. 1996. Truncation of the COOH-terminal region of the paramyxovirus SV5 fusion protein leads to hemifusion but not complete fusion. J. Cell Biol. 135:73-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calain, P., and L. Roux. 1993. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 67:4822-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cathomen, T., B. Mrkic, D. Spehner, R. Drillien, R. Naef, J. Pavlovic, A. Aguzzi, M. A. Billeter, and R. Cattaneo. 1998. A matrix-less measles virus is infectious and elicits extensive cell fusion: consequences for propagation in the brain. EMBO J. 17:3899-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cathomen, T., H. Y. Naim, and R. Cattaneo. 1998. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 72:1224-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coronel, E. C., K. G. Murti, T. Takimoto, and A. Portner. 1999. Human parainfluenza virus type 1 matrix and nucleoprotein genes transiently expressed in mammalian cells induce the release of virus-like particles containing nucleocapsid-like structures. J. Virol. 73:7035-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delchambre, M., D. Gheysen, D. Thines, E. Thiriart, E. Jacobs, E. Verdin, M. Horth, A. Burny, and F. Bex. 1989. The GAG precursor of simian immunodeficiency virus assembles into virus-like particles. EMBO J. 8:2653-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirov, D. G., A. Ono, J. M. Orenstein, and E. O. Freed. 2002. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc. Natl. Acad. Sci. USA 99:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutch, R. E., R. N. Hagglund, M. A. Nagel, R. G. Paterson, and R. A. Lamb. 2001. Paramyxovirus fusion (F) protein: a conformational change on cleavage activation. Virology 281:138-150. [DOI] [PubMed] [Google Scholar]
- 12.Dutch, R. E., and R. A. Lamb. 2001. Deletion of the cytoplasmic tail of the fusion protein of the paramyxovirus simian virus 5 affects fusion pore enlargement. J. Virol. 75:5363-5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouillot-Coriou, N., and L. Roux. 2000. Structure-function analysis of the Sendai virus F and HN cytoplasmic domain: different role for the two proteins in the production of virus particle. Virology 270:464-475. [DOI] [PubMed] [Google Scholar]
- 14.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia-Sastre, A., and P. Palese. 1995. The cytoplasmic tail of the neuraminidase protein of influenza A virus does not play an important role in the packaging of this protein into viral envelopes. Virus Res. 37:37-47. [DOI] [PubMed] [Google Scholar]
- 16.Garrus, J. E., U. K. von Schwedler, O. W. Pornillos, S. G. Morham, K. H. Zavitz, H. E. Wang, D. A. Wettstein, K. M. Stray, M. Cote, R. L. Rich, D. G. Myszka, and W. I. Sundquist. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55-65. [DOI] [PubMed] [Google Scholar]
- 17.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 18.Goff, S. P. 2001. Retroviridae: the retroviruses and their replication, p. 1871-1939. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 19.Gomez-Puertas, P., C. Albo, E. Perez-Pastrana, A. Vivo, and A. Portela. 2000. Influenza virus matrix protein is the major driving force in virus budding. J. Virol. 74:11538-11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harty, R. N., M. E. Brown, J. P. McGettigan, G. Wang, H. R. Jayakar, J. M. Huibregtse, M. A. Whitt, and M. J. Schnell. 2001. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J. Virol. 75:10623-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harty, R. N., M. E. Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He, B., G. P. Leser, R. G. Paterson, and R. A. Lamb. 1998. The paramyxovirus SV5 small hydrophobic (SH) protein is not essential for virus growth in tissue culture cells. Virology 250:30-40. [DOI] [PubMed] [Google Scholar]
- 24.He, B., G. Y. Lin, J. E. Durbin, R. K. Durbin, and R. A. Lamb. 2001. The SH integral membrane protein of the paramyxovirus simian virus 5 is required to block apoptosis in MDBK cells. J. Virol. 75:4068-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He, B., R. G. Paterson, C. D. Ward, and R. A. Lamb. 1997. Recovery of infectious SV5 from cloned DNA and expression of a foreign gene. Virology 237:249-260. [DOI] [PubMed] [Google Scholar]
- 26.Horvath, C. M., and R. A. Lamb. 1992. Studies on the fusion peptide of a paramyxovirus fusion glycoprotein: roles of conserved residues in cell fusion. J. Virol. 66:2443-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasenosky, L. D., G. Neumann, I. Lukashevich, and Y. Kawaoka. 2001. Ebola virus VP40-induced particle formation and association with the lipid bilayer. J. Virol. 75:5205-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin, H., G. Leser, and R. A. Lamb. 1994. The influenza virus hemagglutinin cytoplasmic tail is not essential for virus assembly or infectivity. EMBO J. 13:5504-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin, H., G. P. Leser, J. Zhang, and R. A. Lamb. 1997. Influenza virus hemagglutinin and neuraminidase cytoplasmic tails control particle shape. EMBO J. 16:1236-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Justice, P., W. Sun, Y. Li, Z. Ye, P. R. Grigera, and R. R. Wagner. 1995. Membrane vesiculation function and exocytosis of wild-type and mutant matrix proteins of vesicular stomatitis virus. J. Virol. 69:3156-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamb, R. A., and D. Kolakofsky. 2001. Paramyxoviridae: the viruses and their replication, p. 1305-1340. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 32.Latham, T., and J. M. Galarza. 2001. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J. Virol. 75:6154-6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, Y., L. Luo, M. Schubert, R. R. Wagner, and C. Y. Kang. 1993. Viral liposomes released from insect cells infected with recombinant baculovirus expressing the matrix protein of vesicular stomatitis virus. J. Virol. 67:4415-4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin, G. Y., and R. A. Lamb. 2000. The paramyxovirus simian virus 5 V protein slows progression of the cell cycle. J. Virol. 74:9152-9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Sarrano, J., T. Zang, and P. D. Bieniasz. 2001. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat. Med. 7:1313-1319. [DOI] [PubMed] [Google Scholar]
- 36.Mebatsion, T., M. Konig, and K.-K. Conzelmann. 1996. Budding of rabies virus particles in the absence of the spike glycoprotein. Cell 84:941-951. [DOI] [PubMed] [Google Scholar]
- 37.Mebatsion, T., F. Weiland, and K. K. Conzelmann. 1999. Matrix protein of rabies virus is responsible for the assembly and budding of bullet-shaped particles and interacts with the transmembrane spike glycoprotein G. J. Virol. 73:242-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitnaul, L. J., M. R. Castrucci, K. G. Murti, and Y. Kawaoka. 1996. The cytoplasmic tail of influenza A virus neuraminidase (NA) affects NA incorporation into virions, virion morphology, and virulence in mice but is not essential for virus replication. J. Virol. 70:873-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mountcastle, W. E., and P. W. Choppin. 1977. A comparison of the polypeptides of four measles virus strains. Virology 78:463-474. [DOI] [PubMed] [Google Scholar]
- 40.Murphy, S. K., and G. D. Parks. 1999. RNA replication for the paramyxovirus simian virus 5 requries an internal repeated (CGNNNN) sequence motif. J. Virol. 73:805-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neumann, G., T. Watanabe, and Y. Kawaoka. 2000. Plasmid-driven formation of influenza virus-like particles. J. Virol. 74:547-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108:193-199. [DOI] [PubMed] [Google Scholar]
- 43.Paterson, R. G., T. J. R. Harris, and R. A. Lamb. 1984. Fusion protein of the paramyxovirus simian virus 5: nucleotide sequence of mRNA predicts a highly hydrophobic glycoprotein. Proc. Natl. Acad. Sci. USA 81:6706-6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson, R. G., and R. A. Lamb. 1993. The molecular biology of influenza viruses and paramyxoviruses, p. 35-73. In A. Davidson and R. M. Elliott (ed.), Molecular virology: a practical approach. IRL Oxford University Press, Oxford, England.
- 45.Paterson, R. G., R. A. Lamb, B. Moss, and B. R. Murphy. 1987. Comparison of the relative roles of the F and HN surface glycoproteins of the paramyxovirus simian virus 5 in inducing protective immunity. J. Virol. 61:1972-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Randall, R. E., D. F. Young, K. K. A. Goswami, and W. C. Russell. 1987. Isolation and characterization of monoclonal antibodies to simian virus 5 and their use in revealing antigenic differences between human, canine and simian isolates. J. Gen. Virol. 68:2769-2780. [DOI] [PubMed] [Google Scholar]
- 47.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 48.Robison, C. S., and M. A. Whitt. 2000. The membrane-proximal stem region of vesicular stomatitis virus G protein confers efficient virus assembly. J. Virol. 74:2239-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose, J. K., L. Buonocore, and M. A. Whitt. 1991. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Bio/Technology 10:520-525. [PubMed] [Google Scholar]
- 50.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
- 51.Sanderson, C. M., H. H. Wu, and D. P. Nayak. 1994. Sendai virus M protein binds independently to either the F or the HN glycoprotein in vivo. J. Virol. 68:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt, A. P., B. He, and R. A. Lamb. 1999. Involvement of the cytoplasmic domain of the hemagglutinin-neuraminidase protein in assembly of the paramyxovirus simian virus 5. J. Virol. 73:8703-8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmitt, A. P., G. P. Leser, D. L. Waning, and R. A. Lamb. 2002. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J. Virol. 76:3952-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K. Rose. 1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 17:1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schnell, M. J., J. E. Johnson, L. Buonocore, and J. K. Rose. 1997. Construction of a novel virus that targets HIV-1-infected cells and controls HIV-1 infection. Cell 90:849-857. [DOI] [PubMed] [Google Scholar]
- 56.Sheshberadaran, H., and R. A. Lamb. 1990. Sequence characterization of the membrane protein gene of paramyxovirus simian virus 5. Virology 176:234-243. [DOI] [PubMed] [Google Scholar]
- 57.Stricker, R., and L. Roux. 1991. The major glycoprotein of Sendai virus is dispensable for efficient virus particle budding. J. Gen. Virol. 72:1703-1707. [DOI] [PubMed] [Google Scholar]
- 58.Suomalainen, M., P. Liljestrom, and H. Garoff. 1992. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J. Virol. 66:4737-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takimoto, T., T. Bousse, E. C. Coronel, R. A. Scroggs, and A. Portner. 1998. Cytoplasmic domain of Sendai virus HN protein contains a specific sequence required for its incorporation into virions. J. Virol. 72:9747-9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takimoto, T., K. G. Murti, T. Bousse, R. A. Scroggs, and A. Portner. 2001. Role of matrix and fusion proteins in budding of Sendai virus. J. Virol. 75:11384-11391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Timmins, J., S. Scianimanico, G. Schoehn, and W. Weissenhorn. 2001. Vesicular release of Ebola virus matrix protein VP40. Virology 283:1-6. [DOI] [PubMed] [Google Scholar]
- 62.VerPlank, L., F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. USA 98:7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitt, M. A., L. Chong, and J. K. Rose. 1989. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J. Virol. 63:3569-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao, H., B. Lindqvist, H. Garoff, C. H. von Bonsdorff, and P. Liljestrom. 1994. A tyrosine-based motif in the cytoplasmic domain of the alphavirus envelope protein is essential for budding. EMBO J. 13:4204-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]