Abstract
Hepatitis C virus (HCV) is remarkably efficient in establishing persistent infection, possibly mediated by an impaired immune response to HCV infection. There is compelling evidence that HCV can infect immune cells, such as macrophages, B cells, and T cells. It has been previously reported that HCV core, the first protein expressed during the early phase of viral infection, contains the immunomodulatory function of suppressing host immune responses. This altered function of immune cells caused by HCV infection may explain the ineffective immune response to HCV. To further characterize the immunomodulatory role of HCV core in vivo, we generated transgenic (TG) mice by directing the expression of core protein to T lymphocytes by using the CD2 promoter. T-lymphocyte responses, including the production of gamma interferon and interleukin-2, were significantly diminished in these mice compared to their non-TG littermates. The inhibition of T-lymphocyte responsiveness may be due to the increased susceptibility of peripheral T lymphocytes to Fas-mediated apoptosis. Surprisingly, significant lymphocyte infiltration was observed in the portal tracts of livers isolated from core TG mice, associated with increasing serum alanine aminotransferase levels. Moreover, no intrahepatic lymphocytes or liver damage was found in non-TG littermates and core TG mice bred to Fas-deficient lpr mice. These results suggest that HCV core drives liver injury by increasing Fas-mediated apoptosis and liver infiltration of peripheral T cells.
Hepatitis C virus (HCV) is a serious and growing worldwide threat to human health. It is the major etiologic agent of non-A, non-B hepatitis and infects an estimated 400 million people, more than 3% of the world population. One of the remarkable features of HCV infection is the high rate of viral persistence. More than 80% of HCV-infected individuals develop chronic disease, which can progress to liver cirrhosis and hepatocellular carcinoma. In addition to HCV replication in hepatocytes, immune cells, such as monocytes, B cells, and T cells, can also support viral replication (13, 33, 41), although studies of viral replication in the peripheral lymphocytes of HCV-infected patients have had conflicting results (8). The widespread cellular distribution of CD81 and low-density lipoprotein receptor, putative HCV receptors, is consistent with HCV binding to cells other than hepatocytes (2, 47). However, studies of HCV pathogenesis have been hampered by the lack of a small-animal model.
The immune response to HCV has been extensively characterized and has been found to be significantly impaired in patients chronically infected with HCV. First, HCV persistence and progression of hepatitis were observed in spite of circulating antibody to HCV (14). Second, the production of gamma interferon (IFN-γ) and interleukin-2 (IL-2) was dramatically suppressed in the peripheral T lymphocytes of patients chronically infected with HCV (18, 31, 64). Third, T-lymphocyte responses to HCV gene products have been demonstrated with multiple antigenic stimulations. However, the magnitude of T-lymphocyte responses in patients chronically infected with HCV was dramatically decreased compared to that in patients acutely infected with HCV (12, 30, 31, 58, 61). It is possible that this impaired T-cell response in chronically infected patients may reflect an immunosuppressive mechanism linked to the ability of HCV to establish and maintain persistence after infection.
While little is known about the mechanisms of immune evasion by HCV, one possibility is that HCV generates mutations in the antigenic sites recognized by B and T cells. Alternatively, infection of immune cells, such as macrophages and B and T lymphocytes, may alter their functions, thus suppressing their ability to mount an effective response (1, 41). We have previously demonstrated that HCV core protein, the first protein produced during the early phase of viral infection, can suppress host immune response by inhibiting antiviral cytotoxic-T-lymphocyte (CTL) activity in mice infected with recombinant vaccinia virus expressing core protein (29).
The immunomodulatory function of core protein is intriguing in light of the finding that HCV core can bind to the cytoplasmic domain of tumor necrosis factor receptor (TNFR) family members, such as lymphotoxin-β receptor (35), TNFR1 (66), and Fas (22). The interaction between HCV core and TNFR family members has been reported to modulate the apoptosis of core-expressing cells in response to ligands of TNFR family members, depending on the specific cell type. In the case of T cells, the core protein increased the sensitivity of the cells to Fas-mediated apoptosis (22, 66), which may explain the increased Fas-mediated apoptosis observed in peripheral blood mononuclear cells (PBMC) from patients chronically infected with HCV (19, 60, 62). Interestingly, other lymphotropic viruses, such as human immunodeficiency virus, human T-cell leukemia virus, and cytomegalovirus, have been shown to elicit similar effects (28, 38, 59).
In the present study, we established a TG-mouse system to examine the role of core protein in the regulation of T lymphocytes and the development of liver damage. To this end, we designed a TG-mouse model in which HCV core was expressed in T cells under the control of the CD2 promoter. These mice exhibited suppressed T-lymphocyte responses, including the production of IFN-γ and IL-2. In addition, T lymphocytes derived from core TG mice were more susceptible to Fas-mediated activation-induced apoptosis than were their nontransgenic (NTG) littermates. Surprisingly, core TG mice developed histological changes in the liver that resembled those found in chronically HCV-infected patients. Portal and lobular inflammations with focal aggregates were detectable in TG mice, accompanied by an elevation of the serum alanine aminotransferase (ALT) level. These results suggest that the expression of core protein in T lymphocytes may play a pivotal role in immune dysregulation, recruitment of apoptotic lymphocytes to the liver, and subsequent liver damage.
MATERIALS AND METHODS
Generation of TG mice.
A fertilized oocyte at the single-cell stage, prepared from C57BL/6 (H-2b) × CBA (H-2k) mice, was microinjected with the transgene construct as shown in Fig. 1A. Candidate TG mice, containing the core gene of the Hutchinson strain (genotype 1a) of HCV (29), were analyzed by PCR of tail DNAs using core-specific primers, i.e., a sense primer (5′ AAGAAAAACCAAACGTAACACCA 3′), binding to nucleotides 24 to 46, and an antisense primer (5′ CCAGCTAGGCCGAGAGCCACG 3′), binding to nucleotides 301 to 321. The founder mice were backcrossed with C57BL/6 (H-2b) × CBA (H-2k) mice (Taconic Laboratories) for five generations. To assess the role of Fas in HCV core liver injury, core TG mice were bred with Fas-deficient mice. The mice were used between 6 and 12 weeks of age. All mice were bred in a pathogen-free facility and routinely tested for mouse hepatitis virus and other pathogens. All mice were cared for and handled according to protocols approved by the University of Virginia Institutional Animal Care and Use Committee.
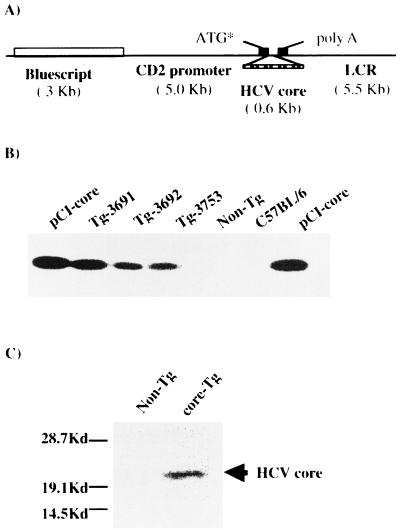
FIG. 1.
Generation of HCV core-expressing TG mice. (A) Diagram of the transgene construct. The HCV core gene was placed downstream of the CD2 promoter. The stippled box indicates cDNA encoding the HCV core protein. A 10.5-kb EcoRI-NotI fragment was introduced into mouse embryos. ATG*, start codon. (B) Expression of core-specific mRNA in TG mice. RT-PCR was performed to examine the expression of the core gene in TG mice, followed by Southern blot analysis using 32P-labeled core probe. RT-PCR analysis was performed on RNA samples isolated from the spleens of mice from three independent TG lines (3691, 3692, and 3753), their NTG littermates, and C57BL/6 mice as a negative control. Plasmid DNA containing the core gene (pCI-core) was used as a positive control. (C) Western blot of core protein. To confirm the expression of core protein, cell lysates obtained from the spleens of core TG and NTG mice were loaded and blotted with anti-core monoclonal antibodies.
Preparation of total RNA and RT-PCR.
Total RNAs were extracted from the cells or organs of TG and control mice with TRIZOL reagent (Gibco BRL, Grand Island, N.Y.) as recommended by the manufacturer. Nucleic acid pellets were suspended in appropriate volumes of diethyl pyrocarbonate-treated water and treated with RNase-free DNase I (Gibco BRL) prior to further analysis. Reverse transcription (RT) of total RNAs was performed with murine leukemia virus reverse transcriptase (Perkin-Elmer, Foster City, Calif.) and a random hexamer primer (Perkin-Elmer) to generate cDNA, according to the manufacturer's instructions. A portion of the cDNA was amplified with 0.5 U of Taq polymerase (Perkin-Elmer) and the HCV core-specific primers described above. To amplify a housekeeping gene, the hypoxanthine phosphoribosyltransferase gene, as an internal control, the cDNA was incubated with a sense primer (5′ GTTGGATACAGGCCAGACTTTGTTG 3′) and an antisense primer (5′ GAGGGTAGGCTGGCCTATGGCT 3′). PCR for the core protein and hypoxanthine phosphoribosyltransferase was carried out at 94°C for 40 s, 60°C for 30 s, and 72°C for 40 s for 35 cycles. The RT-PCR products were electrophoresed through a 1.5% agarose-Tris-acetate-EDTA buffer gel and further analyzed by Southern blotting with an HCV core-specific probe.
Southern blotting.
A standard Southern blotting procedure was utilized to analyze RT-PCR products. Briefly, separated RT-PCR products were transferred to a Zeta-probe GT membrane (Bio-Rad, Hercules, Calif.). To reveal the HCV core, a gel-purified 0.6-kb EcoRI-BamHI fragment of the core DNA was labeled with [32P]dCTP by the random-priming procedure (Amersham Pharmacia, Piscataway, N.J.) and used as a probe. The probe was hybridized with the membrane at 43°C overnight and washed stringently, as suggested in the manufacturer's protocol.
Western blotting.
Cell lysates were prepared with radioimmunoprecipitation assay cell lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 2 mM EDTA, 1% sodium orthovanadate, 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate) supplemented with phenylmethylsulfonyl fluoride (1 mM), aproptonin (2 μg/ml), leupeptin (2 μg/ml), and soybean trypsin inhibitor (37.5 μg/ml) and separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis. The proteins were tranferred to an Immobilon polyvinylidene difluoride membrane (Millipore, Bedford, Mass.) that was subsequently blocked in 5% dry milk in 50 mM Tris-HCl [pH 7.5]-150 mM NaCl-0.25% Tween 20. The membrane was incubated with anti-core antibody generously provided by Michael Lai, University of Southern California. Bound anti-core antibodies were revealed by peroxidase-conjugated goat anti-human immunoglobulin (Boehringer Mannheim, Indianapolis, Ind.) and visualized by enhanced chemiluminescence (Amersham Pharmacia).
Mixed-lymphocyte reaction.
Primary mixed-lymphocyte reactions were performed with spleen responders from core TG mice and their NTG littermates. Purified responder cells (105) were then mixed with 2 × 105 irradiated splenocytes isolated from BALB/c (H-2d) mice (employed as stimulators) and incubated for 4 days at 37°C. The cells were pulsed with 0.5 μCi of [3H]thymidine per well followed by further culture for 16 h. [3H]thymidine incorporation was measured using a betaplate reader (Wallac). For measuring alloreactive CTL activity, 1.5 × 107 purified splenocytes from core TG mice and their NTG littermates were cultured with 3 × 107 BALB/c mouse splenocytes, employed as stimulators, for 5 days at 37°C. The alloreactive CTL activity was measured using a standard 51Cr release assay with the effector to-target ratio (40:1, 13:1, 4:1, and 1:1), as previously described (29). The T cells were incubated with 51Cr-labeled P815 (H-2d) or EL4 (H-2b) for 4 h in 96-well round-bottom plates. The supernatants were harvested and assessed for 51Cr release.
ELISA for cytokine production.
Primary mixed-lymphocyte cultures were prepared using spleens harvested from mice and isolated with Isopaque Ficoll gradient. The T cells were resuspended at 106/ml in Iscove's Dulbecco modified Eagle's medium containing 10 μg of penicillin-streptomycin/ml, 10% fetal calf serum, and 10 mM l-glutamine and stimulated with anti-CD3 antibody for 1 to 6 days. The culture supernatants were analyzed by ELISA (BD PharMingen, San Diego, Calif.) for IFN-γ and IL-2 production.
Flow cytometry analysis.
Thymuses, lymph nodes, and spleens were obtained from core TG mice and their NTG littermates. A single-cell suspension was prepared by homogenizing the tissues with a pestle. Total lymphocytes were determined by trypan blue exclusion. Cells (2 × 106) were incubated on ice for 10 min with anti-FcR antibody and stained in fluorescence-activated cell sorter (FACS) medium (Iscove's medium, 10% newborn calf serum) with phycoerythrin-conjugated anti-CD4, fluorescein isothiocyanate (FITC)-conjugated anti-CD8, phycoerythrin-conjugated anti-CD3, and FITC-conjugated anti-B220 (BD PharMingen) at 4°C for 45 min in a 96-well round-bottom plate. After being washed, the stained cells were measured using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) and analyzed using CellQuest software.
Fas-mediated apoptosis of splenic T lymphocytes.
Purified splenocytes (5 × 106) from core TG mice and their NTG littermates were stimulated with plate-bound anti-CD3 antibody (1 μg/ml) for 24 h at 37°C. To induce Fas-mediated apoptosis, activated T cells were treated with 0.25 μg of Jo-2 anti-mouse Fas monoclonal antibody (PharMingen, San Diego, Calif.)/ml and 0.25 μg of protein G (Sigma, St. Louis, Mo.)/ml for 6 h at 37°C. The percentage of viable cells in the culture was determined by staining the cells with 2 μg of propidium iodide or FITC-annexin V (Roche, Indianapolis, Ind.)/ml, according to the manufacturer's instructions, and subsequent FACS analysis.
Histological and biochemical analysis of liver.
Spleen and liver tissues were harvested from core TG mice and their NTG littermates. The tissues were cut into small pieces, fixed in 10% neutral-buffered formalin for <24 h, and embedded in paraffin. Histological studies were performed on paraffin sections stained with hematoxylin and eosin. Serum samples were collected for subsequent ALT measurements with an Olympus Model 640 automated analyzer.
Detection of HCV core antigen and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay.
The detection of HCV core antigen was performed on 5-μm-thick liver paraffin sections. After deparaffination, samples were permeabilized in 0.01 mM citrate acetic buffer, pH 6.0, with microwave treatment (800 W for 10 min) and blocked with 3% bovine serum albumin and 10% normal goat serum in phosphate-buffered saline for 30 min. The tissue sections were incubated with anti-core rabbit polyclonal antibody diluted 1:400 (American QUALEX, San Clemente, Calif.) at 4°C overnight. After the tissues were washed with phosphate-buffered saline containing 0.2% Tween, biotin-conjugated goat anti-rabbit immunoglobulin G (BD PharMingen) was applied at a 1:1,200 dilution for 30 min, followed by incubation with ABC complex (VECTOR laboratories, Burlingame, Calif.). The reaction was developed with diaminobenzidine (DAB) staining with nickel enhancement peroxidase (Vectastain ABC kit; VECTOR Laboratories) according to the manufacturer's instructions. As a negative control, the primary antibody was tested in NTG littermates. The slides were counterstained with methyl blue.
To detect fragmented DNA in situ, a TUNEL assay was performed on paraffin-embedded sections of spleens and livers according to the manufacturer's instructions (ApopTag manual; Intergen Co., Purchase, N.Y.), and the sections were counterstained with hematoxylin.
Statistics.
A two-tailed Student's t test was used to determine significance.
RESULTS
Generation of core TG mouse lines.
To characterize the immunomodulatory function of HCV core protein in vivo, we generated core TG mice by inserting core-encoding cDNA under the control of the CD2 promoter (Fig. 1A). The expression plasmid contains the 5-kb CD2 promoter and the 5.5-kb locus control region of the human CD2 gene (67). The CD2 promoter has been shown to direct T-cell expression of a desired gene in a position-independent and copy number-dependent manner (54, 67).
Three lines of core TG mice (3691, 3692, and 3753) were identified by PCR. These mice were then backcrossed into C57BL/6 × CBA mice, and their offspring have a stable presence of the transgene throughout five generations. The expression of core mRNA was confirmed by RT-PCR amplification of RNA isolated from the spleens of core TG mice, followed by Southern blot analysis (Fig. 1B). Furthermore, the transcription of the core protein was also determined by Western blot analysis of cell lysates from the spleens of core TG mice. As shown in Fig. 1C, a prominent 21-kDa band was detected, corresponding to HCV core protein (52).
T-lymphocyte responsiveness was inhibited in core TG mice.
Since the immune response to HCV is significantly impaired in patients chronically infected with HCV, infection by HCV of immune cells may alter their function and result in the ineffective immune response to the virus. To determine whether the expression of core protein in T cells alters their function, we examined cell-mediated immune responses in both core TG mice and their NTG littermates. To this end, we assessed T-cell-proliferative and cytolytic responses to allogeneic stimulus by performing a standard mixed-lymphocyte reaction. As shown in Fig. 2A and B, the cytolytic activity and proliferative response of T cells to allogeneic stimulation in core TG mice were reduced to 80 to 90 and 60%, respectively, of those of the NTG littermates. Moreover, core TG mice also failed to generate primary CTL responses against vaccinia virus infection (data not shown). These results suggest that the expression of core protein in T lymphocytes is responsible for inhibiting the generation of T-lymphocyte responses.
FIG. 2.
Immune dysregulation in core TG mice. (A) Proliferation of primary alloreactive T cells. Splenic responders were isolated from core TG or NTG mice and were cocultured with irradiated splenic stimulators from BALB/c (H-2d) or C57BL/6 (H-2b) mice for 4 days. The proliferation of responder cells was measured by uptake of [3H]thymidine. Cell cultures were performed in triplicate. Similar results were obtained in two separate experiments. (B) Alloreactive CTL activity. Splenocytes isolated from core TG and NTG mice were cocultured with irradiated splenocytes from BALB/c (H-2d) mice. After 5 days of coculture, the T cells were assayed for cytolytic activity against P815 target cells (H-2d) with the indicated effector-to-target ratios. (C and D) Splenocytes from core TG and NTG mice were stimulated with anti-CD3 antibody. The supernatants were harvested on days 1, 2, 3, 4, and 5 after stimulation. IL-2 (C) and IFN-γ (D) production was measured by ELISA. The error bars indicate standard deviations.
We next examined the effect of core on the production of the proinflammatory cytokines IFN-γ and IL-2 by peripheral T lymphocytes in response to T-cell receptor (TCR) stimulation using anti-CD3 antibody. The production of IFN-γ and IL-2 was also markedly diminished in core TG mice (80 to 90% after 4 days of stimulation) compared to that of their NTG littermates (Fig. 2C and D). This suggests that HCV core protein may inhibit T-lymphocyte responsiveness in response to TCR stimulation by interfering with T-cell development, activation, and/or differentiation.
HCV core does not interfere with the development of T lymphocytes.
To determine whether core-mediated inhibition of T-lymphocyte responses might be due to defects in the development of T lymphocytes, we examined the developmental status and cellularity of T lymphocytes in TG mice. Thymus- and peripheral lymphoid-organ (i.e., lymph nodes and spleen)-derived CD4+- and CD8+-T-cell populations were examined by FACS. As shown in Fig. 3, the ratios of CD4+ CD8+ double-positive and CD4− CD8− double-negative thymocytes were similar in core TG mice and their NTG littermates. In addition, the ratios of single-positive CD4+ and CD8+ T cells in peripheral lymphoid organs were similar in core TG mice and their NTG littermates. These results demonstrate that HCV core does not interfere with T-cell development, and they are consistent with previous reports of the role of Fas in the deletion of peripheral but not thymic T lymphocytes (55). Therefore, HCV core may alter the activation and/or differentiation of peripheral T lymphocytes.
FIG. 3.
Normal T-cell development and cellularity in core TG mice. Phenotypic analysis was done on thymocytes and peripheral T lymphocytes (spleen and lymph nodes) isolated from core TG mice and their NTG littermates. Purified thymocytes and splenocytes were stained with anti-CD4 and anti-CD8 antibodies. The percentages of cells expressing the indicated markers are shown in each quadrant. The results shown are representative of three independent experiments.
Increased sensitivity of activated peripheral T lymphocytes to Fas-mediated apoptosis in core TG mice.
In light of earlier findings that HCV core can interact with the cytoplasmic domain of Fas (22), the expression of HCV core in T lymphocytes may modulate the Fas-mediated apoptotic pathway. To test this possibility, we performed TUNEL assays of the spleens from core and NTG mice. As shown in Fig. 4A, a large number of TUNEL-positive lymphocytes are detectable in the spleens of core TG mice but are rarely found in their NTG littermates. In addition, since B220, the high-molecular-weight B-cell isoform of CD45, has been reported to serve as a useful marker for dying T cells, we assessed the apoptotic status of HCV core-expressing T lymphocytes by examining the CD3+ B220+ phenotype in peripheral lymphocytes (25). More CD3+ B220+ lymphocytes were detectable in core TG mice than in their NTG littermates, suggesting that the peripheral lymphocytes of core TG mice are more prone to apoptosis (Fig. 4B).
FIG. 4.
Increased apoptosis of T lymphocytes in core TG mice. (A) Apoptosis of splenic T cells by TUNEL assay. Spleen tissues were harvested, formalin fixed, and embedded in paraffin. To detect the fragmented DNA, terminal deoxynucleotidyl transferase was labeled with digoxigenin on the free 3′ OH DNA termini in situ. The labeled DNA fragments are detected using anti-digoxigenin antibody conjugated to peroxidase. The positive signal in the nuclei of splenocytes from core TG mice was clearly visible. No apoptotic lymphocytes were detected in the spleens of NTG mice. (B) Phenotype of T cells in peripheral organs by FACS analysis of CD3 and B220 markers. Double-positive CD3 and B220 cells are indicative of an activated preapoptotic state. Slightly more circulating peripheral T cells exhibit a CD3+ and B220+ phenotype in core TG mice than in their NTG littermates. The percentages of cells expressing the indicated markers are shown in each quadrant. (C) Increased Fas-mediated apoptosis of activated T lymphocytes in core TG mice. Splenocytes isolated from core TG mice and their NTG littermates were stimulated with anti-CD3 antibody for 24 h and treated with anti-Fas antibody for 6 h. The activated T lymphocytes undergoing Fas-mediated apoptosis were determined by staining the cells with propidium iodide. The stained cells present in the gated lymphocyte population were analyzed by flow cytometry. (∗, P < 0.05). The results were reproducible in two independent experiments. The error bars indicate standard deviations.
We further sought to determine whether HCV core increased the susceptibility of activated T lymphocytes to Fas-mediated apoptosis by treating activated T lymphocytes with anti-Fas antibody. T lymphocytes isolated from the spleens of core TG mice were more sensitive to Fas-mediated activation-induced cell death than cells from NTG littermates (Fig. 4C). This suggests that the increased Fas-mediated apoptosis of the T lymphocytes of core TG mice might be responsible for the suppression of T-lymphocyte responses, including the inhibition of IFN-γ and IL-2 production.
Increased liver inflammation and serum ALT levels in core TG mice.
Several studies have reported that apoptotic and activated T lymphocytes accumulate in the liver during the clearance phase of peripheral immune responses (16). These lymphocytes are able to induce hepatocyte death (25, 49), as evidenced by increases in serum ALT levels (26, 49), in a Fas-dependent manner (26). To determine whether these Fas-sensitive peripheral T lymphocytes of core TG mice are recruited to the liver and subsequently induce liver injury, we examined the liver histology and serum ALT levels of core TG and NTG mice. To examine the dependency on functional Fas expression in this procedure, we bred core TG mice with Fas-deficient lpr mice.
Core TG mice demonstrated higher liver inflammatory activity than their NTG littermates: 8 out of 21 versus 0 out of 8, respectively (P < 0.01). The increased number of intrahepatic lymphocytes circulating in sinusoids was also associated with spotty necrosis in the lobular area and more intense inflammation in the portal region. In contrast, no lymphocytic infiltration was found in the livers of core-lpr mice (Fig. 5A). Serum ALT levels in core TG mice were significantly higher than those in their NTG littermates (P < 0.002), indicating that expression of core protein in T cells is responsible for inducing hepatitis (Fig. 5B). In contrast, ALT levels were normal in core-lpr mice, suggesting that Fas may be involved in liver injury induced by HCV core. However, there was no correlation between the grade of liver inflammation and serum ALT levels, as previously reported in patients with chronic hepatitis C (11, 17) and in other mouse models (26, 43). There was no evidence of infection with pathogens, including murine hepatitis virus, in core mice and their NTG littermates (data not shown).
FIG. 5.
Liver pathology in core TG mice. (A) Increased liver inflammation activity in core TG mice. Livers were harvested from core, NTG, and core-lpr mice. Sections of liver tissues were stained with hematoxylin and eosin. A remarkable lymphocytic infiltration was detectable in the portal tract and lobular areas of core TG mice, while no inflammation was detected in their NTG littermates and core-lpr mice. (B) Elevation of ALT levels in core TG mice. Blood was collected from 6- to 12-week-old core TG, NTG, and core-lpr mice to measure the levels of ALT as an indicator of liver damage. Horizontal lines indicate arithmetic mean. The serum ALT levels in core TG mice were significantly higher than those in their NTG littermates and core-lpr mice (∗, P < 0.002). (C) Confirmation of liver injury by TUNEL analysis. Following the same protocol used for the experiments shown in Fig. 4A, livers from core mice and their NTG littermates were analyzed by TUNEL assay. Some intrahepatic lymphocytes recruited in core TG mice were activated and preapoptotic. After their expansion in the liver, they underwent apoptosis (arrows), indicated by red signal in the nucleus. The consequence of this liver inflammation is the induction of apoptosis of bystander hepatocytes (arrowheads) in core TG mice. This phenomenon was not detectable in NTG mice.
The effector function of liver-infiltrating lymphocytes on hepatocyte damage was confirmed by performing TUNEL assays. Numerous intrahepatic lymphocytes were TUNEL positive in core TG mice compared to their NTG littermates (Fig. 5C). Moreover, liver damage is detectable in core TG mice, with most TUNEL-positive hepatocytes around regions of lymphocyte infiltration. No TUNEL-positive hepatocytes were detected in NTG littermates or core-lpr mice (data not shown). This suggests that intrahepatic lymphocytes may induce hepatocyte damage by bystander killing, independent of direct antigen recognition.
Immunohistochemical localization of core protein in T lymphocytes of core TG mouse livers.
To determine whether liver-infiltrating lymphocytes in core TG mice express core protein, immunohistochemical analysis using anti-core antibody was performed on liver tissue obtained from core TG mice and their NTG littermates. As shown in Fig. 6, in the livers of core TG mice, core protein was expressed in the cytoplasm of infiltrated lymphocytes located in the portal tract and some scattered in the lobular area. In contrast, no expression of core was detected in other cell types or in NTG littermates. These results suggest that in core TG mice the lymphocytes localized in the portal area of the liver represent core-expressing T lymphocytes.
FIG. 6.
Detection of HCV core protein in intrahepatic lymphocytes of core TG mice. The expression of core protein in lymphocytes was confirmed by immunohistochemical analysis of the liver. Immunostaining detection was developed by the DAB nickel enhancer method (black). The expression of HCV core protein was not detectable in NTG mice (magnification, ×600). Core protein was present in the cytoplasm of intrahepatic lymphocytes of core TG mice (magnification, ×1,000), particularly those infiltrated in the portal tract (arrows), but absent in hepatocytes (arrowheads).
DISCUSSION
Infection of peripheral blood cells with HCV may play an important role in HCV pathogenesis by altering the function of immune cells and leading to ineffective immune responses to HCV. It is important to point out that chronically HCV-infected patients develop other immunological disorders (lupus, arthritis, cryoglobulinemia, glomerunephritis, non-Hodgkin's lymphoma, and other autoimmune diseases), suggesting the pathological role of HCV replication in immune cells. No animal model so far has been focused on the infection of immune cells by HCV, with the exception of that of Bronowicki et al. (10). Those authors demonstrated the persistence of HCV RNA for 2 months in human mononuclear blood cells inoculated into immunosupressed (SCID) mice. Moreover, several studies indicate that HCV core protein is responsible for suppressing T-lymphocyte responsiveness (20, 27, 29, 65). Although studies aimed at investigating the mechanism of HCV-induced immune dysregulation are crucial to design effective HCV therapeutics, these studies have been hampered by the lack of a small-animal model. To examine the effect of HCV core expressed in extrahepatic sites and to characterize the immunomodulatory role of HCV core protein, we have established a TG-mouse line that directs expression of HCV core protein to T lymphocytes, using the CD2 promoter. Using this TG-mouse model, we observed suppression of CD4+- and CD8+-T-lymphocyte responses, including decreased IFN-γ and IL-2 production. However, the thymic development of T lymphocytes appeared to be normal in core TG mice. Moreover, an increased susceptibility of peripheral T lymphocytes to activation-induced cell death through Fas-mediated apoptosis was observed in core TG mice. Surprisingly, a remarkable lymphocytic infiltration was detectable in the hepatic portal tracts of core TG mice, similar to one of the characteristic histological features of patients chronically infected with HCV. Furthermore, ALT levels in core TG mice were significantly higher than those in their NTG littermates, suggesting that expression of core may induce liver damage.
Interestingly, the suppression of T-lymphocyte responses, including the inhibition of IFN-γ and IL-2 production, is consistent with the status of immune responses in chronically HCV-infected patients. They are characterized by the failure to mount vigorous, sustained, virus-specific CD4+- and CD8+-T-cell responses in the acute phase of HCV infection and are consequently unable to eradicate the virus (12, 31, 48, 56, 61). Therefore, HCV core-mediated inhibition of T-lymphocyte responses may contribute to the ineffective host immune response in chronic HCV infection. However, this observation is not consistent with the reports of other investigators, who failed to detect immune dysregulation in their core TG mice (57). This discrepancy could be due to variation in HCV core expression, as most other core TG-mouse lines direct the transcription of core protein to hepatocytes. The expression of genes under the control of the CD2 promoter have been detected in T cells (21, 54, 67), as confirmed by a CD2-enhanced green fluorescent protein (EGFP)-TG-mouse model, where approximately 95% of peripheral blood EGFP-positive cells were T cells. No expression of EGFP protein or other transgenes have been detected in B cells, monocytes, or neutrophils (54). Furthermore, T-cell proliferation and IL-2 production by EGFP+ cells were comparable to those by EGFP− cells from NTG littermates, indicating that introduction of the transgene did not alter T-cell functions. Since HCV can replicate in immune cells, such as macrophages, B cells, and T cells (9, 10, 51), the HCV core-induced immune dysregulation may depend for its intracellular expression on immune cells rather than hepatocytes. Furthermore, the magnitude of allogeneic responses in CBA (H-2k) versus BALB/c (H-2d) mice was similar to that in C57BL/6 (H-2b) versus BALB/c (H-2d) mice (24). This suggests that suppression of T-lymphocyte responses in core TG mice was not due to minor differences in the genetic background of our murine model but to the immunomodulatory function of HCV core.
An increased susceptibility of splenic T lymphocytes to activation-induced cell death through Fas-mediated apoptosis was detected in core TG mice, associated with a reduction of splenocytes. Moreover, T cells displaying an activated, preapoptotic phenotype circulate in higher proportions in core mice, suggesting a likely immunodysfunction in this population. Compelling evidence indicates that in chronically HCV-infected patients, liver disease severity is related to peripheral-T-cell Fas-mediated apoptosis (19, 60, 62) but not to serum ALT levels or virus load (11, 19, 46). These patients showed a clear association between the increased sensitivity of PBMC to apoptosis, a decrease in their number, and persistence of HCV infection (42, 60). Morever, no genotype difference among genotypes 1, 2, and 3 was detected in the increased sensitivity of PBMC to apoptosis, suggesting an intrinsic characteristic of HCV infection. Therefore, the decrease in the number of PBMC observed in chronically HCV-infected patients might be due, in part, to the acceleration of Fas-mediated apoptosis caused by direct interaction with HCV core.
A striking finding of this study is the induction of liver injury, with a remarkable inflammation in the portal area and the focus of necrosis in the lobular region and high serum ALT levels in core TG mice. This liver pathology resembles that in chronic HCV infection (4, 23). This liver inflammation was not found in young mice of any HCV core TG-mouse lines when expression of core protein was directed to hepatocytes. One possible explanation for this is the genetic background of the mice. In contrast to other studies using C57BL/6, CD1, FVB, or SJL mice to establish core TG mice, our murine model has been generated on a CBA × C57BL/6 background. Another factor might be the different HCV genotype, since the other studies used genotype 1b and our strain is genotype 1a. However, HCV core has been reported to be associated only with the induction of hepatic steatosis in young TG mice and the development of hepatic tumors at 16 months of age (32, 39, 40). The site of HCV core transcription could explain the major discrepancy for liver histology between this study and previous reports. Expression of HCV core in hepatocytes may have a direct effect on lipid metabolism (6, 37, 45, 50), leading to the formation of lipid vacuoles in hepatocytes in TG-mouse models (32, 39, 40, 45). However, expression of HCV core in immune cells may induce liver damage through modulation of the host immune system. The elevation in serum ALT levels reflects the induction of liver injury and was not detectable in other TG-mouse lines, where the expression of HCV core is directed to hepatocytes.
The mechanisms responsible for HCV-mediated liver damage are poorly understood (7). While the mechanism of core-induced liver injury observed in our TG-mouse model has yet to be identified, some hypotheses could explain this result. Several reports provide compelling evidence that the liver is responsible for extrathymic deletion of lymphocytes, recruiting activated and apoptotic T lymphocytes (25, 36, 44, 49). Therefore, it is likely that the increased number of intrahepatic lymphocytes in core TG mice might be the consequence of recruitment of circulating activated preapoptotic peripheral T lymphocytes induced by core protein. The role of those intrahepatic lymphocytes in liver damage has been extensively studied by using a line of OVA-specific CD8+ TCR TG mice, by injecting class I major histocompatibility complex-restricted OVA257-264 peptide (26, 49). Activated T cells are more susceptible to apoptosis upon activation. They disappear from the lymph nodes and spleen and accumulate in the liver, where they undergo clonal expansion followed by apoptosis. Serum ALT levels were elevated upon activation, as OVA-specific CD8+ T cells accumulated in the portal area, suggesting that liver-infiltrating lymphocytes are able to induce liver damage (26, 49), possibly in a Fas-dependent manner (26). This provides a model of autoimmune hepatitis, because hepatocytes are merely bystanders, having little to do with actual antigen presentation.
It is interesting to point out that in chronic hepatitis C, intrahepatic lymphocytes have been shown to have migrated from extrahepatic sites into the liver, where they display effector functions that lead to liver injury (44, 63). Several reports indicate a pathogenic role for autoimmunity in HCV-mediated liver injury, as chronic HCV infection shares features of autoimmune hepatitis type 2. First, intraportal lymphoid aggregates are characteristic of chronic hepatitis C, containing infiltrates of activated CD4+ and CD8+ T cells similar to those in autoimmune hepatitis (4, 23, 53). Second, cytokine-dependent molecules, such as class I and class II HLA and immune adhesion molecules, are predominantly found in hepatocytes and bile duct cells during a natural HCV infection (3, 5). Third, the autoantibodies and immunologically related diseases for chronic hepatitis C are similar to those in autoimmune hepatitis (15, 34).
In this report, we provide evidence that the expression of HCV core protein in T cells induces immune dysregulation by increasing apoptosis of T lymphocytes and that the development of liver damage results from recruitment of these apoptotic lymphocytes into the liver.
Acknowledgments
We thank Chang S. Hahn and Thomas J. Braciale for many helpful discussions and Audrey Eisen-Vandervelde for helping with manuscript preparation. We particularly acknowledge Dimitris Kioussis for providing a plasmid with the CD2 promoter and Michael Lai for providing anti-core monoclonal antibody.
This work was supported by a Cancer Research Institute postdoctoral fellowship to K.A.C.-B. and by National Institutes of Health grant DK57939 to Y.S.H.
REFERENCES
- 1.Afonso, A. M., J. Jiang, F. Penin, C. Tareau, D. Samuel, M. A. Petit, H. Bismuth, E. Dussaix, and C. Feray. 1999. Nonrandom distribution of hepatitis C virus quasispecies in plasma and peripheral blood mononuclear cell subsets. J. Virol. 73:9213-9221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asanza, C. G., C. Garcia-Monzon, G. Clemente, M. Salcedo, L. Garcia-Buey, C. Garcia-Iglesias, R. Banares, E. Alvarez, and R. Moreno-Otero. 1997. Immunohistochemical evidence of immunopathogenetic mechanisms in chronic hepatitis C recurrence after liver transplantation. Hepatology 26:755-763. [DOI] [PubMed] [Google Scholar]
- 4.Bach, N., S. N. Thung, and F. Schaffner. 1992. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology 15:572-577. [DOI] [PubMed] [Google Scholar]
- 5.Banner, B. F., C. Allan, L. Savas, S. Baker, G. Barnard, and H. L. Bonkovsky. 1997. Inflammatory markers in chronic hepatitis C. Virchows Arch. 431:181-187. [DOI] [PubMed] [Google Scholar]
- 6.Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Y. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Brechot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertolino, P., G. Klimpel, and S. M. Lemon. 2000. Hepatic inflammation and immunity: a summary of a conference on the function of the immune system within the liver. Hepatology 31:1374-1378. [DOI] [PubMed] [Google Scholar]
- 8.Boisvert, J., X. S. He, R. Cheung, E. B. Keeffe, T. Wright, and H. B. Greenberg. 2001. Quantitative analysis of hepatitis C virus in peripheral blood and liver: replication detected only in liver. J. Infect. Dis. 184:827-835. [DOI] [PubMed] [Google Scholar]
- 9.Bouffard, P., P. Hayashi, R. Acevedo, N. Levy, and J. Zeldis. 1992. Hepatitis C virus is detected in a monocyte/macrophage subpopulation of peripheral blood mononuclear cells of infected patients. J. Infect. Dis. 166:1276.. [DOI] [PubMed] [Google Scholar]
- 10.Bronowicki, J. P., M. A. Loriot, V. Thiers, Y. Grignon, A. L. Zignego, and C. Brechot. 1998. Hepatitis C virus persistence in human hematopoietic cells injected into SCID mice. Hepatology 28:211-218. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese, F., P. Pontisso, E. Pettenazzo, L. Benvegnu, A. Vario, L. Chemello, A. Alberti, and M. Valente. 2000. Liver cell apoptosis in chronic hepatitis C correlates with histological but not biochemical activity or serum HCV-RNA levels. Hepatology 31:1153-1159. [DOI] [PubMed] [Google Scholar]
- 12.Chang, K. M., R. Thimme, J. J. Melpolder, D. Oldach, J. Pemberton, J. Moorhead-Loudis, J. G. McHutchison, H. J. Alter, and F. V. Chisari. 2001. Differential CD4(+) and CD8(+) T-cell responsiveness in hepatitis C virus infection. Hepatology 33:267-276. [DOI] [PubMed] [Google Scholar]
- 13.Chang, T. T., K. C. Young, Y. J. Yang, H. Y. Lei, and H. L. Wu. 1996. Hepatitis C virus RNA in peripheral blood mononuclear cells: comparing acute and chronic hepatitis C virus infection. Hepatology 23:977-981. [DOI] [PubMed] [Google Scholar]
- 14.Chien, D. Y., Q. L. Choo, R. Ralston, R. Spaete, M. Tong, M. Houghton, and G. Kuo. 1993. Persistence of HCV despite antibodies to both putative envelope glycoproteins. Lancet 342:933.. [DOI] [PubMed] [Google Scholar]
- 15.Clifford, B. D., D. Donahue, L. Smith, E. Cable, B. Luttig, M. Manns, and H. L. Bonkovsky. 1995. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology 21:613-619. [PubMed] [Google Scholar]
- 16.Crispe, I. N., T. Dao, K. Klugewitz, W. Z. Mehal, and D. P. Metz. 2000. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol. Rev. 174:47-62. [DOI] [PubMed] [Google Scholar]
- 17.De Moliner, L., P. Pontisso, G. L. De Salvo, L. Cavalletto, L. Chemello, and A. Alberti. 1998. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut 42:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckels, D. D., N. Tabatabail, T. H. Bian, H. Wang, S. S. Muheisen, C. M. Rice, K. Yoshizawa, and J. Gill. 1999. In vitro human Th-cell responses to a recombinant hepatitis C virus antigen: failure in IL-2 production despite proliferation. Hum. Immunol. 60:187-199. [DOI] [PubMed] [Google Scholar]
- 19.Emi, K., K. Nakamura, K. Yuh, S. Sugyo, H. Shijo, M. Kuroki, and K. Tamura. 1999. Magnitude of activity in chronic hepatitis C is influenced by apoptosis of T cells responsible for hepatitis C virus. J. Gastroenterol. Hepatol. 14:1018-1024. [DOI] [PubMed] [Google Scholar]
- 20.Geissler, M., K. Tokushige, T. Wakita, V. R. Zurawski, Jr., and J. R. Wands. 1998. Differential cellular and humoral immune responses to HCV core and HBV envelope proteins after genetic immunizations using chimeric constructs. Vaccine 16:857-867. [DOI] [PubMed] [Google Scholar]
- 21.Greaves, D. R., F. D. Wilson, G. Lang, and D. Kioussis. 1989. Human CD2 3′-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell 56:979-986. [DOI] [PubMed] [Google Scholar]
- 22.Hahn, C. S., Y. G. Cho, B. S. Kang, I. M. Lester, and Y. S. Hahn. 2000. The HCV core protein acts as a positive regulator of fas-mediated apoptosis in a human lymphoblastoid T cell line.Virology 276:127-137. [DOI] [PubMed] [Google Scholar]
- 23.Hano, H., S. Takasaki, and J. Nakayama. 2000. Autoimmune forms of chronic hepatitis associated with hepatitis C virus (HCV) infection with and without HCV-RNA: histological differences from pure autoimmune hepatitis and chronic hepatitis C. Pathol. Int. 50:106-112. [DOI] [PubMed] [Google Scholar]
- 24.Heber-Katz, E., R. H. Schwartz, L. A. Matis, C. Hannum, T. Fairwell, E. Appella, and D. Hansburg. 1982. Contribution of antigen-presenting cell major histocompatibility complex gene products to the specificity of antigen-induced T cell activation. J. Exp. Med. 155:1086-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang, L., G. Soldevila, M. Leeker, R. Flavell, and I. N. Crispe. 1994. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1:741-749. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy, N. J., J. Q. Russell, N. Michail, and R. C. Budd. 2001. Liver damage by infiltrating CD8+ T cells is Fas dependent. J. Immunol. 167:6654-6662. [DOI] [PubMed] [Google Scholar]
- 27.Kittlesen, D. J., K. A. Chianese-Bullock, Z. Q. Yao, T. J. Braciale, and Y. S. Hahn. 2000. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J. Clin. Investig. 106:1239-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi, N., Y. Hamamoto, N. Yamamoto, A. Ishii, M. Yonehara, and S. Yonehara. 1990. Anti-Fas monoclonal antibody is cytocidal to human immunodeficiency virus-infected cells without augmenting viral replication. Proc. Natl. Acad. Sci. USA 87:9620-9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 30.Lechner, F., N. H. Gruener, S. Urbani, J. Uggeri, T. Santantonio, A. R. Kammer, A. Cerny, R. Phillips, C. Ferrari, G. R. Pape, and P. Klenerman. 2000. CD8+ T lymphocyte responses are induced during acute hepatitis C virus infection but are not sustained. Eur J. Immunol. 30:2479-2487. [DOI] [PubMed] [Google Scholar]
- 31.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191:1499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemon, S. M., H. Lerat, S. A. Weinman, and M. Honda. 2000. A transgenic mouse model of steatosis and hepatocellular carcinoma associated with chronic hepatitis C virus infection in humans. Trans. Am. Clin. Climatol. Assoc. 111:146-156. [PMC free article] [PubMed] [Google Scholar]
- 33.Lerat, H., F. Berby, M. A. Trabaud, O. Vidalin, M. Major, C. Trepo, and G. Inchauspe. 1996. Specific detection of hepatitis C virus minus strand RNA in hematopoietic cells. J. Clin. Investig. 97:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manns, M. P., and E. G. Rambusch. 1999. Autoimmunity and extrahepatic manifestations in hepatitis C virus infection. J. Hepatol. 31(Suppl. 1):39-42. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto, M., T. Y. Hsieh, N. Zhu, T. VanArsdale, S. B. Hwang, K. S. Jeng, A. E. Gorbalenya, S. Y. Lo, J. H. Ou, C. F. Ware, and M. M. Lai. 1997. Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-beta receptor. J. Virol. 71:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehal, W. Z., A. E. Juedes, and I. N. Crispe. 1999. Selective retention of activated CD8+ T cells by the normal liver. J. Immunol. 163:3202-3210. [PubMed] [Google Scholar]
- 37.Moradpour, D., C. Englert, T. Wakita, and J. R. Wands. 1996. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology 222:51-63. [DOI] [PubMed] [Google Scholar]
- 38.Mori, T., K. Ando, K. Tanaka, Y. Ikeda, and Y. Koga. 1997. Fas-mediated apoptosis of the hematopoietic progenitor cells in mice infected with murine cytomegalovirus. Blood 89:3565-3573. [PubMed] [Google Scholar]
- 39.Moriya, K., H. Fujie, Y. Shintani, H. Yotsuyanagi, T. Tsutsumi, K. Ishibashi, Y. Matsuura, S. Kimura, T. Miyamura, and K. Koike. 1998. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat. Med. 4:1065-1067. [DOI] [PubMed] [Google Scholar]
- 40.Moriya, K., H. Yotsuyanagi, Y. Shintani, H. Fujie, K. Ishibashi, Y. Matsuura, T. Miyamura, and K. Koike. 1997. Hepatitis C virus core protein induces hepatic steatosis in transgenic mice. J. Gen. Virol. 78:1527-1531. [DOI] [PubMed] [Google Scholar]
- 41.Muller, H., E. Pfaff, T. Gocser, B. Kallinoski, C. Solbach, and L. Theilmann. 1993. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J. Gen. Virol. 74:669-676. [DOI] [PubMed] [Google Scholar]
- 42.Nasir, A., H. S. Arora, and H. E. Kaiser. 2000. Apoptosis and pathogenesis of viral hepatitis C—an update. In Vivo 14:297-300. [PubMed] [Google Scholar]
- 43.Nishimura, T., and A. Ohta. 1999. A critical role for antigen-specific Th1 cells in acute liver injury in mice. J. Immunol. 162:6503-6509. [PubMed] [Google Scholar]
- 44.Nuti, S., D. Rosa, N. M. Valiante, G. Saletti, M. Caratozzolo, P. Dellabona, V. Barnaba, and S. Abrignani. 1998. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Vα24+ T cells and rapid elimination of effector cells by apoptosis. Eur J. Immunol. 28:3448-3455. [DOI] [PubMed] [Google Scholar]
- 45.Perlemuter, G., A. Sabile, P. Letteron, G. Vona, A. Topilco, Y. Chretien, K. Koike, D. Pessayre, J. Chapman, G. Barba, and C. Brechot. 2002. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 16:185-194. [DOI] [PubMed] [Google Scholar]
- 46.Pianko, S., S. Patella, G. Ostapowicz, P. Desmond, and W. Sievert. 2001. Fas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infection. J. Viral Hepatol. 8:406-413. [DOI] [PubMed] [Google Scholar]
- 47.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 48.Rosen, H. R., C. Miner, A. W. Sasaki, D. M. Lewinsohn, A. J. Conrad, A. Bakke, H. G. Bouwer, and D. J. Hinrichs. 2002. Frequencies of HCV-specific effector CD4+ T cells by flow cytometry: correlation with clinical disease stages. Hepatology 35:190-198. [DOI] [PubMed] [Google Scholar]
- 49.Russell, J. Q., G. J. Morrissette, M. Weidner, C. Vyas, D. Aleman-Hoey, and R. C. Budd. 1998. Liver damage preferentially results from CD8+ T cells triggered by high affinity peptide antigens. J. Exp. Med. 188:1147-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabile, A., G. Perlemuter, F. Bono, K. Kohara, F. Demaugre, M. Kohara, Y. Matsuura, T. Miyamura, C. Brechot, and G. Barba. 1999. Hepatitis C virus core protein binds to apolipoprotein A and its secretion is modulated by fibrates. Hepatology 30:1064-1076. [DOI] [PubMed] [Google Scholar]
- 51.Saleh, M. G., C. J. Tibbs, J. Koskinas, L. M. Pereira, A. B. Bomford, B. C. Portmann, I. G. McFarlane, and R. Williams. 1994. Hepatic and extrahepatic hepatitis C virus replication in relation to response to interferon therapy. Hepatology 20:1399-1404. [DOI] [PubMed] [Google Scholar]
- 52.Santolini, E., G. Migliaccio, and M. N. La. 1994. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J. Virol. 68:3631-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheuer, P. J., P. Ashrafzadeh, S. Sherlock, D. Brown, and G. M. Dusheiko. 1992. The pathology of hepatitis C. Hepatology 15:567-571. [DOI] [PubMed] [Google Scholar]
- 54.Singbartl, K., J. Thatte, M. L. Smith, K. Wethmar, K. Day, and K. Ley. 2001. A CD2-green fluorescence protein-transgenic mouse reveals very late antigen-4-dependent CD8+ lymphocyte rolling in inflamed venules. J. Immunol. 166:7520-7526. [DOI] [PubMed] [Google Scholar]
- 55.Singer, G. G., and A. K. Abbas. 1994. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity 1:365-371. [DOI] [PubMed] [Google Scholar]
- 56.Sobao, Y., H. Tomiyama, S. Nakamura, H. Sekihara, K. Tanaka, and M. Takiguchi. 2001. Visual demonstration of hepatitis C virus-specific memory CD8(+) T-cell expansion in patients with acute hepatitis C. Hepatology 33:287-294. [DOI] [PubMed] [Google Scholar]
- 57.Sun, J., F. Bodola, X. Fan, H. Irshad, L. Soong, S. M. Lemon, and T. S. Chan. 2001. Hepatitis C virus core and envelope proteins do not suppress the host's ability to clear a hepatic viral infection. J. Virol. 75:11992-11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 59.Takizawa, T., S. Matsukawa, Y. Higuchi, S. Nakamura, Y. Nakanishi, and R. Fukuda. 1993. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J. Gen. Virol. 74:2347-2355. [DOI] [PubMed] [Google Scholar]
- 60.Taya, N., Y. Torimoto, M. Shindo, K. Hirai, C. Hasebe, and Y. Kohgo. 2000. Fas-mediated apoptosis of peripheral blood mononuclear cells in patients with hepatitis C. Br. J. Haematol. 110:89-97. [DOI] [PubMed] [Google Scholar]
- 61.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194:1395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toubi, E., A. Kessel, L. Goldstein, G. Slobodin, E. Sabo, Z. Shmuel, and E. Zuckerman. 2001. Enhanced peripheral T-cell apoptosis in chronic hepatitis C virus infection: association with liver disease severity. J. Hepatol. 35:774-780. [DOI] [PubMed] [Google Scholar]
- 63.Valiante, N. M., A. D'Andrea, S. Crotta, F. Lechner, P. Klenerman, S. Nuti, A. Wack, and S. Abrignani. 2000. Life, activation and death of intrahepatic lymphocytes in chronic hepatitis C. Immunol. Rev. 174:77-89. [DOI] [PubMed] [Google Scholar]
- 64.Woitas, R. P., M. Lechmann, G. Jung, R. Kaiser, T. Sauerbruch, and U. Spengler. 1997. CD30 induction and cytokine profiles in hepatitis C virus core-specific peripheral blood T lymphocytes. J. Immunol. 159:1012-1018. [PubMed] [Google Scholar]
- 65.Yao, Z. Q., D. T. Nguyen, A. I. Hiotellis, and Y. S. Hahn. 2001. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J. Immunol. 167:5264-5272. [DOI] [PubMed] [Google Scholar]
- 66.Zhu, N., A. Khoshnan, R. Schneider, M. Matsumoto, G. Dennert, C. Ware, and M. M. Lai. 1998. Hepatitis C virus core protein binds to the cytoplasmic domain of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J. Virol. 72:3691-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhumabekov, T., P. Corbella, M. Tolaini, and D. Kioussis. 1995. Improved version of a human CD2 minigene based vector for T cell-specific expression in transgenic mice. J. Immunol. Methods 185:133-140. [DOI] [PubMed] [Google Scholar]