Abstract
Ebola Zaire virus (EBO-Z) causes severe hemorrhagic fever in humans, with a high mortality rate. It is thought that a vaccine against EBO-Z may have to induce both humoral and cell-mediated immune responses to successfully confer protection. Because it is known that liposome-encapsulated antigens induce both antibody and cellular responses, we evaluated the protective efficacy of liposome-encapsulated irradiated EBO-Z [L(EV)], which contains all of the native EBO-Z proteins. In a series of experiments, mice immunized intravenously with L(EV) were completely protected (94/94 mice) against illness and death when they were challenged with a uniformly lethal mouse-adapted variant of EBO-Z. In contrast, only 55% of mice immunized intravenously with nonencapsulated irradiated virus (EV) survived challenge, and all became ill. Treatment with anti-CD4 antibodies before or during immunization with L(EV) eliminated protection, while treatment with anti-CD8 antibodies had no effect, thus indicating a requirement for CD4+ T lymphocytes for successful immunization. On the other hand, treatment with either anti-CD4 or anti-CD8 antibodies after immunization did not abolish the protection. After immunization with L(EV), antigen-specific gamma interferon (IFNγ)-secreting CD4+ T lymphocytes were induced as analyzed by enzyme-linked immunospot assay. Anti-CD4 monoclonal antibody treatment abolished IFNγ production (80 to 90% inhibition compared to that for untreated mice). Mice immunized with L(EV), but not EV, developed cytotoxic T lymphocytes specific to two peptides (amino acids [aa] 161 to 169 and aa 231 to 239) present in the amino-terminal end of the EBO-Z surface glycoprotein. Because of the highly successful results in the mouse model, L(EV) was also tested in three cynomolgus monkeys. Although immunization of the monkeys with L(EV)-induced virus-neutralizing antibodies against EBO-Z caused a slight delay in the onset of illness, it did not prevent death.
Ebola Zaire virus (EBO-Z), an enveloped, nonsegmented negative-strand RNA virus in the family Filoviridae, causes severe hemorrhagic fever in humans and uniformly lethal infection in nonhuman primates (16). EBO-Z emerged in 1976 and reappeared in 1995 as the causative agent of hospital-based outbreaks in the northern part of the Democratic Republic of Congo, then called Zaire (8, 16). The main route of transmission is through contact with body fluids of patients. EBO-Z infection causes widespread damage to parenchymal cells of vital organs, resulting in severe homeostatic and immune disturbances and leading to death in 80 to 90% of the cases.
Ebola virus virions possess a single surface transmembrane glycoprotein (GP) that plays a crucial role in mediating virus entry into cells. In addition to GP and a smaller secreted GP that is synthesized in abundance early in the infection (25), the genome of Ebola virus codes for other structural proteins (24): the nucleocapsid-associated nucleoprotein (NP), matrix proteins VP24 and VP40, presumed nonstructural proteins VP30 and VP35, and the viral polymerase.
Despite the potential threat of further emergence or spread of EBO-Z, there are presently no approved vaccines or therapeutic agents for humans. A number of preliminary vaccine studies have been performed using guinea pig- and mouse-adapted variants of EBO-Z in the respective rodent hosts. Much of the work (10, 27, 31, 32) has focused on the use of recombinant viral surface GP or viral NP as immunogens, with attention centering on GP as a target of the neutralizing antibody response. Several studies have demonstrated protective efficacy in mice or guinea pigs after immunization with recombinant vaccinia viruses, DNA vaccines, or RNA replicons expressing GP (10, 17, 27, 33). Immunization with NP alone, or with both GP and NP, has also conferred protection in rodents (17, 27). One success has been recently reported in vaccinating nonhuman primates through the use of a prime-boost strategy, using three doses of a DNA vaccine encoding both GP and NP, followed by a boost with recombinant adenovirus expressing GP (26). This study also showed that depletion of CD4+ T lymphocytes but not CD8+ T lymphocytes reduced the EBO-Z-specific T-cell proliferative response. It thus appears that the correct induction of cell-mediated immunity contributes towards a successful immunization against EBO-Z (26).
A role for humoral immunity has been demonstrated by the successful protection of rodents through passive transfer of immune serum or GP-specific monoclonal antibodies (MAbs) (11, 13, 31). However, antibodies have proven to be less successful in conferring protection in nonhuman primates (13, 14). It appears likely that both humoral and cell-mediated immunity play important roles in protection. It has been shown that guinea pigs injected with plasmids encoding GP or secreted GP generated both antibody and cell-mediated responses to the viral GP (33). Although antibody titer correlated with protection, cell-mediated immunity appeared necessary for protection, because antiserum from protected guinea pigs did not inhibit virus replication in vivo or in vitro (33). In a separate study, it was shown that transfer of T cells from NP-immunized mice protected naïve mice against lethal EBO-Z challenge (32). Further research is thus needed to help define the requirements for successful immunization against EBO-Z.
Given the uncertainty as to the immunological requirements for an effective vaccine, we hypothesized that a useful strategy might be to deliver the appropriate EBO-Z antigens by a method that would elicit both antibody and cellular immune responses. We have previously shown that liposomes (L) serve as an efficient delivery system for a variety of antigens and evoke both types of immune response (2, 28, 30). L-encapsulated protein and peptide antigens enter the major histocompatibility complex (MHC) class I pathway and thus are efficient inducers of cytotoxic T lymphocytes (CTLs) (2, 18-21, 23). It was previously shown that immunization of B10.BR mice with irradiated EBO-Z in L containing lipid A induced CTLs specific for EBO-Z GP peptides and that lipid A was necessary for a long-term CTL memory recall response (21). We further hypothesized that L-encapsulated irradiated EBO-Z [L(EV)] virions would be especially immunogenic, since they would simultaneously deliver all the viral proteins to antigen-presenting cells.
In the present study, immunization of BALB/c mice intravenously with L(EV) induced complete protection against challenge with live EBO-Z, even though virus-neutralizing antibodies could not be detected in the sera of these mice. When L(EV) was tested in a cynomolgus monkey model, discordant results were obtained between mice and monkeys. Although immunization with L(EV) induced virus-neutralizing antibodies in the three monkeys studied, the monkeys were not protected against challenge with live EBO-Z.
MATERIALS AND METHODS
Biologic containment.
Infectious material and animals were handled in biological safety level 4 (BSL-4) facilities at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). Laboratory personnel wore positive-pressure protective suits (ILC Dover, Frederica, Del.) equipped with high-efficiency particulate air filters and supplied with umbilically fed air.
Virus and preparation of vaccine.
For preparation of irradiated vaccine, EBO-Z/95 was amplified in Vero E6 cells (Vero C1008; American Type Culture Collection accession number CRL-1586) to a titer in supernatants of approximately 108 PFU/ml. Virus was then concentrated by precipitation with polyethylene glycol, purified by sucrose density gradient centrifugation, and subjected to 6 × 106 rads of γ irradiation from a 60Co source. Safety testing revealed no residual infectious virus. Monkeys were challenged with the same EBO-Z/95 (not inactivated). Challenge studies in mice employed a mouse-adapted variant of EBO-Z/76 (3-5). Viral titers in serum samples were determined as described previously (4). Irradiated virus was encapsulated in L containing 20 μg of lipid A, as previously described (1, 21). The amount of encapsulated antigen was determined by a modified Lowry procedure (29).
Immunization and challenge of mice.
The investigators adhered to the “Guide for the Care and Use of Laboratory Animals,” prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (National Institutes of Health Publication No. 86-23, revised 1996), and used facilities fully accredited by the American Association for Accreditation of Laboratory Animal Care. Six-to-eight-week-old female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and maintained in a pathogen-free facility. Groups of 36 mice were immunized by the intravenous (i.v.) or intramuscular (i.m.) route at 0 and 3 weeks with 1.4 μg of unencapsulated irradiated EBO-Z (EV) alone or with the same quantity of irradiated EBO-Z encapsulated in L [L(EV)] or with empty L (L).
Fifteen mice from each group at 1 week and 15 mice at 4 or 5 weeks after the second immunization were transferred to BSL-4 containment at USAMRIID. The day after arrival, they were injected i.p. with 10 PFU (300 × the 50% lethal dose [LD50]) of mouse-adapted EBO-Z and observed on a daily basis for illness and death. In most experiments, five mice from each group were anesthetized and terminally exsanguinated on day 4 postchallenge, viral titers of sera were determined by plaque assay, and alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were measured using a Kodak 250 chemistry analyzer (Eastman Kodak, Rochester, N.Y.).
Immunization and challenge of monkeys.
Four cynomolgus monkeys weighing 4 to 5 kg, free of antibodies against filoviruses, were transferred to BSL-4 containment. Three animals were immunized on days −84, −56, and −28 before challenge by i.v. inoculation of 1.0 ml of L(EV), which consisted of L containing 194 μg of irradiated EBO-Z and 100 μg of lipid A, while a negative-control animal received 1.0 ml of L, also containing lipid A. On day 0, the monkeys were challenged by i.m. injection with 1,000 PFU of EBO-Z/95. They were observed daily for signs of illness and anesthetized, examined, and bled every second or third day. Serum viral titers were determined by plaque assay, and serum AST and ALT concentrations were measured using a Piccolo portable blood analyzer (Abaxis, Inc., Sunnyvale, Calif.).
Neutralizing antibody determinations.
Pooled prechallenge serum samples from groups of mice and individual prechallenge serum samples from monkeys were tested for the presence of EBO-Z-neutralizing antibodies as previously described (4). Aliquots of EBO-Z/95 in growth medium were mixed with serial dilutions of test sera and incubated at 34°C for 1 h, followed by infecting the monolayers of Vero E6 cells. The 50% plaque reduction titer (PRNT50) was calculated as the reciprocal of the highest dilution of serum that caused a 50% reduction in the number of plaques compared to that of a normal serum control.
Serum antibody determinations.
Ebola virus-specific antibodies were quantitated in pooled mouse sera (1 and 5 weeks after the boost) or individual monkey serum samples (day −56 and day −28) by an enzyme-linked immunosorbent assay (ELISA) as previously described (12). Irradiated EBO-Z/95 (1:1,000 dilution in phosphate-buffered saline [PBS]) was used as the coating antigen. Each sample was tested in duplicate. Affinity-purified peroxidase-labeled goat anti-mouse immunoglobulin (Ig) [IgA + IgG + IgM(H+L)] (Kirkegaard and Perry Laboratories, Gaithersburg, Md.) was used as the secondary antibody. Endpoints were determined by subtracting the mean optical density value obtained using normal mouse or monkey serum from that of the test sera. The highest serum dilution that gave a corrected value of ≥0.20 was considered the endpoint.
Murine CTL assay.
The amino acid sequence of EBO-Z GP was analyzed to identify murine CTL epitopes, based on the motifs specific for the MHC allele H-2D, using two different computer programs (Patterns, University of Wisconsin Genetics Computer Group Sequence Analysis Program, and HLA peptide binding predictions) (6, 22). Two sequences at the amino-terminal end of EV-GP were identified as specific for the MHC class I allele H-2Kd by this program: LYDRLASTVI (peptide P1, specific for Kd) and EYLFEVDNL (peptide P2, specific for Kd and Kb). These peptides were synthesized by SynPep Corp., Dublin, Calif.
One, two, and five weeks after the booster immunization, splenic lymphocytes (effector cells) were collected from three mice from each group. Effector cells were stimulated for 5 days with or without antigen (10 μg of P1 or P2 per ml) in the presence of T STIM culture supernatant (Collaborative Biomedical Products, Bedford, Mass.), as described previously (21). In some experiments, effector cells were also cultured with irradiated Ebola virus (3,000 PFU/5 × 106 cells/1.5 ml). Effector cells were collected 5 days later and analyzed for the presence of CTLs. Target cells consisted of syngeneic P815 (H-2d) cells alone or incubated with either 50 μg of P1 or P2 or incubated with 100 μg of an unrelated H-2kd-specific CTL epitope (CYASGWGSI) from human prostate-specific antigen or allogeneic EL-4 (H-2b) cells. In some experiments, P815 cells were infected with recombinant vaccinia virus expressing Ebola virus GP or with wild-type vaccinia virus (kindly provided by Kevin Anderson, USAMRIID) at a multiplicity of infection of 10 (21). Target cells were labeled for 1 h with 100 μCi of 51Cr as Na2CrO4 per 106 cells (NEN Life Science Products, Boston, Mass.). CTL assays were performed, and the percentage of specific lysis was calculated as previously described (21). Each assay was performed at least three times. The results shown are means of triplicate wells from a representative experiment.
In vivo T-cell depletion in mice.
Mice were divided into three groups for treatment with monoclonal antibodies (MAbs). All groups were immunized i.v. with L(EV) on day 0 and on day 21 and challenged on day 28. In the first set of experiments, one group received daily injections of 0.5 to 1 mg of either anti-CD4 (GK1.5) or anti-CD8 (53.10.72) MAbs (50% ammonium sulfate precipitate of ascites fluid). The injections were given on days −3, −2, and −1 and on days 17 through 20 (3 days before the first immunization and 4 days before the second immunization). The second group received weekly injections of anti-CD4 or anti-CD8, beginning 4 days after the first immunization and continued through the week of challenge. The third group received daily injections of both anti-CD4 and anti-CD8 on days −3, −2, and −1 and on days 17 through 20 (3 days before the first immunization and 4 days before the second immunization). The positive-control group was immunized with L(EV) but not treated with MAbs. The negative-control groups consisted of naïve mice or mice immunized with L alone or treated with MAbs alone.
In the second set of experiments, mice were immunized i.v. with L(EV) on day 0 and on day 21 and challenged on either day 28 or on day 56. One group received daily injections of 0.5 to 1 mg of either anti-CD4 or anti-CD8 MAbs. The injections were given on days −3, −2, and −1 and on days 17 through 20 (3 days before the first immunization and 4 days before the second immunization). The second group received daily injections of either anti-CD4 or anti-CD8 on days 25 through 27 (3 days prior to challenge on day 28). The third group of mice received daily injections of either anti-CD4 or anti-CD8 on days 53 through 55 (3 days prior to challenge on day 56). The positive- and the negative-control groups were the same as in experiment 1.
The effectiveness of cell depletion was analyzed by flow cytometry either on day 25 or on day 61 (5 to 6 days after the last MAb treatment) using either peripheral blood lymphocytes or spleen cells. Lymphocytes were stained with fluorescent antibodies to CD4, CD8, and CD3 (BD PharMingen, San Diego, Calif.) and analyzed on a FACSCalibur apparatus (Becton Dickinson, San Jose, Calif.) using CellQuest software.
Cytokine ELISPOT.
Spleen cells secreting IFNγ were analyzed by enzyme-linked immunospot assay (ELISPOT). Ninety-six-well nitrocellulose-backed MultiScreen-IP sterile plates (Millipore, Bedford, Mass.) were coated overnight at 4°C with 10 μg of anti-gamma interferon (IFNγ) (clone RMGG-1; BioSource International, Inc., Camarillo, Calif.)/ml in sterile PBS. The wells were blocked with sterile PBS-0.5% bovine serum albumin for 30 min at 37°C and washed with PBS-0.025% Tween 20 (wash solution) followed by sterile RPMI-1640 complete medium. Single cell suspensions were prepared from the spleens (three mice/group) of naïve and immunized mice. Cells (2 × 106/well) were plated on anti-IFNγ-coated plates and incubated for 18 h at 37°C in a humidified CO2 incubator. In some cases, CD4+ and CD8+ T cells were purified from naïve and immunized mice by using T-cell enrichment columns (R and D Systems, Inc., Minneapolis, Minn.). Purified T cells were plated at a concentration of 2 × 105 cells/well. Cells were incubated with or without irradiated EBO-Z (360 ng/ml) and peptide P1 or P2 (5 to 10 μg/ml). Plates were washed with the wash solution followed by distilled water and overlaid with 0.125 μg of biotinylated anti-IFNγ (clone XMG 1.2; BD PharMingen)/ml and incubated at room temperature for 2 h. The plates were then washed and incubated with a 1:1,000 dilution of avidin-conjugated alkaline phosphatase (Vector Laboratories, Burlingame, Calif.) for 2 h at room temperature. The plates were washed, and bound IFNγ was detected by the addition of 5-bromo-4-chloro-3-indolylphosphate (BCIP)/nitroblue tetrazolium (NBT) (Kirkegaard and Perry Labs, Gaithersburg, Md.). The plates were washed with water, and the individual spots were visualized and counted the next day using a stereo binocular microscope. The average number of spots/number of cells plated was plotted.
Disclaimer.
The views, opinions, and/or findings contained herein are those of the authors and should not be construed as an official Department of the Army position, policy, or decision unless so designated by other documentation.
RESULTS
Antibody response in mice induced after immunization with L(EV).
Using pooled sera from mice bled 1 or 5 weeks after the second immunization, antibody responses to EBO-Z were analyzed by ELISA and PRNT50 assays (Table 1). Antibodies specific to EBO-Z could be detected by ELISA 1 and 5 weeks after the second immunization, with antibody titers being fivefold higher 5 weeks after the boost. L(EV) elicited a 10-fold stronger antibody response than EV (endpoint titers of 500 versus 5,000) 5 weeks after the boost, irrespective of the route of immunization. No neutralizing antibodies were detected in any of the groups at either 1 or 5 weeks after the second immunization. This result is consistent with those of other studies with rodents, in which anti-Ebola virus-neutralizing antibodies were low or undetectable in animals that proved to be resistant to EBO-Z challenge (17, 27).
TABLE 1.
Antibody response specific to EBO-Z in micea
| Immunogen (immunization method) | Wk 1d
|
Wk 5
|
||
|---|---|---|---|---|
| ELISAb | PRNT50c | ELISAb | PRNT50c | |
| L(EV) i.v. | 1,000 | <20 | 5,000 | <20 |
| L(EV) i.m. | 1,000 | <20 | 5,000 | <20 |
| EV i.v. | NDd | ND | 500 | <20 |
| EV i.m. | ND | ND | 500 | <20 |
| L i.v. | <100 | <20 | <100 | <20 |
| L i.m. | <100 | <20 | <100 | <20 |
BALB/c mice were immunized i.v. or i.m. with L(EV), or EV or L and bled 1 and 5 weeks after the second immunization. Sera were pooled and analyzed for EBO-Z-specific and neutralizing antibodies (see Materials and Methods).
Data shown represent the endpoint titers for ELISA.
PRNT50 values represent reciprocal 50% endpoint dilutions for neutralizing antibodies.
ND, not determined.
Cellular immune response to L(EV) in mice.
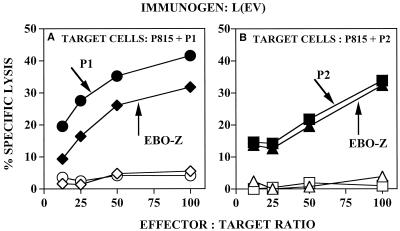
Figure 1 shows the presence of CTLs 2 weeks after the second i.v. immunization with L(EV). Effector spleen cells from naïve or L(EV)-immunized mice were cultured for 5 days with peptide P1, peptide P2, or irradiated EV and then analyzed for the presence of CTLs by using either peptide P1- or P2-pulsed syngeneic P815 cells as targets. Effector cells from L(EV)-immunized mice cultured with EV recognized both peptide P1- and P2-pulsed target cells and gave recall CTL responses reaching 30% to 40% specific lysis (Fig. 1A and B). Similarly, cells cultured with peptide P2 recognized P2-pulsed target cells (Fig. 1B). Less than 5% lysis was seen (Fig. 1A and B) with naïve effector cells cultured under conditions identical to those for the effector cells from L(EV)-immunized mice. These results confirm that the lysis seen with effector cells from L(EV)-immunized mice (Fig. 1A and B) was specific for Ebola virus GP peptides P1 and P2 and was not due to in vitro artificial induction of CTLs. In contrast to the CTL responses obtained after immunization with L(EV), peptide-specific CTL responses were not detected in spleen cells from mice immunized with EV or with L (data not shown).
FIG. 1.
CTL response to Ebola virus 2 weeks after the boost. BALB/c mice were immunized i.v. with 1.4 μg of L(EV) at 0 and 3 weeks or left untreated. Splenic lymphocytes from immunized mice were collected 2 weeks after the boost and stimulated in vitro with EBO-Z GP peptide 1 (•), EBO-Z GP peptide 2 (▪), or irradiated EBO-Z (⧫ and ▴), and splenic lymphocytes from untreated mice were stimulated in vitro with EBO-Z GP peptide 1 (○), EBO-Z GP peptide 2 (□), or irradiated EBO-Z (◊ and ▵), as described in Materials and Methods. Standard 5-h CTL assays were performed using 51Cr-labeled syngeneic (H-2d) P815 cells pulsed with EBO-Z GP peptide 1 or 2. Effector/target ratios ranged from 12.5:1 to 100:1. Percentages of specific lysis were calculated as described in Materials and Methods. Data represent the means of triplicate cultures of a representative experiment.
To ensure that the CTL response was EBO-Z specific and to demonstrate that peptides P1 and P2 are potential CTL epitopes of Ebola virus GP, splenocytes taken from BALB/c mice 5 weeks after the second immunization were cultured with either peptide P1 or P2 and then tested against P815 target cells infected with either recombinant vaccinia virus expressing EBO-Z GP or with wild-type vaccinia virus. Irrespective of the route of immunization, in comparison with the lysis seen with wild-type vaccinia virus-infected control targets, more-specific lysis of target cells infected with vaccinia virus GP was obtained with effector cells from L(EV)-immunized mice cultured with either peptide P1 (Fig. 2A and G) or P2 (Fig. 2B and H). The specificity of lysis was greater when cells were cultured with P2 (Fig. 2B and H). In contrast, no specific lysis of vaccinia virus GP-infected targets was observed with effector cells from mice immunized with EV (Fig. 2C, D, I, and J) or with L (Fig. 2E, F, K, and L). These results confirm that CTLs specific for EBO-Z GP peptides P1 and P2 were induced upon immunization with L(EV) but not by immunization with EV or L.
FIG. 2.
CTL response to EBO-Z 5 weeks after the second immunization, using recombinant vaccinia virus-infected target cells. BALB/c mice were immunized i.m. (A through F) or i.v. (G through L) with 1.4 μg of L(EV) (A, B, G, and H) or with 1.4 μg of EV (C, D, I, and J) or L (E, F, K, and L) at 0 and 3 weeks. Splenic lymphocytes were collected 5 weeks after the boost and stimulated in vitro with either EBO-Z GP peptide 1 or 2, as described in Materials and Methods. Standard 5-h CTL assays were performed using peptide-cultured effector cells and 51Cr-labeled syngeneic (H-2d) P815 cells infected with either recombinant vaccinia virus expressing Ebola virus GP (• and ▪) or wild-type control vaccinia virus (○ and □). Effector/target ratios ranged from 12.5:1 to 100:1. Percentages of specific lysis were calculated as described in Materials and Methods. Data represent the means of triplicate cultures of a representative experiment.
Protective efficacy of L(EV) in mice.
The most direct means of evaluating L(EV) as a successful vaccine in mice was to assess the ability of L(EV) to confer protection against weight loss and death after lethal EBO-Z challenge. Two experiments (Table 2) were performed, in which groups of mice were immunized twice, either by the i.m. or by the i.v. route. Mice were then challenged with 300 LD50 of mouse-adapted EBO-Z at 1, 4, or 5 weeks after the second immunization. The two experiments gave similar results (Table 2) (Fig. 3A). All naïve mice (negative controls) died after challenge. In contrast, immunization with L(EV) by the i.v. route completely prevented illness and death in 64/64 mice. Mice immunized i.m. with L(EV) were partially protected. Even though all mice in the latter group became ill after challenge, 33/43 (77%) survived, a result differing significantly from that for the placebo group. Immunization with EV was even less protective, with an overall survival rate of 55% for i.v. and 45% for i.m. immunization. The percent survival differed significantly from that of the placebo group when mice were challenged 1 week but not 5 weeks after the boost (Table 2). In individual experiments, because of small group sizes, groups immunized with L(EV) i.m. or with EV i.v. or i.m. did not differ significantly from each other in total numbers of mice surviving and in mean times to death (Table 2). In all experiments, the average duration of survival of those immunized mice that died after challenge tended to be longer than that of negative controls, reflecting partial protection (Fig. 3A). Although the results of immunization with EV i.v. were statistically significant compared to those with L (P < 0.001), only 40% of the mice were protected against live EBO-Z challenge. The results of immunization with L(EV) were statistically significant compared to those with EV i.v. (P < 0.001), and 100% protection was obtained. Encapsulation of irradiated EBO-Z in L is thus superior to immunization with EV alone.
TABLE 2.
Immunization with L(EV) induces protection in BALB/c micea
| Expt no. | Challenge week | Immunogen (immunization method) or status | No. morbid (ruffled fur) vs total no. | No. surviving vs total no. | % Survival | Significance vs L i.v. (P)b | MTDc ± SD |
|---|---|---|---|---|---|---|---|
| 1 | 1 | L(EV) i.v. | 0/10 | 10/10 | 100 | <0.001 | |
| L(EV) i.m. | 10/10 | 10/10 | 100 | <0.001 | |||
| L i.v. | 10/10 | 0/10 | 0 | 6.0 ± 0.94 | |||
| L i.m. | 10/10 | 0/10 | 0 | 5.3 ± 0.67 | |||
| Naive | 10/10 | 0/10 | 0 | 5.2 ± 0.79 | |||
| 4 | L(EV) i.v. | 0/14 | 14/14 | 100 | <0.001 | ||
| L(EV) i.m. | 13/13 | 11/13 | 85 | <0.001 | 7.5 ± 0.71 | ||
| L i.v. | 14/14 | 0/14 | 0 | 5.7 ± 0.95 | |||
| L i.m. | 14/14 | 0/14 | 0 | 5.8 ± 0.42 | |||
| Naive | 10/10 | 0/10 | 0 | 5.4 ± 0.84 | |||
| 2 | 1 | L(EV) i.v. | 0/20 | 20/20 | 100 | <0.001 | |
| L(EV) i.m. | 10/10 | 7/10 | 70 | <0.01 | 8.7 ± 0.58 | ||
| EV i.v. | 10/10 | 7/10 | 70 | <0.01 | 6.7 ± 0.58 | ||
| EV i.m. | 10/10 | 5/10 | 50 | <0.05 | 7.2 ± 1.1 | ||
| L i.v. | 10/10 | 0/10 | 0 | 6.4 ± 0.84 | |||
| L i.m. | 10/10 | 0/10 | 0 | 5.8 ± 0.92 | |||
| 5 | L(EV) i.v. | 0/20 | 20/20 | 100 | <0.001 | ||
| L(EV) i.m. | 10/10 | 5/10 | 50 | <0.05 | 9.7 ± 1.5 | ||
| EV i.v. | 10/10 | 4/10 | 40 | <0.05 | 7.5 ± 1.5 | ||
| EV i.m. | 10/10 | 4/10 | 40 | <0.05 | 6.7 ± 1.2 | ||
| L i.v. | 10/10 | 0/10 | 0 | 5.9 ± 1.1 | |||
| L i.m. | 10/10 | 0/10 | 0 | 5.8 ± 1.1 |
BALB/c mice were immunized with L(EV), EV, or L on days 0 and 21 or left untreated. Mice were challenged on day 28, 49, or 56 with 10 PFU (300 LD50) of mouse-adapted EBO-Z.
Values represent results obtained using Fisher's exact test.
MTD, mean time to death of animals that died. SD, standard deviation.
FIG. 3.
Protective efficacy of L(EV) in mice. Groups of 15 BALB/c mice were either immunized with L(EV) or EV or L on days 0 and 21 or left untreated. Five weeks after the second immunization, mice were challenged with 300 LD50 of mouse-adapted EBO-Z. (A) Percent survival of groups of 15 mice immunized and challenged as described above. (B) Viral titer in serum of five mice from each group killed on day 4 postchallenge. Each symbol represents an individual mouse. (C) Serum AST and ALT levels of pooled serum samples obtained on day 4 from mice challenged 1 week (• and ⧫) or 5 weeks (○ and ◊), respectively, after the second immunization. Normal values represent pooled serum from five naïve mice.
In the second experiment, the titer of circulating virus in the serum (five mice per group) was determined on day 4 postchallenge. While control mice immunized with L developed titers in the range of 109 PFU/ml, no virus was detected in sera of mice immunized i.v. with L(EV) (Fig. 3B). Three of the mice immunized i.m. with L(EV) also remained free of viremia, while the other two had titers around 103 PFU/ml. In contrast, all animals immunized i.v. with EV showed viremias in the range of 103 to 106 PFU/ml and those immunized i.m. had titers in the range of 105 to 108 PFU/ml.
Serum AST concentrations showed a pattern resembling that of viremia, reflecting the degree of viral replication and hepatic cell injury (Fig. 3C). Negative-control mice immunized with L had marked AST elevations on day 4. In contrast, animals immunized i.v. or i.m. with L(EV), as well as those immunized i.v. with EV, showed no increase in AST levels compared to those of sera from normal mice. Animals immunized i.m. with EV had intermediate values.
Contribution of T-cell subsets to protection.
Complete protection in mice was achieved by i.v. administration of L(EV) not only 1 week but also 5 weeks after the booster immunization (Table 2). To further understand the mechanism of solid protection induced, we assessed the function of CD4+ and CD8+ T lymphocytes in inducing resistance to EBO-Z challenge.
Mice were injected with anti-CD4 (GK1.5) or anti-CD8 (53.10.72) MAbs either before or after soon after i.v. immunization with L(EV). Flow cytometry studies (Fig. 4, experiment 1) showed that treatment with anti-CD8 and anti-CD4 antibodies caused a 99.9% reduction in the levels of circulating CD8+ T cells (Fig. 4, experiment 1) and splenic CD4+ T cells (Fig. 4, experiment 2), respectively. Mice treated with anti-CD4 antibodies either before or soon after i.v. immunization with L(EV) developed high viral titers on day 4 postchallenge, became ill, lost weight, and died (Table 3). In contrast, mice treated with anti-CD8 MAbs remained healthy after challenge and had very low levels of circulating virus on day 4, ranging from undetectable to 103 PFU/ml (Table 3).
FIG. 4.
Flow cytometric analysis of lymphocytes from L(EV)-immunized mice. Peripheral blood lymphocytes (Experiment 1) or splenic lymphocytes (Experiment 2) from naïve mice or anti-CD4-treated mice or anti-CD8-treated mice and then immunized with L(EV) were stained with fluorescein isothiocyanate-labeled anti-CD3, anti-CD4, and anti-CD8. Nonspecific labeling (shaded area, experiment 1) was determined by using an isotype-matched, fluorescein isothiocyanate-labeled antibody. Cells were analyzed on a FACSCalibur instrument using CellQuest software. The percentages of positive stained cells are shown (Experiment 2). Peripheral blood lymphocytes or spleens were processed individually from three mice/group. The data shown are from an individual mouse from each group.
TABLE 3.
Requirement of T-cell subsets for protectiona
| Expt no. | Vaccine | Antibody | Treatment days | Challenge day | No. surviving vs total no. | MTD ± SDb | Log10 (viremia)c (significance [P])d |
|---|---|---|---|---|---|---|---|
| 1 | L(EV) | None | None | 28 | 10/10 | >21 | 1.7 |
| L(EV) | Anti-CD4 | −3, −2, −1, 17 to 20 | 28 | 0/10 | 6.6 ± 0.52 | 7.8 (<0.00001) | |
| L(EV) | Anti-CD8 | −3, −2, −1, 17 to 20 | 28 | 10/10 | >21 | 2.7 (>0.05) | |
| L(EV) | Anti-CD4 + anti-CD8 | −3, −2, −1, 17 to 20 | 28 | 0/10 | 6.4 ± 0.52 | ND | |
| L(EV) | Anti-CD4 | 4, 11, 18, 25 | 28 | 1/10 | 7.1 ± 0.33 | 7.7 (<0.00001) | |
| L(EV) | Anti-CD8 | 4, 11, 18, 25 | 28 | 9/9 | >21 | 1.8 (>0.05) | |
| L | None | None | 28 | 0/10 | 5.0 ± 0.67 | 8.3 (<0.00001) | |
| None | Anti-CD4 | −3, −2, −1, 17 to 20 | 28 | 0/10 | 5.2 ± 0.79 | ND | |
| None | Anti-CD8 | −3, −2, −1, 17 to 20 | 28 | 0/10 | 5.7 ± 1.2 | ND | |
| 2 | L(EV) | None | None | 28 | 10/10 | >21 | |
| L(EV) | Anti-CD4 | −3, −2, −1, 17 to 20 | 28 | 0/10 | 6.6 ± 0.55 | ||
| L(EV) | Anti-CD8 | −3, −2, −1, 17 to 20 | 28 | 10/10 | >21 | ||
| L(EV) | Anti-CD4 | 25, 26, 27 | 28 | 10/10 | >21 | ||
| L(EV) | Anti-CD8 | 25, 26, 27 | 28 | 10/10 | >21 | ||
| None | None | None | 28 | 0/10 | 5.8 ± 1.0 | ||
| None | Anti-CD4 | 25, 26, 27 | 28 | 0/10 | 6.2 ± 0.79 | ||
| None | Anti-CD8 | 25, 26, 27 | 28 | 0/10 | 6.7 ± 0.48 | ||
| L(EV) | None | None | 56 | 10/10 | >21 | ||
| L(EV) | Anti-CD4 | 53, 54, 55 | 56 | 9/10 | 7.0 | ||
| L(EV) | Anti-CD8 | 53, 54, 55 | 56 | 10/10 | >21 | ||
| None | None | None | 56 | 0/10 | 5.3 ± 0.68 |
Mice were immunized with L(EV) on days 0 and 21 and challenged on either day 28 or 56 with 10 PFU (300 LD50) of mouse-adapted EBO-Z. Mice received either anti-CD4 or anti-CD8 MAbs or no treatment on the indicated days. Controls were either vaccinated with L(EV) and not treated or not vaccinated but treated with MAbs or immunized with L alone. In experiment 1, five mice from some of the groups were killed on day 32 (4 days after challenge) and serum viral titers were determined.
MTD, mean time to death in days of animals that died. SD, standard deviation.
Log10 (mean serum viral titer) on day 4 postchallenge.
Significance of difference in mean serum viral titer from that of the L(EV)-immunized, untreated group, by two-way t test. ND, not done.
Similar results were obtained when the experiment was repeated with anti-CD4 and anti-CD8 antibody administration 3 days prior to immunization and 3 days prior to the booster injection (Table 3). However, treatment with either anti-CD4 or anti-CD8 MAbs 3 days before a live Ebola virus challenge did not abolish protection (Table 3). Furthermore, 5 weeks after the booster immunization, treatment with anti-CD4 antibodies 3 days before a live Ebola virus challenge also did not abolish protection (Table 3).
IFNγ production by CD4+ T cells.
The above results clearly indicate the importance of CD4+ T cells in inducing protection during the induction phase following L(EV) priming and boosting. To determine if these CD4+ T cells were making IFNγ, naïve and immune spleen cells as well as purified CD4+ and CD8+ T cells were tested by ELISPOT assay for the production of IFNγ. As shown in Fig. 5A, in vitro restimulation of spleen cells obtained from mice 3 weeks after the L(EV) boost resulted in a three- to fivefold increase in IFNγ production over spontaneous background levels. Purified CD4+ T cells from L(EV)-immunized mice also produced IFNγ (four- to fivefold increase over background) in response to EV and the putative CTL epitopes, peptides P1 and P2. In contrast, there was no IFNγ production by purified immune CD8+ T cells in response to in vitro stimulation with the antigen or the peptides. There was a further increase in the number of cells stimulated to produce IFNγ even 7 weeks after the boost (Fig. 5B). Again the response was specific to CD4+ T cells. No IFNγ production was elicited from naïve spleen cells or purified naïve CD4+ or CD8+ T cells in response to in vitro stimulation with the antigens. To conclusively prove that IFNγ production was due to CD4+ T cells, spleen cells were prepared from immunized mice or mice pretreated with either anti-CD4 or anti-CD8 antibodies and then immunized and boosted with L(EV). IFNγ production was seen only in immunized mice that were either untreated (three- to fourfold increase compared to background) or treated with anti-CD8 antibodies (seven- to eightfold increase over background). Treatment of mice with anti-CD4 antibodies either before or soon after i.v. immunization with L(EV) eliminated the IFNγ response (Fig. 5C). These results were similar to those obtained by using purified immune CD4+ and CD8+ T cells, as shown in Fig. 5A and B. IFNγ production from CD4+ T cells correlated with protection (Table 3), in that protection was consistently seen in mice immunized with L(EV) and either left untreated or treated with anti-CD8 antibodies. The results of challenge with live virus, together with the ELISPOT data, strongly suggest a role for CD4+ T cells and IFNγ in inducing protection.
FIG. 5.
Production of IFNγ by CD4+ T cells. BALB/c mice were immunized i.v. with L(EV) or pretreated with anti-CD4 or anti-CD8 MAbs and then immunized with L(EV). Spleen cells from immunized mice (three mice/group) were isolated 3 weeks (A) and 7 weeks (B) after the boost. Total immune and naïve spleen cells, as well as purified CD4+ T cells and CD8+ T cells, were purified and stimulated in vitro with EV, P1, and P2 for 18 h. The number of cells activated to secrete IFNγ was determined by ELISPOT assay. (C) Spleen cells from L(EV)-immunized anti-CD4- and anti-CD8-pretreated BALB/c mice were isolated 2 weeks after the boost and processed as described above for determination of antigen-specific IFNγ spots. Naïve spleen cells served as the negative control.
Immunization and challenge of monkeys.
The success of i.v. immunization with L(EV) in mice encouraged us to test the same preparation in a limited experiment in cynomolgus monkeys. Three animals immunized i.v. with L(EV) (monkeys 1, 2, and 3) developed EBO-Z-specific ELISA titers of 800, 400, and 400, respectively, and PRNT50 titers of 40, 80, and 40, respectively, on day −28 before challenge (Table 4). No antibodies were detected in a control animal immunized with L (monkey 4).
TABLE 4.
Pre- and postchallenge parameters for cynomolgus monkeysa
| Monkey | Day −28
|
Day +5
|
Day of death | ||
|---|---|---|---|---|---|
| PRNT50b | ELISA (titers)c | Log10 (viremia)d | AST (U/ml)e | ||
| 1 | 40 | 800 | 6.6 | 68 | 7 |
| 2 | 80 | 400 | 2.3 | 31 | 7 |
| 3 | 40 | 400 | <1.7 | 36 | 11 |
| Control | <10 | <100 | 7.0 | 774 | 7 |
Cynomolgus monkeys were immunized as described in Materials and Methods. Monkeys were bled on day −28 with respect to EBO-Z challenge and on day +5 postchallenge. Individual serum samples were analyzed by ELISA for antibodies, for neutralizing antibodies, for circulating virus, and for AST enzyme.
Reciprocal endpoint.
Endpoint titer against EBO-Z/95.
Log10 (circulating virus titer).
Aspartate aminotransferase concentration.
On day 4 after challenge with EBO-Z/95, monkeys 1 to 3 were still active but monkey 4 (control) appeared ill. On day 5, all animals were febrile and monkey 4 had a rash over most of its body. On day 6, monkeys 1, 2, and 4 all appeared depressed and dehydrated, and all three were found dead the next morning. Monkey 3 was still active on day 7 but was severely ill by day 10, with a rash on the upper and lower limbs, and was found dead the morning of day 11. The three immunized monkeys showed a delay in onset of viremia, corresponding to the delay in onset of illness, with respect to that of the negative control (Fig. 6A). A similar delay was observed in the increase in serum AST concentrations (Fig. 6B) and ALT concentrations (not shown).
FIG. 6.
Postchallenge parameters of cynomolgus monkeys immunized with L(EV) and challenged with EBO-Z/95. Three monkeys were immunized with L(EV) (monkeys 1, 2, and 3) and one with L (monkey 4). (A) Log10 (serum viral concentration). Values on day 7 are postmortem for monkeys 1, 2, and 4. (B) Serum AST concentrations of the same animals; postmortem sera were not tested.
DISCUSSION
Several animal studies demonstrate an apparent requirement for both cell-mediated and humoral responses for the induction of protection against live Ebola virus challenge (10, 27, 33). Development of an effective Ebola virus vaccine should therefore include antigen preparations that induce not only humoral immunity but also cell-mediated immune responses. The results of the present study demonstrate that immunization with L(EV) induces complete protection against lethal EBO-Z challenge in BALB/c mice and that the protective response is dependent on IFNγ-producing CD4+ T lymphocytes. However, the mechanism of protective immune responses elicited is still unclear. It is possible that protection is dependent on three major elements: IFNγ, CD4+ T lymphocytes, and nonneutralizing antibodies.
Based on the previous successful induction of antibody and CTL responses to several L-encapsulated antigens containing lipid A (20, 21, 23, 30), we hypothesized that our immunization strategy of delivering all EBO-Z proteins by encapsulating irradiated EBO-Z in L containing lipid A would provide protection against subsequent viral challenge. In the present study, mice immunized i.v. with L(EV) were completely protected against a 300-LD50 EBO-Z challenge. In contrast to previous studies (17, 27), in which mice immunized with DNA or replicon vaccines became viremic postchallenge, L(EV) prevented the development of viremia. Our inability to detect neutralizing antibodies in L(EV)-immunized mice is consistent with the results of other vaccine studies, as well as with the recent report that mice are protected by GP-specific MAbs that fail to show activity in PRNT50 assays (17, 27, 31).
We identified two peptides (LYDRLASTVI and EYLFEVDNL), consisting of ten and nine amino acid sequences, respectively, in the amino terminus of the Ebola virus GP protein (amino acids [aa] 161 to 169 and aa 231 to 239) as CTL epitopes in BALB/c (H-2d) mice. CTLs specific to these two GP peptides of Ebola virus were induced only in those mice that had been immunized with L(EV) but not in mice immunized with EV. The fact that L(EV) induced GP-specific CTLs suggests that L(EV) may also induce CTLs specific for other EBO-Z proteins, including NP that may also be involved in conferring protection.
In a recent study, CD8+ CTLs have been shown to protect mice immunized with a single Ebola virus protein (NP) against Ebola virus challenge (32). In the present work, all the EBO-Z viral proteins were delivered by encapsulating irradiated Ebola virus in L-containing lipid A, as opposed to immunization with a single protein. Under these circumstances, treatment with anti-CD8 MAbs did not eliminate protection, since more than one protective mechanism was probably active. Furthermore, our studies use BALB/c mice that differ from C57BL/6 mice at the MHC. Therefore, the CTL epitopes and the CTL responses would be different due to the MHC restriction. Using L(EV), the presence of CD8+ CTLs in B10.BR mice has previously been demonstrated (21). However, in that study the ability of CTLs to protect against live Ebola virus challenge was not evaluated. Also, in another study (31), MAbs to Ebola virus GP were sufficient for protecting C57BL/6 mice from a lethal Ebola virus challenge. The results for all these studies again emphasize the strain differences and argue that several mechanisms are involved in inducing protection against Ebola virus infections.
Our study demonstrates that establishment of protective immunity required the presence of CD4+ T cells during the vaccination period, since administration of anti-CD4 MAbs before and during L(EV) immunization prevented induction of a protective immune response. This contrasted with the result obtained when anti-CD4 or -CD8 MAbs were given after vaccination, in which case the MAbs had no significant effect. A similar result was obtained with the Toxoplasma gondii murine model system. In that study, C57BL/6 mice treated with anti-CD4 MAbs during vaccination showed a complete loss of resistance to challenge, whereas anti-CD4 given after vaccination was ineffective in preventing the loss of resistance (7).
In the present study, it is not certain which function is being eliminated by antibody treatment, since there are a range of types of CD4+ T cells. Thus, the critically important cells could be either MHC class I- or II-restricted CD4+ T cells that are specific to GP or to another viral protein. It is also possible that antibody treatment ablates CD4+ NK1.1 T cells that play a critical role in nonspecific resistance to infection. Antigen-specific IFNγ production in vitro was seen only with immune CD4+ T cells. The responses could be recalled even 7 weeks after the boost. It is possible that immunization with L(EV) drives the lymphocytes towards a dominant IFNγ-producing CD4+ T cell. It will be interesting to learn, in future research, whether immunity induced by recombinant GP or NP is eliminated by treatment with anti-CD4 or anti-CD8 antibodies.
The results with mice thus encouraged us to test the same approach in nonhuman primates in a limited experiment. Although, in the single experiment performed, the immunized animals did develop neutralizing antibodies against EBO-Z, they showed little evidence of protection against subsequent viral challenge. Since we did not perform CTL assays on the monkeys, we cannot report on the efficacy of L(EV) in inducing cell-mediated immune responses in primates.
The discordant results between mice and monkeys, with respect to both antibody induction and protection, highlight the difficulty of extrapolating vaccine data from rodents to primates. In general, the outcome of viral challenge in immunized animals is determined by two factors: the vigor of innate antiviral resistance to infection and the strength of the acquired antigen-specific immune response. Rodents and primates differ markedly in their innate resistance to filovirus infections (3). This difference is best illustrated by the fact that EBO-Z isolates from human patients do not cause significant disease in mature mice or guinea pigs. These viruses must first be adapted to lethal virulence through serial animal-to-animal passage (4, 9). Nonhuman primates, in contrast, are susceptible to uniformly lethal infection with very low doses of nonpassaged isolates of EBO-Z.
The outcome of our preliminary experiment in monkeys provides two lessons. The first is that the presence of neutralizing antibodies does not guarantee protection against EBO-Z infection. The fact that the monkeys showed only minimal resistance to challenge is consistent with previous studies demonstrating a weak prophylactic or therapeutic effect of passively transferred neutralizing antiserum and adds some support to the argument that antibodies may be less important than cellular immune mechanisms for protection.
The second lesson is that proper presentation of Ebola virus proteins may be the most fruitful route to vaccination to elicit a strong protective response. Even though our vaccine showed partial efficacy in its present form, standardization of the type of adjuvant and the dose administered could result in better protection in monkeys. An immunization scheme based on GP and NP has been reported to protect monkeys at a very low challenge dose (26). This fact encourages us to continue our research to evaluate L encapsulation or novel liposomal oil-in-water emulsions (15) as a method for efficiently delivering recombinant GP or other Ebola virus proteins for presentation to both the humoral and cellular arms of the immune system.
Acknowledgments
We thank Kevin Anderson for providing the vaccinia virus constructs. We thank Julie Wilson for performing the ELISA and PRNT assays from the mouse sera. We acknowledge the excellent technical assistance of Elaine Morrison in performing all immunization and bleeding procedures in mice.
REFERENCES
- 1.Alving, C. R., S. Shichijo, I. Mattsby-Baltzer, R. L. Richards, and N. M. Wassef. 1993. Preparation and use of liposomes in immunological studies, p. 317-343. In G. Gregoriadis (ed.), Liposome technology. CRC Press, Inc., Boca Raton, Fl.
- 2.Alving, C. R., V. Koulchin, G. M. Glenn, and M. Rao. 1995. Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol. Rev. 145:5-31. [DOI] [PubMed] [Google Scholar]
- 3.Bray, M. 2001. The role of the type I interferon response in the resistance of mice to filovirus infection. J. Gen. Virol. 82:1365-1373. [DOI] [PubMed] [Google Scholar]
- 4.Bray, M., K. Davis, T. Geisbert, C. Schmaljohn, and J. Huggins. 1998. A mouse model for evaluation of prophylaxis and therapy of Ebola hemorrhagic fever. J. Infect. Dis. 178: 651-661. [DOI] [PubMed] [Google Scholar]
- 5.Bray, M., S. Hatfill, L. Hensley, and J. Huggins. 2001. Haematological, biochemical and coagulation changes in mice, guinea pigs and monkeys infected with a mouse-adapted variant of Ebola Zaire virus. J. Comp. Pathol. 125:243-253. [DOI] [PubMed] [Google Scholar]
- 6.Chain, B. M., L. Sealy, D. R. Katz, and M. Brinks. 1994. Antigen processing and presentation, p. 177-194. In P. J. Delves (ed.), Cellular immunology LabFax. Academic Press, New York, N.Y.
- 7.Denkers, E. Y., T. Scharton-Kersten, S. Barbieri, P. Caspar, and A. Sher. 1996. A role for CD4+NK1.1+ T lymphocytes as major histocompatibility complex class II independent helper cells in the generation of CD8+ effector function against intracellular infection. J. Exp. Med. 184:131-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann, H., W. Slenczka, and H. D. Klenk. 1996. Emerging and reemerging of filoviruses. Arch. Virol. Suppl. 11:77-100. [DOI] [PubMed] [Google Scholar]
- 9.Gibb, T., M. Bray, T. Geisbert, K. Steele, K. Davis, and N. Jaax. 2001. Pathogenesis of experimental Ebola Zaire virus infection in BALB/c mice. J. Comp. Pathol. 125:233-242. [DOI] [PubMed] [Google Scholar]
- 10.Gilligan, K. J., J. B. Geisbert, P. B. Jahrling, and K. Anderson. 1997. Assessment of protective immunity conferred by recombinant vaccinia viruses to guinea pigs challenged with Ebola virus, p. 87-92. In F. Brown, D. Burton, P. Doherty, J. Mekalanos, and E. Norrby (ed.), Vaccines. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 11.Gupta, M., S. Mahanty, M. Bray, R. Ahmed, and P. E. Rollin. 2001. Passive transfer of antibodies protects immunocompetent and immunodeficient mice against lethal Ebola virus infection without complete inhibition of viral replication. J. Virol. 75:4649-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hevey, M., D. Negley, J. B. Geisbert, P. B. Jahrling, and A. Schmaljohn. 1997. Antigenicity and vaccine potential of Marburg virus glycoprotein expressed by baculovirus recombinants. Virology 239:206-216. [DOI] [PubMed] [Google Scholar]
- 13.Jahrling, P. B., J. Geisbert, J. R. Swearengen, G. P. Jaax, T. Lewis, J. W. Huggins, J. J. Schmidt, J. W. LeDuc, and C. J. Peters. 1996. Passive immunization of Ebola virus-infected cynomolgus monkeys with immunoglobulin from hyperimmune horses. Arch. Virol. Suppl. 11:135-140. [DOI] [PubMed] [Google Scholar]
- 14.Jahrling, P. B., T. W. Geisbert, J. B. Geisbert, J. R. Swearengen, M. Bray, N. K. Jaax, J. W. Huggins, J. W. LeDuc, and C. J. Peters. 1999. Evaluation of immune globulin and recombinant interferon-alpha 2b for treatment of experimental Ebola virus infections. J. Infect. Dis. 179(Suppl. 1):S224-S234. [DOI] [PubMed] [Google Scholar]
- 15.Muderhwa, J. M., G. R. Matyas, L. E. Spitler, and C. R. Alving. 1999. Oil-in-water liposomal emulsions: characterization and potential use in vaccine delivery. J. Pharm. Sci. 88: 1332-1339. [DOI] [PubMed] [Google Scholar]
- 16.Peters, C. J., A. Sanchez, P. E. Rollin, T. G. Ksiazek, and F. A. Murphy. 1996. Filoviridae: Marburg and Ebola viruses, p. 1161-1176. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology. Lippincott-Raven, Philadelphia, Pa.
- 17.Pushko, P., M. Bray, G. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and A. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 18.Rao, M., S. W. Rothwell, N. M. Wassef, R. E. Pagano, and C. R. Alving. 1997. Visualization of peptides derived from liposome-encapsulated proteins in the trans Golgi area of macrophages. Immunol. Lett. 59:99-105. [DOI] [PubMed] [Google Scholar]
- 19.Rao, M., and C. R. Alving. 1998. Class I presentation of liposomal antigens, p. 15-25. In D. D. Lasic and D. Papahadjopoulos (ed.), Medical applications of liposomes. Elsevier, New York, N.Y.
- 20.Rao, M., and C. R. Alving. 2000. Delivery of lipids and liposomal proteins to the cytoplasm and Golgi of antigen-presenting cells. Adv. Drug Deliv. Rev. 41:171-188. [DOI] [PubMed] [Google Scholar]
- 21.Rao. M., G. R. Matyas, F. Grieder, K. Anderson, P. B. Jahrling, and C. R. Alving. 1999. Cytotoxic T lymphocytes to Ebola Zaire virus are induced in mice by immunization with liposomes containing lipid A. Vaccine 17:2991-2998. [DOI] [PubMed] [Google Scholar]
- 22.Romero, P., G. Corradin, I. F. Luescher, and J. L. Maryanski. 1991. H-2Kd restricted antigenic peptides share a simple binding motif. J. Exp. Med. 174:603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothwell, S. W., N. M. Wassef, C. R. Alving, and M. Rao. 2000. Proteasome inhibitors block entry of liposome-encapsulated antigens into the classical MHC class I pathway. Immunol. Lett. 74:141-152. [DOI] [PubMed] [Google Scholar]
- 24.Sanchez, A., M. P. Kiley, B. P. Holloway, and D. D. Auperin. 1993. Sequence analysis of the Ebola virus genome: organization, genetic elements, and comparison with the genome of Marburg virus. Virus Res. 29:215-240. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez, A., S. G. Trappier, B. W. J. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 93:3602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Zang, and G. J. Nabel. 2000. Development of a protective vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 27.Vanderzanden, L., M. Bray, D. Fuller, T. Roberts, D. Custer, K. Spik, P. Jahrling, J. Huggins, A. Schmaljohn, and C. Schmaljohn. 1998. DNA vaccines expressing either the GP or NP genes of Ebola virus protect mice from lethal challenge. Virology 246:134-144. [DOI] [PubMed] [Google Scholar]
- 28.Verma, J. N., M. Rao, S. Amselem, U. Krzych, C. R. Alving, S. J. Green, and N. M. Wassef. 1992. Adjuvant effects of liposomes containing lipid A: enhancement of liposomal antigen presentation and recruitment of macrophages. Infect. Immun. 60:2438-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wassef, N. M., C. R. Alving, and R. L. Richards. 1994. Liposomes as carriers for vaccines. Immunomethods 4:217-222. [DOI] [PubMed] [Google Scholar]
- 30.White, W. I., D. R. Cassatt, J. Madsen, S. J. Burke, R. M. Woods, N. M. Wassef, C. R. Alving, and S. Koenig. 1995. Antibody and cytotoxic T-lymphocyte responses to a single liposome-associated peptide antigen. Vaccine 13:1111-1122. [DOI] [PubMed] [Google Scholar]
- 31.Wilson, J. A., M. Hevey, R. Bakken, S. Guest, M. Bray, A. L. Schmaljohn, and M. K. Hart. 2000. Epitopes involved in antibody-mediated protection from Ebola virus. Science 287:1664-1666. [DOI] [PubMed] [Google Scholar]
- 32.Wilson, J. A., and M. K. Hart. 2001. Protection from Ebola virus mediated by cytotoxic T lymphocytes to the viral nucleoprotein. J. Virol. 75:2660-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu, L., A. Sanchez, Z.-Y. Yang, S. R. Zaki, E. G. Nabel, S. T. Nichol, and G. J. Nabel. 1998. Immunization for Ebola virus infection. Nat. Med. 4:37-42. [DOI] [PubMed] [Google Scholar]