Abstract
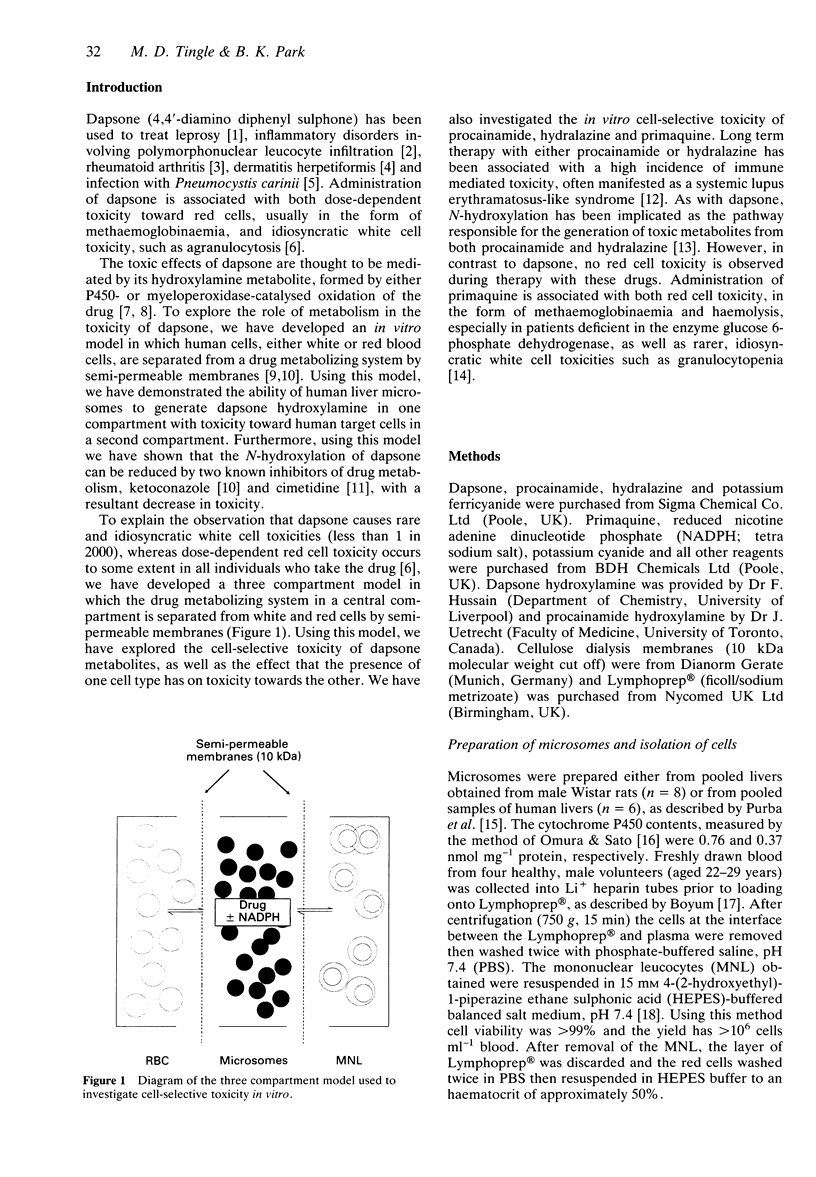
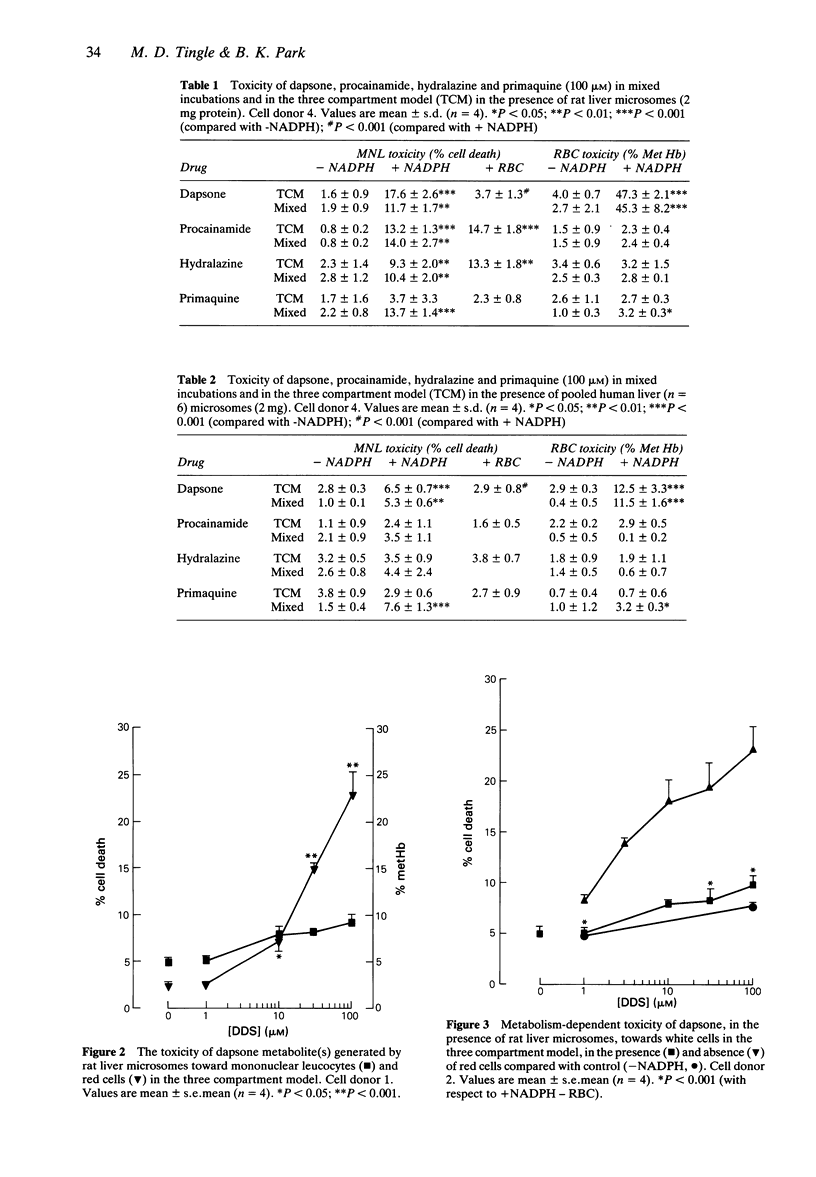
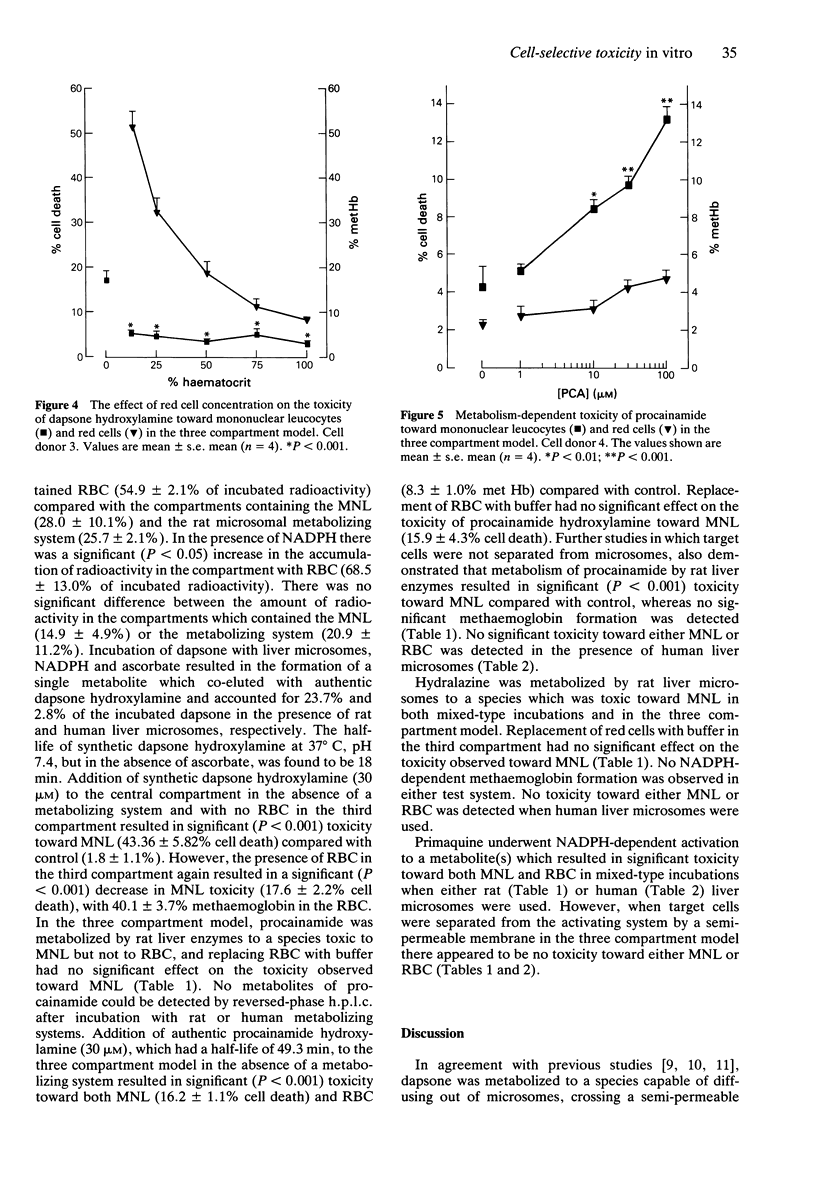
1. N-hydroxylation is thought to be an essential step in the haemotoxicity of dapsone (DDS). To investigate both metabolism-dependent and cell-selective drug toxicity in vitro we have developed a three-compartment system in which an hepatic drug metabolizing system is contained within a central compartment separated by semipermeable membranes from compartments containing mononuclear leucocytes (MNL) and red blood cells (RBC). 2. Metabolism of dapsone (100 microM) by rat liver microsomes resulted in toxicity to RBC cells (47.3 +/- 2.1% methaemoglobin), but there was no significant toxicity toward MNL (3.7 +/- 1.3% cell death) compared with control values (1.6 +/- 0.9%). However, when RBC were replaced with buffer in the third compartment there was significantly greater (P < 0.001) white cell toxicity (17.6 +/- 0.6% cell death), demonstrating the protection of MNL by RBC. Metabolism of dapsone by human liver microsomes again resulted in RBC toxicity (12.5 +/- 3.3% methaemoglobin) but no significant MNL toxicity (2.9 +/- 0.8% cell death). Replacement of RBC resulted in a significant (P < 0.001) increase in MNL toxicity (6.5 +/- 0.7% cell death). Addition of synthetic dapsone hydroxylamine (30 microM) in the absence of a metabolizing system and with no RBC in the third compartment resulted in significant (P < 0.001) toxicity toward MNL (43.36 +/- 5.82% cell death) compared with control (1.8 +/- 1.1%). The presence of RBC in the third compartment resulted in a significant (P < 0.001) decrease in MNL toxicity (17.6 +/- 2.2% cell death), with 40.1 +/- 3.7% methaemoglobin in the RBC.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey E., Brooks A. G., Bird I., Farmer P. B., Street B. Monitoring exposure to 4,4'-methylenedianiline by the gas chromatography-mass spectrometry determination of adducts to hemoglobin. Anal Biochem. 1990 Nov 1;190(2):175–181. doi: 10.1016/0003-2697(90)90177-b. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Separation of lymphocytes, granulocytes, and monocytes from human blood using iodinated density gradient media. Methods Enzymol. 1984;108:88–102. doi: 10.1016/s0076-6879(84)08076-9. [DOI] [PubMed] [Google Scholar]
- Coleman M. D., Breckenridge A. M., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite by human hepatic microsomal enzymes. Br J Clin Pharmacol. 1989 Oct;28(4):389–395. doi: 10.1111/j.1365-2125.1989.tb03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glader B. E., Conrad M. E. Hemolysis by diphenylsulfones: comparative effects of DDS and hydroxylamine-DDS. J Lab Clin Med. 1973 Feb;81(2):267–272. [PubMed] [Google Scholar]
- Grindulis K. A., McConkey B. Rheumatoid arthritis: the effects of treatment with dapsone on hemoglobin. J Rheumatol. 1984 Dec;11(6):776–778. [PubMed] [Google Scholar]
- Harrison J. H., Jr, Jollow D. J. Role of aniline metabolites in aniline-induced hemolytic anemia. J Pharmacol Exp Ther. 1986 Sep;238(3):1045–1054. [PubMed] [Google Scholar]
- Kramer P. A., Glader B. E., Li T. K. Mechanism of methemoglobin formation by diphenylsulfones. Effect of 4-amino-4'-hydroxyaminodiphenylsulfone and other p-substituted derivatives. Biochem Pharmacol. 1972 May 1;21(9):1265–1274. doi: 10.1016/0006-2952(72)90288-2. [DOI] [PubMed] [Google Scholar]
- Lang P. G., Jr Sulfones and sulfonamides in dermatology today. J Am Acad Dermatol. 1979 Dec;1(6):479–492. doi: 10.1016/s0190-9622(79)80088-2. [DOI] [PubMed] [Google Scholar]
- Lee B. L., Medina I., Benowitz N. L., Jacob P., 3rd, Wofsy C. B., Mills J., 5th Dapsone, trimethoprim, and sulfamethoxazole plasma levels during treatment of Pneumocystis pneumonia in patients with the acquired immunodeficiency syndrome (AIDS). Evidence of drug interactions. Ann Intern Med. 1989 Apr 15;110(8):606–611. doi: 10.7326/0003-4819-110-8-606. [DOI] [PubMed] [Google Scholar]
- Link C. M., Theoharides A. D., Anders J. C., Chung H., Canfield C. J. Structure-activity relationships of putative primaquine metabolites causing methemoglobin formation in canine hemolysates. Toxicol Appl Pharmacol. 1985 Nov;81(2):192–202. doi: 10.1016/0041-008x(85)90155-3. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Perry H. M., Jr, Tan E. M., Carmody S., Sakamoto A. Relationship of acetyl transferase activity to antinuclear antibodies and toxic symptoms in hypertensive patients treated with hydralazine. J Lab Clin Med. 1970 Jul;76(1):114–125. [PubMed] [Google Scholar]
- Powell R. D. The chemotherapy of malaria. Clin Pharmacol Ther. 1966 Jan-Feb;7(1):48–76. doi: 10.1002/cpt19667148. [DOI] [PubMed] [Google Scholar]
- Purba H. S., Maggs J. L., Orme M. L., Back D. J., Park B. K. The metabolism of 17 alpha-ethinyloestradiol by human liver microsomes: formation of catechol and chemically reactive metabolites. Br J Clin Pharmacol. 1987 Apr;23(4):447–453. doi: 10.1111/j.1365-2125.1987.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley R. J., Roberts P., Coleman M. D., Kitteringham N. R., Park B. K. Bioactivation of dapsone to a cytotoxic metabolite: in vitro use of a novel two compartment system which contains human tissues. Br J Clin Pharmacol. 1990 Sep;30(3):417–426. doi: 10.1111/j.1365-2125.1990.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim E. Drug-induced immune-complex disease. Complement Inflamm. 1989;6(2):119–126. doi: 10.1159/000463084. [DOI] [PubMed] [Google Scholar]
- Spielberg S. P. Acetaminophen toxicity in human lymphocytes in vitro. J Pharmacol Exp Ther. 1980 May;213(2):395–398. [PubMed] [Google Scholar]
- Streeter A. J., Timbrell J. A. Enzyme-mediated covalent binding of hydralazine to rat liver microsomes. Drug Metab Dispos. 1983 May-Jun;11(3):179–183. [PubMed] [Google Scholar]
- Sugiyanto, Scharping C. E., McManus M. E., Birkett D. J., Holder G. M., Ryan A. J. The formation of proximate carcinogens from three polycyclic aromatic compounds by human liver microsomes. Xenobiotica. 1992 Nov;22(11):1299–1307. doi: 10.3109/00498259209053158. [DOI] [PubMed] [Google Scholar]
- Tingle M. D., Coleman M. D., Park B. K. An investigation of the role of metabolism in dapsone-induced methaemoglobinaemia using a two compartment in vitro test system. Br J Clin Pharmacol. 1990 Dec;30(6):829–838. doi: 10.1111/j.1365-2125.1990.tb05448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. D., Coleman M. D., Park B. K. The effect of preincubation with cimetidine on the N-hydroxylation of dapsone by human liver microsomes. Br J Clin Pharmacol. 1991 Jul;32(1):120–123. doi: 10.1111/j.1365-2125.1991.tb05623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. P., Sweetman B. J., Woosley R. L., Oates J. A. Metabolism of procainamide to a hydroxylamine by rat and human hepatic microsomes. Drug Metab Dispos. 1984 Jan-Feb;12(1):77–81. [PubMed] [Google Scholar]
- Uetrecht J., Sokoluk B. Comparative metabolism and covalent binding of procainamide by human leukocytes. Drug Metab Dispos. 1992 Jan-Feb;20(1):120–123. [PubMed] [Google Scholar]
- Uetrecht J., Zahid N., Shear N. H., Biggar W. D. Metabolism of dapsone to a hydroxylamine by human neutrophils and mononuclear cells. J Pharmacol Exp Ther. 1988 Apr;245(1):274–279. [PubMed] [Google Scholar]
- Wheeler J. F., Adams L. E., Mongey A. B., Roberts S. M., Heineman W. R., Hess E. V. Determination of metabolically derived nitroprocainamide in the urine of procainamide-dosed humans and rats by liquid chromatography with electrochemical detection. Drug Metab Dispos. 1991 May-Jun;19(3):691–695. [PubMed] [Google Scholar]
- Zuidema J., Hilbers-Modderman E. S., Merkus F. W. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986 Jul-Aug;11(4):299–315. doi: 10.2165/00003088-198611040-00003. [DOI] [PubMed] [Google Scholar]


