Abstract
The envelope glycoprotein of human immunodeficiency virus type 1 (HIV-1), gp160, is synthesized as a protein precursor that when proteolytically cleaved yields two subunits, gp120 and gp41. gp120 is the surface glycoprotein on HIV-1 responsible for binding to CD4, and gp41 is the transmembrane glycoprotein involved in the membrane fusion process. gp41 is divided into the N-terminal fusion peptide, the heptad repeat 1 (HR1) and HR2 regions, and the C-terminal transmembrane region, which are collectively responsible for virus fusion and entry into the cell. Synthetic peptides derived from the HR2 and HR1 regions of HIV-1LAI have been shown to prevent virus-cell fusion and infection in vitro. In phase II clinical trials in HIV patients, data revealed that T20 has antiviral efficacy and is well tolerated. Similar results were obtained in vitro with HIV-2 and simian immunodeficiency virus, supporting the conservation of the gp41 ectodomain among lentiviruses. Feline immunodeficiency virus (FIV) infection in the cat has been used as a model to develop potential antivirals for HIV. To determine if synthetic gp40 analogs capable of inhibiting FIV infection could be identified, 15 overlapping 35-amino-acid peptides derived from the C-terminal HR2 domain of FIV gp40 were synthesized. These peptides were tested for efficacy against FIV in a syncytium-forming assay with FIV-infected CrFK cells and HeLa cells expressing the FIV receptor CXCR4. Several peptides exhibited activity at the nanogram level. Antiviral activity was confirmed by suppression of reverse transcriptase in a FIV feline CD4+-T-cell (FCD4-E) acute-infection assay. These data demonstrate that synthetic peptides derived from the HR2 domain of the FIV gp41 protein are effective inhibitors of FIV infection.
The envelope protein, gp160, of human immunodeficiency virus type 1 (HIV-1) is synthesized as the polyprotein precursor and is proteolytically cleaved to yield two subunits, gp120 and gp41. The gp120 subunit is a surface glycoprotein (SU) responsible for receptor binding, and gp41 is a transmembrane glycoprotein (TM) responsible for viral membrane fusion with the host cell membrane (7). The gp41 subunit contains different functional regions in its ectodomain: the fusion peptide (FP), heptad repeat 1 (HR1), HR2, and the transmembrane region.
When gp120 of human immunodeficiency virus (HIV) binds to CD4 receptors, it induces conformational changes in gp41 that expose the hydrophobic region of gp41, which is essential for membrane fusion activity (8). Proteolysis studies have shown that the coiled C- and coiled N-terminal regions of the gp41 ectodomain interact to form a highly stable helical trimeric complex of heterodimers with the HR1 and HR2 regions oriented in an antiparallel manner. The interaction of these domains is required for membrane fusion and infection of the host cell (1, 3, 4, 12, 13). When analogous experiments were performed with the gp41 ectodomain of simian immunodeficiency virus (SIV), the results were almost identical, supporting the idea that these functional regions of the gp41 ectodomain are conserved among lentiviruses (1, 13).
Coiled-coil or helical domains appear to be a generalized feature of viral fusion proteins, and this motif has been exploited in generating antiviral fusion agents for a number of viruses, including HIV and several members of the paramyxovirus family (11, 22, 23).
Studies with HIV-1 demonstrate that synthetic peptides representing discrete regions of the fusion protein (gp41) effectively block virus-mediated cell-cell fusion, as well as virus infection of T cells, by blocking the HR1-HR2 interaction that is necessary for fusion. Two synthetic peptides, designated T21 (DP107) and T20 (DP178), were shown to be potent inhibitors of HIV-1-mediated cell-cell fusion (22, 23). T21, derived from an amino acid sequence adjacent to the gp41 fusion peptide sequence, was shown by circular dichroism to have a stable coiled-coil structure in phosphate-buffered saline and also was able to block HIV infection and fusion at concentrations of <5 μg/ml (22). Importantly, when a proline residue was substituted within the amino acid sequence of T21, the coiled-coil structure was lost and the mutant T21 no longer blocked fusion or infection (22). This finding establishes a clear structure-function relationship between the coiled-coil conformation and the antiviral properties of T21.
The peptide T20, derived from an amino acid sequence just amino terminal to the gp41 transmembrane region, completely blocked virus-mediated cell-cell fusion at 1 to 2 ng/ml (23). Although analysis of the T20 primary amino acid sequence predicts a coiled-coil structure, at pH 7 the synthetic peptide contains only about 20% helical structure as determined by circular dichroism (12). Nonetheless, this peptide displays unprecedented antiviral activity (23).
Similar HR2 peptides have been designed, tested, and demonstrated to be active inhibitors of SIV and HIV-2 (S. Barney, personal communication). In addition, Carr and Kim identified a coiled-coil domain as the key structural element involved in triggering influenza hemagglutinin-mediated membrane fusion (2).
As the Env protein of FIV, a related lentivirus, is processed in a manner similar to those of HIV and SIV (7), and as FIV causes cell fusion (6, 19, 24), its gp40 protein (TM) was expected to have similar heptad repeat structure-function motifs. Experiments were initiated with FIV based on the idea that the HIV-1 gp41 ectodomain and the fusion process are similar for all lentiviruses. Therefore, the antiviral activity observed using synthetic peptides from the C-terminal region of the gp41 ectodomain (for inhibiting HIV and SIV) must also be present in FIV. To test this idea, computerized antiviral searching technology (CAST), an amino acid sequence motif search strategy based on the predicted secondary structure of T20 and T21, was used to define areas in FIV Env that were homologous to the domains in the gp41 protein of HIV based on which the HIV antiviral peptides were designed. Overlapping 35-amino acid (aa) peptides from the HR2 region in FIV were designed. Selected peptides from this walk were synthesized and tested for antiviral activity. The results of this study demonstrate that synthetic peptides selected from the T20-like domain of FIV are effective antivirals in vitro.
MATERIALS AND METHODS
Cell lines and cell culture.
The CrFK-P+ cell line (Crandell feline kidney cells persistently infected with a CrFK-adapted strain of FIV-Petaluma) (stock number ATCC-CCL-94; American Type Culture Collection [ATCC]) was maintained in Dulbecco's modified Eagle medium (high glucose; Gibco BRL-Life Technologies, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/ml), penicillin (100 IU/ml), l-glutamine (2 mM), HEPES (15 mM), and sodium pyruvate (2 mM) at 37°C in 5% CO2. The HeLa cell line (stock number ATCC-CCL-2; ATCC) was maintained in Dulbecco's modified Eagle medium (high glucose) supplemented as described above. The feline CD4-E (FCD4-E) cell line (an interleukin 2 [IL-2]-dependent feline CD4+ lymphocyte cell line described by English et al. [6]) was maintained in RPMI 1640 medium (Gibco BRL-Life Technologies) supplemented with 10% FBS, streptomycin (100 μg/ml), penicillin (100 IU/ml), l-glutamine (2 mM), HEPES (15 mM), sodium pyruvate (2 mM), β-mercaptoethanol (2.5 × 10−5 M), and recombinant human IL-2 (rhIL-2) (100 U/ml; catalog number 0801023; Zeptometrix Corporation Cellkines) at 37°C in 7% CO2. MOLT4 cells (stock number ATCC-CRL-1582; ATCC) were maintained in RPMI 1640 medium supplemented with 20% FBS, streptomycin (100 μg/ml), penicillin (100 IU/ml), and l-glutamine (2 mM). CEM4/IIIb cells (provided by Trimeris, Inc.) were maintained in RPMI 1640 medium supplemented with 10% FBS, streptomycin (100 μg/ml), penicillin (100 IU/ml), and l-glutamine (2 mM).
Virus isolates.
FIV-NCSU1 (clade A), isolated from a naturally FIV-infected, feline leukemia virus-seronegative cat, was used in the infectivity assay and has been described previously (19). A virus stock was prepared by infecting FCD4-E cells with this isolate and harvesting the supernatant. Supernatant with a high 50% tissue culture infective dose (3.54 × 106) was filtered (0.2-μm pore size) and stored at −80°C. FIV-Petaluma (clade A) was used to chronically infect CrFK cells as previously described (16, 25). FIV-PPR (clade A) is a molecular clone derived from a cat in San Diego, Calif. (17). FIV-TM2 (clade B) is an isolate from Japan (14).
CAST identification of anti-FIV peptides.
CAST is an algorithm that identifies helical motifs from primary amino acid sequences based on the predicted secondary structure (11). This computer search strategy has been used successfully to identify biologically active peptides in both the T21-like (HR1) and T20-like (HR2) domains of several viruses, including respiratory syncytial virus, measles virus, human parainfluenza virus 3, SIV, HIV-1, and HIV-2 (11, 22). Domains within the FIV envelope that were predicted to form helical structures similar to those of T20 and T21 in HIV-1 gp41 were identified by analyzing the primary amino acid sequences of the FIV gp40 of Petaluma, TM2, PPR, and NCSU1 virus isolates using the CAST motif searching strategy. Potential helical domains identified in these publicly available sequences were compared with the gp40 sequence of FIV-NCSU1. Overlapping peptides, 35 aa in length, from FIV-NCSU1 were designed from the area identified as the T20-like domain (HR2), and 15 peptides from this walk were selected to be synthesized. Once purified, the peptides were tested for antiviral activity in the FIV syncytial assay and the FCD4-E infectivity reverse transcriptase (RT) assay. The most potent compounds were also checked for cross-reactivity in the HIV syncytial assay.
Peptide synthesis.
Peptides were synthesized at Trimeris, Inc., on a Rainin Symphony Multiplex multiple peptide synthesizer. Rink-amide 4-methylbenzhydryl amine resin (Nova Biochemicals) served as the solid phase and provided carboxy-terminal blockade. Briefly, each 9-fluorenylmethoxy cabonyl amino acid (Rainin or Nova Biochemicals) that is appropriately protected was activated. After coupling, the new N-terminal amino acid residue was deprotected. The resins were washed in preparation for the next cycle and addition of another residue. Following complete synthesis, the amino terminus was blocked through automated acetylation on the synthesizer. Peptides were purified by reverse-phase high-pressure liquid chromatography. Verification of peptide purity and composition was performed by analytic high-pressure liquid chromatography (Trimeris, Inc.). The peptides were each given a unique identifier beginning with T. Peptide concentrations were determined using the method of Edelhoch (5).
FIV syncytial assay.
FIV mediates syncytium formation. The assay described here utilizes HeLa cells, as they express CXCR4 (24), the only known receptor for FIV, and have been shown to fuse with persistently infected CrFK cells (24). HeLa cells were plated in a 96-well flat-bottom plate at a concentration of 10,000 per well in 100 μl of HeLa medium and allowed to adhere overnight. The following day, the culture medium was aspirated and peptide was added in a dilution series in 50 μl of medium. CrFK-P+ cells (5 × 102 in 150 μl of medium) were added to give reproducible numbers of syncytia. After 24 h, the plates were fixed and stained with crystal violet (stock stain, 2.5 g of crystal violet, 1.25 g of Giemsa stain, 500 ml of 80% methanol; working solution, 200 ml of stock and 200 ml of 80% methanol). Stained syncytial plaques (fused cells that are five cell diameters or greater) are counted using a stereo dissecting microscope. Dose-response curves are generated, and 50 and 90% effective concentrations (EC50 and EC90) for the peptides are determined. When the number of syncytia formed within 24 h is compared to the number of CrFK-P+ cells plated, there is a linear correlation between the number of infected cells plated and the number of syncytia produced, suggesting that only approximately 10 to 14% of the CrFK-P+ cells create syncytia.
FCD4-E infectivity assay for RT.
The FCD4-E lymphocyte infectivity assay was used to assess the abilities of peptides to inhibit productive FIV infection as measured by RT production. FCD4-E cells were plated at 50,000 to 100,000 per well in 100 μl of RPMI 1640 medium (supplemented with 10% FBS, 10 mM HEPES, 2 mM l-glutamine, 0.075% sodium bicarbonate, 2.5 × 10−5 M 2-mercaptoethanol, penicillin [100 U/ml], streptomycin [100μg/ml], and IL-2 [100 U/ml; kindly provided by the Biological Resources Branch of the National Institutes of Health]). The compounds to be tested were added in 50 μl of FCD4-E medium (without rhIL-2), and then NCSU1 virus at a multiplicity of infection of 0.1 was added in 50 μl of FCD4-E medium (without rhIL-2). After incubation for 5 days at 37°C in 5% CO2, samples of the supernatant were harvested, lysed in Triton X-100 (final concentration, 1%), and assayed for RT activity (23).
RT assay.
Samples of supernatant treated with Triton X-100 were assayed for RT activity as described previously (15). Briefly, 10 μl of a test sample was added to 50 μl of RT reaction mixture and incubated in a 37°C water bath for 2 h. The RT reaction buffer (for 20 samples) consisted of 859.5 μl of double-distilled H2O, 50 μl of 1 M Tris buffer (pH 8.0), 20 μl of 0.25 M MgCl2, 7.5 μl of 1 M potassium chloride (KCl), 2 μl of 1 M dithiothreitol, 10 μl of poly(A) · oligo(dT), 50 μl of Nonidet P-40, and 1 μl of α-32P-labeled-ATP. The products (10 μl) of each reaction were spotted on D81 ion-exchange paper in triplicate and allowed to dry for 30 min. The papers were washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) four times for 5 min each time and allowed to dry for another 30 min. The RT blots were read by a Molecular Dynamics PhosphorImager and analyzed by ImageQuant. The values were corrected for background, and pixel intensity values were used to quantitate positive versus negative wells and to determine EC50s and EC90s. The viral titer derived at each concentration of peptide was determined using the Reed-Muench (18) calculation and compared to the infected control titer. The concentration of peptide that reduced the viral titer by 1 log unit is the concentration of peptide at which Vn/VO is 0.1, where Vn equals the titer in the presence of concentration x of inhibitor and VO equals the titer in the absence of inhibitor. The Karber method (10) was used to determine the EC50 in relation to the pixel intensity determined for each well.
HIV syncytial assay.
The HIV syncytial assays were performed as described by Wild et al. (22). Dose-response curves were generated, and the EC50s were calculated as stated above. In brief, MOLT-4 cells (∼7 × 104) were incubated with CEM4 cells (1.0 × 104 to 1.2 × 104) chronically infected with HIV-1 IIIb in 96-well plates (half-area cluster plates) in 80 μl of culture medium. Serial dilutions of peptide inhibitors were added in 10 μl of deionized water, and the inhibitor-cell mixtures were incubated for 18 to 24 h at 37°C and 5% CO2. Multinucleated cells, syncytia, were counted using an inverted microscope.
Cytotoxicity assay.
Measurement of cell viability in the presence and absence of potential antiviral compounds was carried out using the metabolic reduction of XTT {2,3-bis[2-methoxy-4-nitro-5-sulfonphenyl]-5-[(phenylamino)carbonyl]-2H-tetrazoliumhydroxide} to a soluble brown product (XTT-Formazan) in surviving and metabolizing cells as previously described (20). Previously prepared monolayers (HeLa cells) were incubated with and without titrated peptide solutions for 24 h. At the end of incubation, XTT was added. Following color development, the A450 of each well was measured and compared to those of untreated controls. The results were expressed as a percentage of the absorbance of the untreated control. The 50% toxic dose (the concentration at which XTT reduction is equal to 50% of the untreated control) was determined by the Karber method (10). The selectivity index was calculated by dividing the 50% toxic dose of the compound by the EC50 of the same compound.
RESULTS
Identification of gp40 HR2 FIV peptides.
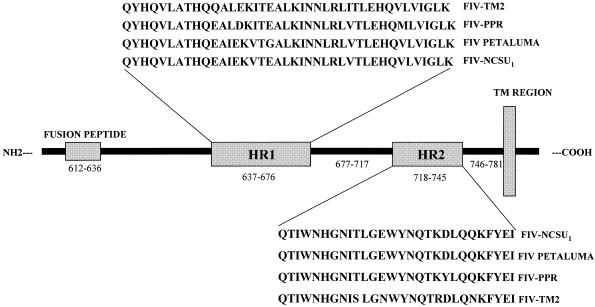
In this study, we used CAST to identify conserved heptad repeat domains in the FIV gp40 sequences from the Petaluma, TM2, and PPR isolates of FIV. The following T21-like (HR1) and T20-like (HR2) regions were identified by this approach: FIV-Petaluma, aa 650 to 680 (HR1) and 722 to 749 (HR2); FIV-TM2, aa 640 to 679 (HR1) and 721 to 748 (HR2); and FIV-PPR, aa 639 to 668 (HR1) and 720 to 747 (HR2). The gp40 sequences from the FIV isolates were aligned with the FIV-NCSU1 isolate, and the heptad repeat regions for the FIV-NCSU1 isolate were determined to be aa 631 to 676 (HR1) and 718 to 745 (HR2) (Fig. 1). HR1 peptides were not synthesized. Fifteen HR2 peptides for the NCSU1 isolate were synthesized (Fig. 2). A peptide-scanning method that involved synthesizing overlapping peptides covering the HR2 region of FIV gp40 was used to identify biologically active peptides. A 35-aa peptide (covering five heptads) was used as a standard length based on the lengths of HIV T20 and T21 (36 and 36 aa, respectively) and for ease of synthesis (11). It has been shown that peptides from this area have greater antiviral activity than peptides from the HR1 domain (11, 12, 22). Figure 2 shows the FIV-NCSU1 HR2 peptide walks.
FIG. 1.
Map of NCSU1, Petaluma, PPR, and TM2 FIV gp41 proteins showing HR1 and HR2 as defined by CAST.
FIG. 2.
FIV-NCSU1 HR2 peptide walks. The overlapping walks were designed to walk through the HR2 domain that was defined by CAST by moving a single amino acid over in the amino-terminal-to-carboxy-terminal direction. This walk covers additional amino acids on both the amino and carboxy ends of the HR2 domain. The walks begin with aa 714 and proceed through aa 776. The sequences depicted here are the peptides that were actually synthesized and tested against virus.
HR2 peptides block FIV-mediated cell membrane fusion.
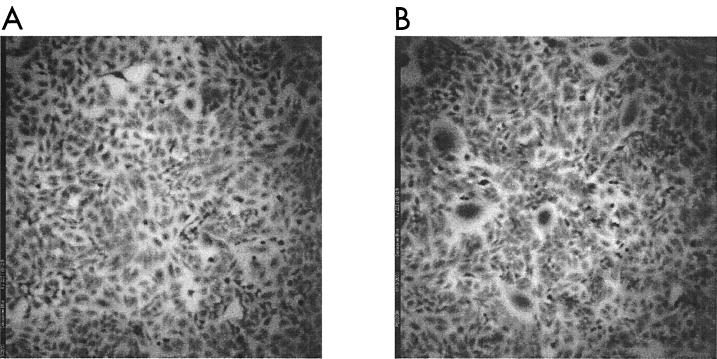
Peptide preparations were analyzed for the ability to prevent syncytium formation caused by the fusion of persistently infected CrFK cells with CXCR4-positive HeLa cells in the syncytial plaque-forming assay. Figure 3A shows the uninfected HeLa monolayer, and Fig. 3B shows an example of the syncytial plaques seen in the infected CrFK HeLa coculture. Figure 4 shows a typical fusion inhibition assay with peptide T1569.
FIG. 3.
FIV syncytial plaque-forming assay measures FIV-induced cell-cell fusion. (A) Uninfected HeLa cell monolayer. (B) HeLa cell monolayer infected by overlaying it with persistently infected CrFK cells at a concentration of 500 per well and incubating it overnight at 37°C.
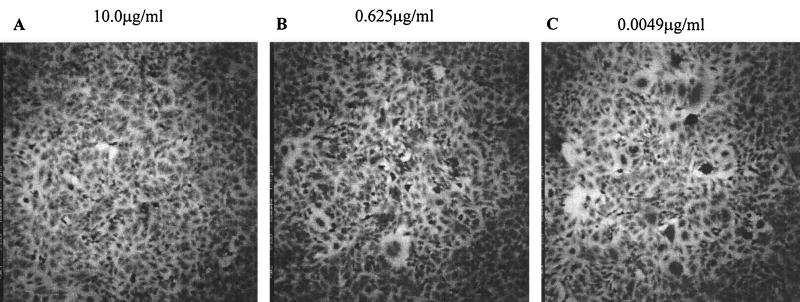
FIG. 4.
HR2 peptides inhibit FIV-mediated cell fusion. T1569 is used here to give an example of the inhibition seen with an active peptide. (A) Complete inhibition of virus infection. (B) As the peptide concentration is diluted, syncytium formation is observed. (C) Complete loss of virus inhibition by T1569 compared to the infected control seen in Fig. 3B. The T1569 concentration is shown above each panel.
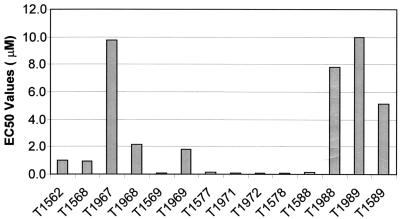
The EC50s presented in Table 1 indicate that the peptides tested exhibited a range of antiviral activities. T1971 and T1972 had the greatest antiviral activities of the peptides tested and exhibited EC50s of 0.01 μM each. The most carboxy-terminal peptide, T1566, adjacent to the transmembrane region, showed no activity up to 2.346 μM. Although active, T1562, the most amino-terminal peptide in the walk, showed significantly decreased antiviral activity (>27-fold higher) compared to the most active peptides, T1971 and T1972. The antiviral activity (EC50) for each peptide was plotted in the order that they appear in the walk, amino terminal to carboxy terminal. As can be seen in Fig. 5, the peptides toward the center of the walk are significantly more active than those at either the amino-terminal or carboxy-terminal end.
TABLE 1.
FIV HR2 peptides in the FIV syncytial plaque-forming assay
| Peptide | MWa | EC50 (μM)b | EC90 (μM)b |
|---|---|---|---|
| T1562 | 4,337 | 0.318 | 1.236 |
| T1568 | 4,353 | 0.259 | 0.971 |
| T1967 | 3,972 | 2.454 | >2.518 |
| T1968 | 4,044 | 0.586 | 2.067 |
| T1569 | 4,228 | 0.026 | 0.117 |
| T1969 | 4,002 | 0.435 | 1.413 |
| T1577 | 4,270 | 0.050 | 0.162 |
| T1971 | 4,270 | 0.012 | 0.033 |
| T1972 | 4,340 | 0.012 | 0.054 |
| T1578 | 4,341 | 0.020 | 0.090 |
| T1588 | 4,341 | 0.030 | 0.125 |
| T1988 | 4,307 | 1.990 | >2.322 |
| T1989 | 4,308 | >2.321 | >2.321 |
| T1589 | 4,367 | 1.145 | >2.290 |
| T1566 | 4,262 | >2.346 | >2.346 |
MW, molecular weight.
EC50s and EC90s for each peptide were determined using the Karber method. The most active peptides are boldfaced.
FIG. 5.
EC50 for each peptide in the syncytium formation assay. The peptides are in order from amino terminal to carboxy terminal. The graph represents the area of increased activity in the HR2 peptide walk.
Cytotoxicities of HR2 peptides.
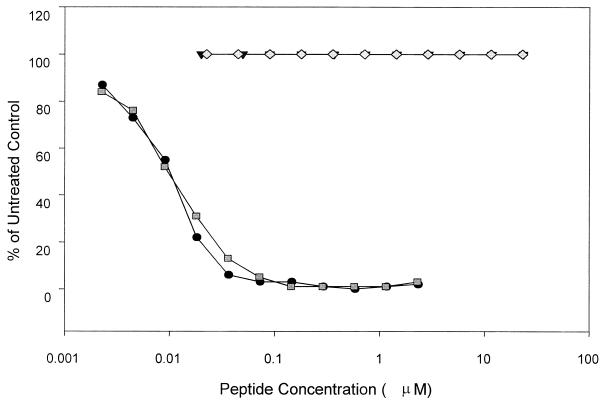
Selected peptides, T1971 and T1972, were assessed for potential toxicity by examining their ability to interrupt the basic metabolism of healthy cells. As previously observed for HIV peptides from this region of gp41, analysis of the FIV peptides in the XTT assay demonstrated no cytotoxic effects at concentrations of the peptide tested up to 23 μM (selectivity index for T1971, >1,961; selectivity index for T1972, >1,852). The results in Fig. 6 show the cytotoxic potentials of T1971 and T1972 compared with the antiviral activities of the same peptides. Comparing the effective concentrations (percentage of infected control) of the peptides with the XTT metabolisms (percentage of untreated control) of the same two peptides demonstrates that the antiviral activities observed for the peptides cannot be attributed to cytotoxic effects of the peptides on the cell.
FIG. 6.
Comparison of FIV peptide antiviral activities versus cytotoxicities. Cytotoxicity is plotted as a percentage of those of the untreated controls (T1971 and T1972). Antiviral activity as measured by syncytium formation is given as a percentage of those of the infected controls (T1971 and T1972).
FCD4-E infectivity assay for RT.
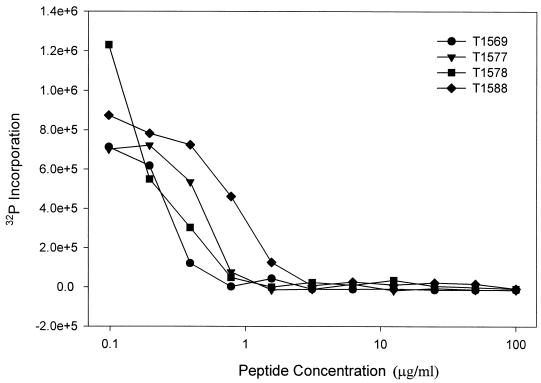
The FIV infectivity assay was used to demonstrate that the peptides are not only effective in preventing virus-induced cell-cell fusion but are also effective in preventing productive infection by FIV, as measured by RT synthesis. Selected peptides were analyzed for the ability to inhibit FIV RT production as a measure of inhibition of virus infection. Figure 7 shows that the selected peptides inhibit FIV infection of FCD4-E cells in a dose-dependent manner. As shown in Table 2, the four peptides tested (T1569, T1577, T1578, and T1588) that were most effective at inhibiting RT production in the FIV-FCD4-E infection assay were also the most active in preventing syncytium formation.
FIG. 7.
FIV infection of FCD4-E cells is inhibited by selected HR2 peptides. The dose-response curves for T1569, T1577, T1578, and T1588 activities against RT production are plotted as the amount of 32P incorporation versus peptide concentration. The EC50s are given in Table 2.
TABLE 2.
Comparison of activities of selected HR2 peptides in plaque-forming and RT infectivity assays
| Peptide | Plaque-forming assaya
|
RT infectivity assaya
|
||
|---|---|---|---|---|
| EC50 (μM) | EC90 (μM) | EC50 (μM) | EC90 (μM) | |
| T1569 | 0.026 | 0.117 | 0.055 | 0.106 |
| T1577 | 0.050 | 0.162 | 0.098 | 0.177 |
| T1578 | 0.020 | 0.090 | 0.053 | 0.157 |
| T1588 | 0.030 | 0.125 | 0.168 | 0.423 |
EC50s and EC90s for each peptide were determined using the Karber method.
Specificities of FIV-derived peptides for FIV versus HIV-1.
The specificities of the FIV HR2 peptides for FIV versus HIV were examined by testing selected peptides in an HIV fusion assay. The HIV syncytial assay measures HIV-1-induced fusion of chronically infected CEM4/IIIb cells with uninfected MOLT4 cells. The ability of a peptide to block this fusion event is measured by counting the number of syncytia (≥5 times the diameter of uninfected cells) compared to the number of syncytia in untreated infected controls. The majority of the FIV HR2 peptides tested exhibited no inhibitory effect on HIV-induced cell fusion at 2.3 μM or below (Table 3). However, T1577 and T1967 showed some activity, with EC50s of 1.933 and 2.053 μM, respectively (Table 3). T1577 has an EC50 of 0.050 μM in the FIV fusion assay, which is more active by >38-fold against the FIV virus isolates tested than against HIV. Interestingly, T1967 has an EC50 of 2.454 μM in the FIV plaque-forming assay, which is less than its EC50 in the HIV fusion assay. When the reverse experiment was done, testing the HIV-1 HR2 peptides T20 and T649 (Table 3) in the FIV syncytial assay, similar results were observed. T20 had an EC50 of 17.587 μM, while T649 exhibited no activity at concentrations up to 22 μM. T20 and T649 both exhibit EC50s of approximately 0.0004 μM in cell-cell fusion assays against HIV-1 IIIb.
TABLE 3.
Activities of FIV HR2 peptides against HIV-1 and HIV HR2 peptides against FIVa
| Target virus | Peptide | EC50 (μM) | EC90 (μM) |
|---|---|---|---|
| HIV-1 | T1569 | >2.365 | >2.365 |
| T1577 | 1.933 | >2.342 | |
| T1967 | 2.053 | >2.518 | |
| T1968 | >2.473 | >2.473 | |
| T1971 | >2.342 | >2.342 | |
| T1972 | >2.304 | >2.304 | |
| T1988 | >2.322 | >2.322 | |
| T1989 | >2.321 | >2.321 | |
| FIV | T20 | 17.587 | >22.262 |
| T649 | >22.070 | >22.070 |
EC50s for FIV HR2 peptides in the HIV fusion assay and for HIV HR2 peptides in the FIV syncytial plaque-forming assay. The EC50s for each peptide were determined using the Karber method. The most active peptides are boldfaced.
DISCUSSION
The HIV TM, gp41, can be divided into different functional regions or domains: FP, HR1, HR2, the transmembrane region, and the cytoplasmic domain. The heptad repeat domains have been shown to interact, a crucial step for viral fusion and entry into the cell (1, 3, 4, 12, 13). Crystal structure analyses by Chan et al. (3) and Weissenhorn et al. (21) demonstrated that the HR2 domain interacts with the trimeric HR1 domain as a six-helix bundle (3, 13). The HR1 domain is a parallel coiled-coil in the interior of the bundle, and the HR2 domain is packed in an antiparallel manner in the grooves of the HR1 trimer. Peptides designed from the HR1 and HR2 domains (T21 and T20, respectively) have been shown to be potent inhibitors of HIV infection of susceptible cells (12, 23).
The goal of the studies reported here was to define the antiviral or biological activities of the HR2 peptides derived from the FIV gp40 protein. We hypothesized that biological activity is indicative of the HR2 peptides binding to the HR1 domain in FIV gp40, as had previously been demonstrated with HIV. Thus, by analogy to the HIV system, inhibition of FIV-induced fusion and infectivity indicates that HR2 peptides bind to the HR1 domain. To address this question, we determined the areas in the FIV TM (gp40) that are similar to the predictive α-helical domains of HIV. This was accomplished by using a computer motif searching program, CAST, based on HIV helical-domain sequences to identify potential helical heptad repeat regions in FIV gp40 sequences (Fig. 2). HIV peptides from the HR2 domain are more potent than peptides from the HR1 domain (11, 12, 23). Therefore, in this study, only peptides derived from the HR2 domains of FIV gp40 were synthesized and tested for antiviral activity against FIV. The initial experiments in the FIV syncytial assay, using CrFK-P+ and HeLa cells, took advantage of the capability of FIV Env to bind to the CXCR4 receptors present on HeLa cells, causing cell fusion and syncytium formation (24). CXCR4 is also a coreceptor for the syncytium-inducing strains of HIV (24). Table 1 shows that peptides from the HR2 domain of FIV exhibit activity against FIV, as demonstrated by inhibition of syncytium formation. The most active peptides are T1971 and T1972, and both exhibit EC50s of 0.01 μM. As with HIV-inhibitory peptides, the trend for the more active FIV HR2 peptides to be located towards the center of the walk and less active peptides to be located at the outer edges of the walk reflects the primary potential interaction sites between the FIV HR1 and HR2 domains. Whether this interaction is trimeric in FIV is not known. Nevertheless, the knowledge that this interaction is a trimer in both HIV (3, 13) and SIV (1) supports the assumption that this would also be true for FIV. However, these peptides were not as active against FIV as T20 is against HIV. By using a fluorescent probe that displays little fluorescence in aqueous solutions and more fluorescence when bound to hydrophobic groups, Jones et al. detected and quantified changes in fluorescence upon the interaction of gp120-gp41-expressing cells and appropriate target cells in the HIV fusion process (9). By utilizing this method, these investigators measured a lag time of approximately 10 min from the maximum fluorescence, due to the conformational changes induced by gp120 binding to CD4 and the appropriate coreceptor in HIV, to the actual start of lipid mixing of the membranes. The transient stage shown in the Kim-Chan model for HIV fusion (4) is supported by this observation. It is possible that this transient intermediate stage lasts for a shorter time in FIV fusion and therefore may require more peptide to compete with the interaction of the HR1 and HR2 domains.
Testing in the FCD4-E infectivity assay using the NCSU1 isolate was done to demonstrate that productive virus infection, as measured by RT, is blocked in the presence of the antiviral peptides that were active in FIV cell-cell fusion assays. The results shown in Fig. 7 and Table 2 confirm that the peptides T1569, T1577, T1578, and T1588 are effective inhibitors of FIV infection as measured by RT production. A comparison of EC50s from the syncytium-forming assay and the infectivity assay is also shown in Table 2. The differences in EC50s suggest that less peptide is required for prevention of virus-induced cell-cell fusion than for FIV infectivity. A similar difference in activity has also been observed for T20 in the fusion assay versus the infectivity assay, although the reasons for this are not yet understood (23). The fusion and infectivity assays are very different, utilizing different cell lines that may account for the observed differences in the EC50s. However, another possible explanation may be reflected in the lengths of time required by the different assays. The fusion assay is a 24-h assay, and the infectivity assay requires 5 days for its incubation period. It is possible that the peptides begin to degrade over time, which would ultimately require more peptide to achieve 50% inhibition.
It has been shown that the NCSU1 FIV isolate is unable to infect CrFK cells (26). Therefore, CrFK cells that were persistently infected with the Petaluma isolate of FIV were used in the syncytium-forming assay. Because the peptides were designed using the NCSU1 isolate gp40 sequence as a template, there was a question as to whether the activities of these peptides against FIV-Petaluma are different from their activities against FIV-NCSU1. Sequence analysis of the two virus isolates showed that only 1 aa was different in the HR1 domain in the Petaluma strain compared to the NCSU1 strain, and no differences were observed in the HR2 domain. There were two amino acid changes observed in the peptide walk outside the HR2 domain. At position 758, there is a K→T change, and at position 771 there is a G→R change. The K change to a T alters the hydrophobicity of the amino acid residue at this site from −3.9 to −0.7, and the G-to-R change alters the hydrophobicity of this amino acid residue from −0.4 to −4.5. These differences appeared not to affect the activities of the peptides, as the peptides most active against FIV-Petaluma in the syncytium-forming assay were also the most active against FIV-NCSU1 in the infectivity assay.
The specificity of the FIV peptides for FIV compared to HIV was addressed by examining their activities against HIV-1 IIIb in a fusion assay. T20 has been tested against a variety of viruses, such as HIV-2 and SIV, and was found to be largely inactive against them. Therefore, it was not unexpected that the FIV peptides were not very active against HIV. As shown in Table 3, two peptides (T1577 and T1967) exhibited some effects on HIV fusion. The EC50 for T1577 in the HIV fusion assay was >38-fold higher than the EC50 observed in the FIV syncytium-forming assay. Conversely, testing of the HIV HR2 peptides T20 and T649 (both highly active against HIV-1) in the FIV fusion assay demonstrated a similar lack of activity against FIV. T20 has an EC50 of 0.0004 μM in the HIV fusion assay (23), while in the FIV syncytial assay, the EC50 is 17.587 μM, an 88,000-fold difference (Table 3). The specificities of the peptides seen here reflect how the peptides are designed. In each case, the peptide that was active for a specific virus was designed based on that virus' sequence. If the primary amino acid sequence of HIV and FIV heptad repeat regions exhibited greater sequence homology, peptides from these regions would most probably display greater antiviral cross-reactivity.
The specificity results are of interest because they demonstrate that, although lentiviruses may have HR2 regions that are similar in secondary structure and function (i.e., important for virus fusion), the primary amino acid sequence of the HR2 domain is a key factor in determining the antiviral activities of these peptides.
In summary, peptide sequences have been successfully identified within discrete regions of the FIV gp40 that are capable of suppressing FIV fusion and infectivity. The searching strategy, implemented by CAST and used successfully in the past to identify common secondary-structure motifs that are analogous to those found in HIV gp41 (11) and in a variety of other viruses (11), has now been shown to be successful for FIV. Studies have demonstrated that enveloped viruses in many cases share a common mechanism that enables the virus to fuse with its host cell membrane (11). The influx of structural and functional information on the HIV-1 fusion process has shown that viral entry is a valid target for antivirals (1, 3, 4, 12, 13). A small-animal model for HIV infection continues to be an important requirement for use in developing therapies for treatment of HIV infection and AIDS in humans. This study demonstrates another area of similarity between HIV and FIV and further supports the potential for FIV as a model for HIV.
Acknowledgments
This work was supported by Public Health Services grant AI43858.
REFERENCES
- 1.Blacklow, S. C., M. Lu, and P. S. Kim. 1995. A trimeric subdomain of the simian immunodeficiency virus envelope glycoprotein. Biochemistry 34:14955-14962. [DOI] [PubMed] [Google Scholar]
- 2.Carr, C. M., and P. S. Kim. 1993. A spring-loaded mechanism for the conformational change of influenza hemagglutinin. Cell 73:823-832. [DOI] [PubMed] [Google Scholar]
- 3.Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. [DOI] [PubMed] [Google Scholar]
- 4.Chan, D. C., and P. S. Kim. 1998. HIV entry and its inhibition. Cell 93:681-684. [DOI] [PubMed] [Google Scholar]
- 5.Edelhoch, H. 1967. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry 6:1948-1954. [DOI] [PubMed] [Google Scholar]
- 6.English, R. V., C. M. Johnson, D. H. Gebhard, and M. B. Tompkins. 1993. In vivo lymphocyte tropism of feline immunodeficiency virus. J. Virol. 67:5175-5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields, B. N., D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.). 1996. Virology. Raven Press, New York, N.Y.
- 8.Hart, T. K., R. Kirsh, H. Ellens, R. W. Sweet, D. M. Lambert, S. R. Petteway, Jr., J. Leary, and P. J. Bugelski. 1991. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 88:2189-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones, P. L. S. J., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 Envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 10.Karber, G. 1931. Beitreg zur kollektiven behandlung pharmakologischer reihenversuche. Arch. Exp. Pathol. Pharmakol. 162:480-483. [Google Scholar]
- 11.Lambert, D. M., S. Barney, A. L. Lambert, K. Guthrie, R. Medinas, D. E. Davis, T. Bucy, J. Erickson, G. Merutka, and S. R. Petteway, Jr. 1996. Peptides from conserved regions of paramyxovirus fusion (f) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 93:2186-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawless, M. K., S. Barney, K. I. Guthrie, T. B. Bucy, S. R. Petteway, Jr., and G. Merutka. 1996. HIV-1 membrane fusion mechanism: structural studies of the interactions between biologically-active peptides from gp41. Biochemistry 35:13697-13708. [DOI] [PubMed] [Google Scholar]
- 13.Lu, M., S. C. Blacklow, and P. S. Kim. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat. Struct. Biol. 2:1075-1082. [DOI] [PubMed] [Google Scholar]
- 14.Maki, N., T. Miyazawa, M. Fukasawa, A. Hasegawa, M. Hayami, K. Miki, and T. Mikami. 1992. Molecular characterization and heterogeneity of feline immunodeficiency virus isolates. Arch. Virol. 123:29-45. [DOI] [PubMed] [Google Scholar]
- 15.Novotney, C., R. V. English, J. Housman, M. G. Davidson, M. P. Nasisse, C. R. Jeng, W. C. Davis, and M. B. Tompkins. 1990. Lymphocyte population changes in cats naturally infected with feline immunodeficiency virus. AIDS 4:1213-1218. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotrophic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 17.Philips, T. R., R. L. Talbott, C. Lamont, S. Muir, K. Lovelace, and J. H. Elder. 1990. Comparison of two host cell range variants of feline immunodeficiency virus. J. Virol. 64:4605-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reed, L. J., E. Read, and H. Muench. 1938. Am. J. Hyg. 27:493-497. [Google Scholar]
- 19.Tompkins, M. B., P. D. Nelson, R. V. English, and C. Novotney. 1991. Early events in the immunopathogenesis of feline retrovirus. J. Am. Vet. Med. Assoc. 199:1311-1315. [PubMed] [Google Scholar]
- 20.Weislow, O. S., R. Kiser, D. L. Fine, J. Bader, R. H. Shoemaker, and M. R. Boyd. 1989. New soluble-formazan assay for HIV-1 cytopathic effects: application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 81:577-586. [DOI] [PubMed] [Google Scholar]
- 21.Weissenhorn, W., A. Dessen, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1997. Atomic structure of the ectodomain from HIV-1 gp41. Nature 387:426-430. [DOI] [PubMed] [Google Scholar]
- 22.Wild, C., T. Oas, C. McDanal, D. Bolognesi, and T. Matthews. 1992. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc. Natl. Acad. Sci. USA 89:10537-10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wild, C. T., D. C. Shugars, T. K. Greenwell, C. B. McDanal, and T. J. Matthews. 1994. Peptides corresponding to a predictive α-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc. Natl. Acad. Sci. USA 91:9770-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett, B. J., L. Picard, M. Hosie, J. D. Turner, K. Adema, and P. R. Clapham. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto, J. K., F. Sparger, E. W. Ho, P. R. Anderson, T. P. O'Connor, C. P. Nandell, L. Lowenstine, R. Munn, and N. C. Pedersen. 1988. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am. J. Vet. Res. 48:1246-1258. [PubMed] [Google Scholar]
- 26.Yang, J. S., R. V. English, J. W. Ritchey, M. G. Davidson, T. Wasmoen, J. K. Levy, D. H. Gebhard, M. B. Tompkins, and W. A. F. Tompkins. 1996. Molecularly cloned feline immunodeficiency virus NCSU1 JSY3 induces immunodeficiency in specific-pathogen-free cats. J. Virol. 70:3011-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]