Abstract
The adenovirus large E1A protein activates transcription from early viral promoters by a mechanism that requires a forty amino acid zinc finger activation domain in E1A conserved region 3 (CR3). Recent results indicate that activation by a Gal4 DNA-binding domain-E1A-CR3 fusion requires an interaction between the E1A-CR3 zinc finger and the Sur2 subunit of the mammalian Mediator (of transcription) complex. Although several host proteins have been shown to bind stably to E1A proteins in adenovirus-infected and -transformed cells, an in vivo interaction with Mediator complex subunits has not been described previously. Using immunoprecipitation and gel filtration analyses of nuclear extracts prepared from HeLa cells infected with adenovirus 5 or mutants that express either large or small E1A specifically and from adenovirus 5-transformed cells, we report here that large E1A, but not small E1A, binds to Mediator complex in vivo. Only ∼1 to 10% of large E1A is bound to Mediator complex at 18 h postinfection and in transformed cells, probably explaining why Mediator complex subunits were not identified among cellular E1A-binding proteins described earlier. Surprisingly, even though extracted Mediator can quantitatively bind to an E1A-CR3 affinity column, only on the order of 1% of cellular Mediator complex is bound by E1A in vivo. Much of the large E1A bound to Mediator in 293 cells is in a stable complex that includes RNA polymerase II, leading us to suggest that the interaction of E1A-CR3 with Mediator stabilizes the interaction of Mediator with the polymerase. This stabilization of the interaction between Mediator and RNA polymerase II may contribute to the mechanism of activation by E1A-CR3.
The adenovirus large E1A protein, one of the first eukaryotic activators discovered, activates transcription from the early viral promoters E1B, E2, E3, and E4 (2, 18, 26, 27, 41). Activation requires the 49-amino-acid E1A conserved region 3 (CR3) (19, 26, 41). CR3 contains an N-terminal 40-amino-acid transcriptional activation domain defined by its ability to activate transcription when bound near a promoter by a fused DNA-binding domain (21, 24). The E1A-CR3 activation domain is thought to be bound to adenovirus early promoters through protein-protein interactions between the C-terminal ∼10 residues of CR3 and the DNA-binding domains of several cellular transcription factors that bind to the viral early promoters (22). The CR3 activation domain includes a four-cysteine zinc finger of unknown structure (7) required for both activation of viral early gene transcription by large E1A (39) and activation by an E1A-CR3-DNA-binding domain fusion (21, 24).
Based on these results, cellular proteins involved in the mechanism of E1A transcriptional activation were anticipated to interact with large E1A protein specifically, as opposed to smaller E1A proteins that lack CR3 translated from alternatively spliced E1A mRNAs (3, 29, 34). Although E1A proteins were found to bind multiple cellular proteins, including the retinoblastoma (Rb) and the Rb-related proteins p107 and p130, p300 and the closely related CBP (1, 10, 11), and CtBP (31), no cellular proteins shown to be involved in the mechanism of E1A-CR3 activation have been identified that bind in vivo specifically to large E1A and not smaller E1A proteins in adenovirus-infected or -transformed cells.
Recent studies have shown that E1A-CR3 binds the mammalian Mediator (of transcription) complex (23, 30) in vitro and that this interaction is required for activation by Gal4-E1A-CR3 and by large E1A in the context of a viral infection. By using a glutathione S-transferase (GST)-CR3 affinity column, E1A-CR3 was found to bind a protein kinase that phosphorylates the RNA polymerase II (Pol II) large subunit carboxy-terminal heptapeptide repeat domain (CTD) in a HeLa cell nuclear extract, whereas a C174S point mutant defective for binding Zn2+ and for activating transcription did not (17). This CTD kinase was determined to be CDK8 (12), a homolog of Saccharomyces cerevisiae Srb10, a subunit of the yeast holoenzyme (20, 28). CDK8 was further shown to be associated with a complex of ∼1.5 MDa, leading to the suggestion that E1A-CR3 binds to the mammalian counterpart of the yeast Mediator (of transcription) subcomplex of the yeast holoenzyme (12).
Similarly, GST fusions to wild-type E1A-CR3 and point mutants that activate transcription, but not to activation-defective point mutants, were found to bind directly to the human homolog of Caenorhabditis elegans Sur2 (5). Whereas E1A-CR3 was initially found to bind monomeric Sur2 partially purified from HeLa nuclear extract, most of the Sur2 in the extract was found to be associated with an ∼1.5-MDa human Mediator (of transcription) complex that also bound specifically to the E1A-CR3 affinity column (5). Purification of the human Mediator complex showed that it contains ∼30 subunits, including CDK8 (5). This finding agreed well with the earlier report that E1A-CR3 binds an ∼1.5-MDa protein complex containing CDK8 and the suggestion that this complex is the human homolog of the yeast Mediator (of transcription) complex (12). The finding that the Mediator complex is required for activation of transcription by Gal4-E1A-CR3 in vitro directly demonstrated the importance of Mediator for the E1A-CR3 activation mechanism (5). Similar mammalian Mediator complexes also were identified by others, either because they bind to activation domain affinity columns or because they include CDK8 (23, 30). Yeast Mediator is required for the function of most, if not all, transcriptional activators in yeast and for the function of many repressors (20, 28). Similarly, mammalian Mediator complexes are required for high-level activation by mammalian activators in vitro (23, 30).
To analyze further the link between CR3 activation and its interaction with human Mediator via the Sur2 subunit, we isolated murine embryonic stem (ES) cells with knockout mutations of both copies of the sur2 gene (35). Activation by Gal4-E1A-CR3 was completely defective in these sur2−/− cells but could be rescued by expression of human Sur2 (35). Expression of E2 mRNA following infection with adenovirus 2 (Ad2) was also extremely defective in sur2−/− compared to wild-type ES cells (35). Gal4-E1A-CR3 was able to recruit Mediator complex to a promoter with Gal4-binding sites from nuclear extract prepared from wild-type ES cells but not from sur2−/− cells (35). We concluded that activation by Gal4-E1A-CR3 requires the recruitment of Mediator complex to a promoter via E1A-CR3-Sur2 interaction (35).
These results on the interaction between E1A-CR3 and the human Mediator complex (5, 12) via the Sur2 Mediator subunit (5), the requirement of Mediator complex for activation by Gal4-E1A-CR3 in vitro (5), and the dependence of E1A-CR3 activation on the Sur2 Mediator subunit in vivo (35) imply that the large E1A protein interacts with human Mediator complex in vivo during the infection of human cells. Yet, as discussed above, Mediator subunits were not found previously among the E1A-binding proteins observed in adenovirus-infected or -transformed cells. In the work reported here, we characterize complexes formed between large E1A and the human Mediator complex in Ad5-infected HeLa cells and in Ad5-transformed 293 cells (13). Although clear evidence for a large E1A-Mediator complex was observed, only a small fraction of total large E1A protein, ∼1 to 10%, was associated with Mediator complex, explaining why subunits of the complex were not detected in earlier studies of E1A-associated proteins.
MATERIALS AND METHODS
Cell culture, viral infection, and nuclear extract preparation.
HeLa and 293 cell suspension cultures were maintained in exponential growth at 37°C in Dulbecco modified Eagle medium with 10% iron-supplemented bovine calf serum. Ad5 mutants pm975 and dl1500 were described previously (26, 27). Mutant viruses were grown and titrated by plaque assay on 293 cells (13). For preparation of nuclear extracts from adenovirus-infected HeLa cells, 10 to 20 liters of exponentially growing HeLa cells were infected with Ad5, pm975, or dl1500 at a multiplicity of infection of 10 by adding virus stock to the suspension culture without concentrating the cells. At 18 h postinfection, cells were collected by centrifugation at 3,000 rpm for 10 min in a DPR-6000 IEC centrifuge at 4°C. Nuclear extracts were prepared as described previously (8), except that the final dialysis in 0.1 M KCl D buffer (20 mM HEPES [pH 7.9], 20% [vol/vol] glycerol, 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 10 mM β-mercaptoethanol) was omitted because the protein precipitate that forms during this dialysis contains a significant fraction of extracted Mediator subunits. Nuclear extract was stored in 1-ml aliquots at −70°C, thawed, and centrifuged at 14,000 rpm for 10 min at 4°C in an Eppendorf centrifuge, and the supernatant was used for immunoprecipitaton or 3 ml was applied to a 100-ml Superose 6 column run in 300 mM KCl D buffer.
Immunoprecipitation and immunoblotting.
A portion (1 ml) of undialyzed nuclear extract was diluted with an equal volume of 300 mM KCl D buffer plus 1% NP-40, followed by incubation with 20 μl of agarose-conjugated antibody (anti-E1A rabbit immunoglobulin G [IgG] or normal rabbit IgG [Santa Cruz Biotechnology] in Fig. 2 and 6; anti-CDK8 goat antibody or normal goat IgG [Santa Cruz Biotechnology] in Fig. 3) for 16 h at 4°C. After centrifugation at 8,000 rpm for 5 min in a Eppendorf centrifuge, the agarose beads were washed two times with 1 ml of 300 mM KCl D buffer plus 0.5% NP-40, one time with phosphate-buffered saline, and then eluted with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and subjected to Western blotting. Antibodies to Med150 and Med95 were generous gifts from Leonard Freedman and Robert Roeder, respectively. Antibodies to CDK8 and cyclin C were from NeoMarkers. Anti-Sur2 monoclonal antibody was from BD Biosciences. Antibodies to Med220, Med6, and P300 were from Santa Cruz Biotechnology. Monoclonal antibody to Pol II large subunit CTD (8WG16) was from Thompson et al. (37). In Fig. 4C, 50 μl of four column purified Mediator fraction (38) was diluted with 100 μl of 300 mM KCl D buffer plus 0.5% NP-40 and immunoprecipitated with 10 μl of protein A-agarose beads conjugated to anti-E1A mouse monoclonal antibody M73 (15). In Fig. 5, 100 μl of nuclear extract from Superose 6 column fractions (1 ml) were subjected to immunoblotting. Immunoblots were developed by using enhanced chemiluminescence reagents from Pierce.
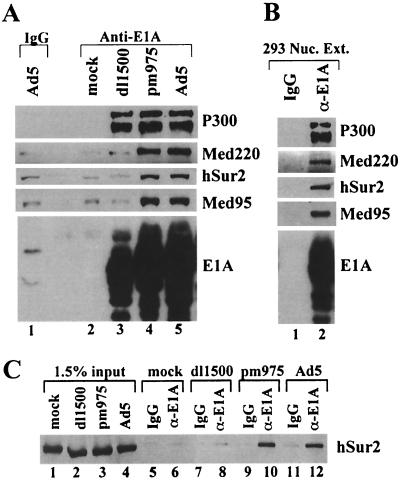
FIG. 2.
Immunoprecipitation of Mediator complex by anti-E1A antibody. (A) Nuclear extracts from mock-, dl1500-, pm975-, or Ad5-infected HeLa cells were immunoprecipitated with anti-E1A antibody (lanes 2 to 5) or normal rabbit IgG (lane 1). Immunoprecipitates were analyzed by Western blotting with the indicated antibodies. (B) 293 cell nuclear extract was immunoprecipitated with anti-E1A antibody (lane 2) or normal rabbit IgG (lane 1). Immunoprecipitates were analyzed by Western blotting. (C) The Sur2 Mediator subunit immunoprecipitated by anti-E1A antibody or normal rabbit IgG, as indicated (lanes 5 to 12), was compared to that of 1.5% of the nuclear extracts used in the immunoprecipitation reactions (lanes 1 to 4).
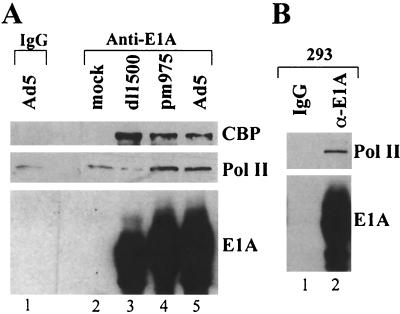
FIG. 6.
RNA Pol II associated with large E1A protein. Nuclear extract prepared from mock- and virus-infected HeLa cells (A) and from 293 cells (B) was immunoprecipitated with anti-E1A antibody or normal rabbit IgG, and immunoprecipitates were analyzed by Western blotting with the indicated antibodies.
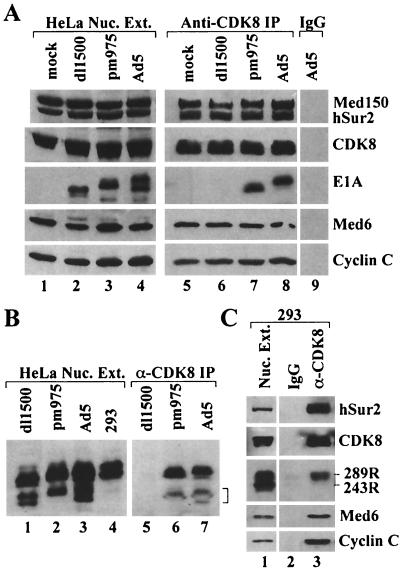
FIG. 3.
Immunoprecipitation of E1A by anti-CDK8 antibody. (A) Nuclear extracts of mock-, dl1500-, pm975-, and Ad5-infected HeLa cells (lanes 1 to 4), anti-CDK8 immunoprecipitates of these extracts (lanes 5 to 8), and a control immunoprecipitate prepared with normal goat IgG (lane 9) were analyzed by Western blotting with the indicated antibodies. For lanes 5 to 8, the exposure time of the E1A Western blot was four times longer than for the Mediator subunit Western blots. (B) Long exposure of E1A Western blots of nuclear extracts and anti-CDK8 immunoprecipitates. The region of the gel corresponding to E1A proteins translated from multiply spliced E1A mRNAs is indicated by a bracket at the right. (C) Western blots of nuclear extract and immunoprecipitates from 293 cells. The E1A Western of lanes 2 and 3 was exposed four times longer than the Mediator subunit Westerns.
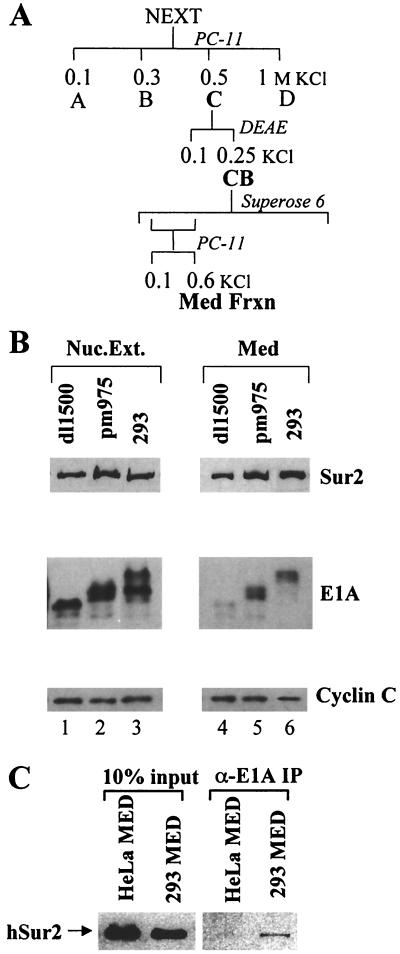
FIG. 4.
E1A present in purified Mediator from infected HeLa cells and 293 cells. (A) Scheme for chromatographic purification of Mediator complex. (B) Immunoblots of nuclear extracts (lanes 1 to 3) and purified Mediator from dl1500- and pm975-infected HeLa cells and 293 cells (lanes 4 to 6). (C) Western blot of Sur2 in purified Mediator from uninfected HeLa and 293 cells and of anti-E1A immunoprecipitates of the purified Mediator preparations. The two lanes at the left contained 10% of the Mediator preparation volumes used in the immunoprecipitations.
FIG. 5.
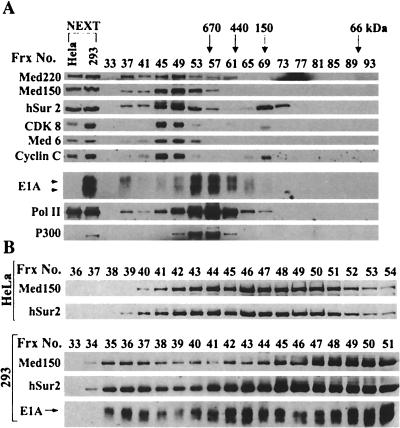
Gel filtration chromatography of E1A and Mediator subunits in 293 cell nuclear extract. (A) 293 cell nuclear extract was fractionated on Superose 6 as described in Materials and Methods. Every fourth column fraction was analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. Column fractions in which the peaks of protein standards (670, 440, 150, and 66 kDa) eluted are indicated at the top of the panel. Uninfected HeLa and 293 nuclear extract were loaded in the left two lanes as standards. (B) High-resolution analysis of the >1-MDa Superose 6 fractions of HeLa and 293 cell nuclear extracts. A total of 100 μl of each 1-ml Superose 6 fraction was subjected to immunoblotting with the indicated antibodies.
Purification of Mediator complex.
Nuclear extracts were prepared from 20 liters of uninfected 293 cells or HeLa cells infected with dl1500 or pm975 and fractionated by chromatography on phosphocellulose and DEAE-Sepharose as described previously (8). Briefly, nuclear extracts were bound to a Whatman P11 phosphocellulose column (10 mg of protein per ml of P11) equilibrated in 0.1 M KCl D buffer. The column was washed with four column volumes 0.1 M KCl D buffer and then step-eluted with two column volumes 0.3, 0.5, and 1 M KCl D buffer. Peak protein fractions eluted at 0.5 M KCl D buffer were dialyzed into 0.1 M KCl D buffer and bound to a DEAE-Sepharose column. After being washed with four column volumes of 0.1 M KCl D buffer, protein was step eluted with 0.3 M KCl D buffer. Next, 2 ml of peak protein fractions was chromatographed on a 100-ml Superose 6 gel filtration column, and 1-ml fractions were collected. The Mediator complex peak was assayed by immunoblotting with anti-Sur2, anti-CDK8, and anti-cyclin C antibodies. Peak fractions were pooled and applied to a 0.3-ml P11 column equilibrated with 0.3 M KCl D buffer. Bound protein was step eluted with 0.6 M KCl D buffer. Peak protein fractions were dialyzed into 0.1 M KCl D buffer.
RESULTS
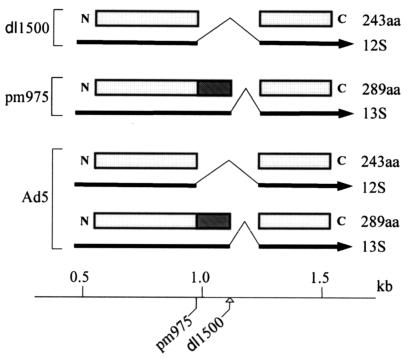
To search for an association between large E1A protein and Mediator complex, nuclear extracts from adenovirus-infected HeLa cells were subjected to immunoprecipitation with an anti-E1A antibody and the immunoprecipitates were analyzed by immunoblotting with antibodies to Mediator subunits. As a control for association specifically with the large E1A protein, cells were infected with Ad5 mutant dl1500 (26), as well as wild-type Ad5, and mutant pm975 (27). Two prominent alternatively spliced E1A mRNAs are expressed during the early phase of infection, 13S and 12S, encoding the large (289-amino-acid) and small (243-amino-acid) E1A proteins, respectively (Fig. 1) (3, 29). Mutant dl1500 contains a nine-base-pair deletion at the 5′ splice site of the 13S mRNA intron, resulting in production of the 12S mRNA exclusively during the early phase of infection (26). Mutant pm975 contains a T→G mutation in the invariant GT at the 5′ end of the 12S mRNA intron, leading to exclusive production of the 13S mRNA during the early phase (27). The mutation results in a synonymous codon at this position in the 13S mRNA. E1A mRNAs with an additional splice are expressed during the late phase of infection (34). However, the dl1500 deletion is expected to prevent splicing of any E1A mRNA containing CR3.
FIG. 1.
Structure of early E1A mRNAs and proteins from wild-type and mutant adenoviruses. The bottom line represents the adenovirus genome demarcated in kilobase pairs from the left end. The locations of the pm975 and dl1500 mutations are indicated. The exons of the E1A mRNAs are represented by the lines joined by caret symbols. Arrowheads indicate the 3′ ends of the mRNAs. The open rectangles represent the common regions of the large (289aa) and small (243aa) E1A proteins. The filled rectangle represents the unique region of the large E1A protein, equivalent to the N-terminal 46 amino acids of the 49-amino-acid conserved region 3 (19). Mutant pm975 contains a T→G mutation in the invariant GT at the 5′ end of the 12S mRNA intron and consequently expresses only the 13S mRNA. Mutant dl1500 contains a nine-base-pair deletion encompassing the 5′ splice site of the 13S mRNA intron and consequently expresses only the 12S mRNA.
Nuclear extracts were prepared at 18 h postinfection, when E1A proteins reach their maximal concentration (33). Immunoblotting of anti-E1A immunoprecipitates with antibodies specific for Mediator complex subunits Med220, Sur2, and Med95 revealed signals well above background for Ad5 and pm975 but not for mock-infected cells or dl1500 (Fig. 2A). P300, which is bound by the N-terminal region common to both the large and small E1A proteins was readily detected in the immunoprecipitate from all virus-infected cells. Specific immunoprecipitation of the Mediator subunits CDK8, cyclin C, Med6, and PAQ (25) were also observed in Ad5- and pm975-infected, but not in mock- or dl1500-infected, cell extracts (data not shown). These results indicate that large E1A, but not small E1A, is associated with Mediator complex in adenovirus-infected cells, as expected since E1A CR3 present exclusively in large E1A binds to the Sur2 Mediator subunit (5). Mediator subunits were also specifically immunoprecipitated by anti-E1A antibody from nuclear extract of Ad5-transformed 293 cells (Fig. 2B). We estimated the fraction of Mediator complexes associated with large E1A protein by comparing the intensity of the Sur2 band in the immunoblot of total nuclear extract to the Sur2 band in the anti-E1A immunoprecipitate (Fig. 2C). Approximately 50% of both large and small E1A proteins were immunoprecipitated in these analyses (data not shown). Based on this and the data in Fig. 2C, we estimate that only on the order of 1% of the total Mediator complex in the nuclear extract from Ad5- and pm975-infected cells was associated with large E1A protein.
Nuclear extract was also subjected to immunoprecipitaton with an antibody to Mediator subunit CDK8 that can immunoprecipitate the Mediator complex (5, 14, 38). Immunoblotting of the immunoprecipitate revealed equivalent amounts of Mediator subunits Med150, Sur2, Med6, and cyclin C, as well as CDK8, in all anti-CDK8 immunoprecipitates (Fig. 3A). However, E1A proteins were only detected in anti-CDK8 immunoprecipitates from Ad5- and pm975-infected cells. These results confirm that the large E1A protein, and not the small E1A protein, is associated with Mediator complex in adenovirus-infected cells. The large E1A protein from pm975-infected cells had a faster mobility in the SDS gel than the large E1A protein from Ad5-infected cells (Fig. 3A, lanes 7 and 8). The mobility of E1A proteins in such a gels is influenced by E1A phosphorylation (9, 16). We find that the extent of E1A phosphorylation, as judged by SDS gel mobility, varies between different preparations of infected-cell nuclear extract. E1A proteins from 293 cells invariably had slower mobility than E1A proteins from infected HeLa cells (see Fig. 3B, lanes 1 to 4, and Fig. 4B, lanes 1 to 3). The nuclear extract from pm975-infected cells used in the analysis of Fig. 3 contained predominantly a faster-migrating form of large E1A protein than in the Ad5-infected cells (Fig. 3A, lanes 3, 4, 7, and 8). The results of Fig. 3A, lanes 7 and 8, indicate that the extent of large E1A phosphorylation, as assayed by the influence on large E1A mobility in an SDS gel, did not significantly affect the association of large E1A with the Mediator complex.
During the late phase of Ad5 infection, some E1A mRNAs have a second intron removed. This intron is spliced out of the 5′ exon of the early E1A mRNAs, producing messengers that are translated into smaller E1A proteins than observed during the early phase (34). These smaller E1A proteins were apparent in long exposures of Western blots of nuclear extract prepared 18 h postinfection (Fig. 3B, lanes 1 to 3). E1A proteins in this smaller size class were also immunoprecipitated with anti-CDK8 antibody from Ad5- and pm975-infected cells, but not from dl1500-infected cells (Fig. 3B, lanes 5 to 7). Since the nine-base-pair deletion in dl1500 should prevent the inclusion of CR3 in multiply spliced E1A mRNAs, these results are consistent with the conclusion that E1A proteins require CR3 to bind Mediator complexes.
293 cell nuclear extract was also immunoprecipitated with anti-CDK8 antibody (Fig. 3C). Consistent with the results for virus-infected HeLa cells, only large E1A, and not small E1A, was specifically immunoprecipitated.
The large E1A protein also copurified with Mediator complex by column chromatography. Mediator complexes from dl1500- and pm975-infected HeLa cells and from 293 cells were partially purified as shown in Fig. 4A. The purified Mediator fraction from pm975-infected HeLa cells and 293 cells contained readily detectable amounts of E1A proteins, whereas the Mediator fraction prepared from dl1500-infected HeLa cells contained only a trace amount. Consistent with the results shown in Fig. 2 and 3, the 293 cell Mediator fraction contained large E1A and not small E1A. To confirm that large E1A in the purified 293 Mediator fraction was associated with Mediator complexes, the purified Mediator fraction from 293 cells was subjected to immunoprecipitation with anti-E1A monoclonal antibody M73 (15) (Fig. 4C). As a control for the specificity of the immunoprecipitation, the Mediator fraction from uninfected HeLa cells was subjected to the same procedure. The Sur2 Mediator subunit was specifically immunoprecipitated from the 293 cell Mediator fraction but not from the uninfected HeLa cell Mediator fraction. As for total nuclear extract from Ad5- and pm975-infected cells, only a very small fraction (∼1%) of the total Mediator in the purified fraction was complexed to E1A.
To analyze further the association of E1A proteins with cellular proteins, 293 cell nuclear extract was fractionated by gel filtration chromatography on a Superose 6 column. Column fractions were subjected to immunoblotting with antibodies to E1A, P300, Mediator subunits, and the large subunit of Pol II (Fig. 5A). By far, most E1A proteins, both large and small, coeluted with P300 in fractions corresponding to a mass of 700 to 900 kDa. E1A proteins in these fractions are likely present in a protein complex that includes P300 or the related protein CBP since these proteins coimmunoprecipitate with anti-E1A antibody from cell extracts (1, 16, 42) (Fig. 2A). As for HeLa cell nuclear extract (38), most of the Sur2 protein eluted in fractions corresponding to a mass of ∼1.5 MDa with other Mediator subunits, whereas ∼30% of the Sur2 eluted in fractions corresponding to monomeric Sur2 (150 kDa). A peak of E1A coeluting with the main peak of Mediator complexes in fractions 45 and 49 was not apparent. However, large E1A specifically, and not small E1A, was detected in fraction 37, just after the void volume of the column (fraction 33). A small fraction of Mediator subunits Med220, Med150, and Sur2 and a small fraction of Pol II also eluted in fraction 37. Treatment of 293 nuclear extract with DNase I did not eliminate the observation of large E1A, Mediator subunits, and Pol II in this high-molecular-weight (>2-mDa) fraction.
When nuclear extract from uninfected HeLa cells is subjected to chromatography on Superose 6, Mediator subunits and Pol II are generally not detected to a similar extent in fractions corresponding to the region of the elution profile just beyond the void volume of the column (38). To analyze this region of the column further, each fraction corresponding to 1% of the column volume was analyzed by immunoblotting with antibodies to Med150, Sur2, and E1A (Fig. 5B). Most Med150 and Sur2 in uninfected HeLa nuclear extract peaked in fractions 41 to 51. No Med150 or Sur2 was apparent in fraction numbers below 39. In contrast, in the analysis of 293 nuclear extract, in addition to the major Med150 and Sur2 peak in fractions 41 to 51, a second peak of Med150 and Sur2 was apparent in fractions 35 to 37. A peak of E1A in fractions 35 to 37 was greatly enriched for large over small E1A. Another peak of E1A in fractions 40 to 45 was also enriched for large E1A. However, the exposure required to clearly reveal the low level of E1A in fractions 35 to 37 was overexposed for fractions 42 and 43, making the relative amount of small E1A in these fractions appear artificially high compared to what was observed in a shorter exposure (data not shown). By far, most of the E1A proteins, both large and small, peaked in fractions 49 to 61 (Fig. 5A).
The results of Fig. 5 indicate that a fraction of large E1A in 293 cells is associated with a >2-MDa complex and that this complex may also contain Mediator subunits and Pol II. A complex of Mediator and Pol II in this size range was far less abundant in nuclear extract from uninfected HeLa cells.
To test further for the presence of large E1A in a complex with a small fraction of total cell Pol II, anti-E1A immunoprecipitates of nuclear extract from virus-infected HeLa cells and 293 cells were assayed by immunoblotting with antibody against the large Pol II subunit (Fig. 6). Specific immunoprecipitation of Pol II was clearly observed with 293 nuclear extract (Fig. 6B). Pol II immunoprecipitated from Ad5- and pm975-infected HeLa cell nuclear extract was reproducibly above the background level observed after immunoprecipitation of Ad5-infected HeLa nuclear extract with control normal rabbit IgG and of mock- and dl1500-infected HeLa nuclear extract with anti-E1A antibody (Fig. 6A). These results are consistent with the suggestion based on the 293 nuclear extract Superose 6 gel filtration analysis (Fig. 5A and B) that a small fraction of large E1A in 293 cells is in a very high molecular weight complex (>2 MDa) that includes Mediator subunits and Pol II. The results of Fig. 6A suggest that this is also true for large E1A in adenovirus-infected cells. As discussed below, these observations indicate that the interaction between E1A-CR3 and Mediator stabilizes a previously observed weak interaction between human Mediator complex and Pol II.
DISCUSSION
As discussed in the introduction, recent studies demonstrated that transcriptional activation by a Gal4-E1A-CR3 fusion protein requires binding of the multisubunit mammalian Mediator complex via a direct interaction between E1A-CR3 and the Sur2 Mediator subunit (4, 35). However, whereas previous studies identified a number of cellular proteins bound by full-length E1A proteins in adenovirus-infected and -transformed cells, Mediator complex subunits were not observed (10, 11, 40, 42). The recent characterization of mammalian Mediator complexes and the development of antibodies against Mediator subunits have now allowed us to examine directly the association of E1A proteins with Mediator as reported here.
The results presented above demonstrate an association between large E1A and Mediator complex in productively infected HeLa cells and in transformed 293 cells. The specific association in vivo of large E1A, but not small E1A, is consistent with the specific in vitro interaction between E1A-CR3 and the Sur2 Mediator subunit (5). Although the in vivo association of large E1A with Mediator complex was clearly observed by several different approaches, only a very small fraction of the large E1A protein in Ad5-infected cells at 18 h postinfection (unpublished results) and in 293 cells (Fig. 5A) was associated with Mediator complex. This probably explains why Mediator subunits were not identified in earlier analyses of E1A-associated proteins. It is possible that a much larger fraction of large E1A is associated with Mediator complex at early times postinfection when much lower concentrations of E1A are present.
Similarly, only a very small fraction of Mediator complex (∼1%) was associated with large E1A in Ad5- and pm975-infected HeLa cells (Fig. 2C) and in 293 cells (Fig. 4C). This was surprising in light of earlier observations that the in vitro interaction between E1A-CR3 and Sur2 is extremely stable and that Mediator complex in HeLa nuclear extract can be depleted by passage over a GST-E1A-CR3 column (5). Consequently, there is a great excess of large E1A that does not associate with Mediator complex in vivo even though the extracted Mediator complex can bind to E1A-CR3 in vitro. Perhaps most Mediator complexes are inhibited from interacting with large E1A in vivo because they are bound to cellular activators in association with cellular promoters. Alternatively, posttranslational modifications of large E1A, such as phosphorylation, that occur in vivo may inhibit the interaction of most large E1A proteins with Mediator in vivo. Such potential inhibitory posttranslational modifications likely would not be present on the GST-E1A-CR3 protein that can deplete Mediator complexes from a HeLa nuclear extract since it is expressed in Escherichia coli. However, as discussed above, phosphorylation of E1A at sites that cause a decrease in the mobility of the protein during SDS-PAGE do not inhibit the interaction with Mediator. But E1A proteins are phosphorylated at multiple sites, some of which have little affect on gel mobility (9, 16). Another possible explanation for why only a small fraction of large E1A is associated with Mediator complexes is that its association with other proteins, such as Rb family members, P300, CBP, or CtBP, through interactions with E1A sequences outside of CR3 (11, 31, 40) might sterically block or otherwise inhibit the interaction of CR3 with Mediator complexes.
Analysis of E1A-containing complexes in 293 cell nuclear extract by Superose 6 gel filtration revealed a large complex of >2 MDa containing large E1A, Mediator subunits, and Pol II (Fig. 5A). This observation indicates that the interaction between E1A-CR3 and Mediator stabilizes an interaction between Mediator and Pol II. S. cerevisiae Mediator binds to the yeast Pol II CTD (20, 28). Mammalian Mediator complexes also interact with Pol II in vitro in a low-salt buffer containing 150 mM KCl, although the interaction does not require the large-subunit CTD (36). Similarly, immunoprecipitation of Mediator subunits from HeLa nuclear extract coprecipitates Pol II in a low-salt buffer containing 50 mM KCl but not in a buffer containing 300 mM KCl (14). Consistent with this, we did not observe a significant fraction of Pol II eluting with Mediator complexes from a Superose 6 gel filtration column run in a buffer containing 300 mM KCl (38). In contrast to this, as mentioned above, we did observe a complex larger than the Mediator complex (>2 MDa) that contained large E1A, Mediator subunits, and Pol II during gel filtration of 293 nuclear extract in buffer containing 300 mM KCl (Fig. 5A). Immunoblotting did not reveal general Pol II transcription factors TFIIB or TFIIF in the >2-MDa complex (unpublished results). Similar, very high molecular weight complexes containing Mediator subunits, Pol II, and large E1A were also observed after fractionation of Ad5- and pm975-infected, but not dl1500-infected, HeLa nuclear extract. However, a distinct peak separate from the main Mediator complex peak at ∼1.5 MDa was not observed clearly with virus-infected HeLa nuclear extracts, probably because of lower amounts of these >2-MDa complexes compared to 293 nuclear extract (unpublished results). Perhaps formation of the >2-MDa complex occurs more efficiently with the unique form(s) of the large E1A protein made in 293 cells that migrates more slowly in an SDS gel than E1A protein made in infected HeLa cells (Fig. 4B). Nonetheless, the reproducible immunoprecipitation of Pol II with anti-E1A antibody from Ad5- and pm975-infected, but not dl1500-infected, HeLa nuclear extract (Fig. 6) supports the occurrence of a complex stable to 300 mM KCl containing large E1A, Mediator, and Pol II in adenovirus-infected HeLa cells, as well as in adenovirus-transformed 293 cells.
This observation of a stable complex between the E1A-CR3 activation domain, Mediator, and Pol II in vivo is similar to the binding of Pol II to Mediator observed in vitro in response to the vitamin D3 receptor when bound to its specific activating ligand (6). This stabilization of the interaction between Mediator and Pol II induced by the binding of an activation domain to Mediator may directly stimulate transcription by promoting the association of Pol II with a promoter.
Several observations indicate that E1A-CR3 associates with the Mediator complex by binding to the Sur2 subunit. (i) Approximately 30% of the Sur2 in HeLa nuclear extract is apparently monomeric as judged by its elution from Superose 6 (5, 38). This monomeric Sur2 binds to an E1A-CR3 affinity column, demonstrating a direct interaction between E1A-CR3 and Sur2 (35). (ii) Mediator complex in nuclear extract from wild-type ES cells binds to E1A-CR3, but Mediator complex in nuclear extract from sur2 knockout ES cells does not (35). (iii) Overexpression of Sur2 in a transient-transfection assay inhibits (squelches) E1A-CR3 activation (5). Other mammalian activators must interact with other Mediator subunits since multiple other activators function normally in the sur2 knockout cells (35). The finding that E1A binds Mediator complex through an interaction with the Sur2 subunit raises the question of why adenovirus E1A-CR3 evolved to interact with this particular Mediator subunit. Sur2 was initially discovered in C. elegans because sur2 mutations inhibit vulval cell development in response to a Ras-mitogen-activated protein kinase (MAPK) signal transduction pathway (32). We found that activation by the mammalian Elk-1 transcription factor, normally dependent on its phosphorylation by MAPKs, is nearly completely defective in sur2 knockout murine ES cells, whereas multiple other activation domains function normally (35). Perhaps E1A-CR3 cooperates with specific cellular transcription factors such as Elk-1 that also activate by interacting with Sur2 to synergistically activate transcription from specific viral or cellular promoters. Or perhaps cellular regulation of Sur2 function in the context of normal human tissues influences viral host interactions in specific cells such as the well-known, but poorly understood, latency of adenovirus in adenoid tonsil lymphoid tissue. Further studies will be required to address these questions.
Acknowledgments
This work was supported by USPHS grant CA25235.
We thank Carol Eng for excellent technical assistance and Mike Carey and members of the Berk lab for helpful discussions.
REFERENCES
- 1.Arany, Z., D. Newsome, E. Oldread, D. M. Livingston, and R. Eckner. 1995. A family of transcriptional adaptor proteins targeted by the E1A oncoprotein. Nature 374:81-84. [DOI] [PubMed] [Google Scholar]
- 2.Berk, A. J., F. Lee, T. Harrison, J. Williams, and P. A. Sharp. 1979. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell 17:935-944. [DOI] [PubMed] [Google Scholar]
- 3.Berk, A. J., and P. A. Sharp. 1978. Structure of the adenovirus 2 early mRNAs. Cell 14:695-711. [DOI] [PubMed] [Google Scholar]
- 4.Boyer, T. G., and A. J. Berk. 1993. Functional interaction of adenovirus E1A with holo-TFIID. Genes Dev. 7:1810-1823. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, T. G., M. E. Martin, E. Lees, R. P. Ricciardi, and A. J. Berk. 1999. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature 399:276-279. [DOI] [PubMed] [Google Scholar]
- 6.Chiba, N., Z. Suldan, L. P. Freedman, and J. D. Parvin. 2000. Binding of liganded vitamin D receptor to the vitamin D receptor interacting protein coactivator complex induces interaction with RNA polymerase II holoenzyme. J. Biol. Chem. 275:10719-10722. [DOI] [PubMed] [Google Scholar]
- 7.Culp, J. S., L. C. Webster, D. J. Friedman, C. L. Smith, W. J. Huang, F. Y. Wu, M. Rosenberg, and R. P. Ricciardi. 1988. The 289-amino-acid E1A protein of adenovirus binds zinc in a region that is important for trans-activation. Proc. Natl. Acad. Sci. USA 85:6450-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dignam, J. D., P. L. Martin, B. S. Shastry, and R. G. Roeder. 1983. Eukaryotic gene transcription with purified components. Methods Enzymol. 101:582-598. [DOI] [PubMed] [Google Scholar]
- 9.Dumont, D. J., M. L. Tremblay, and P. E. Branton. 1989. Phosphorylation at serine 89 induces a shift in gel mobility but has little effect on the function of adenovirus type 5 E1A proteins. J. Virol. 63:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyson, N., and E. Harlow. 1992. Adenovirus E1A targets key regulators of cell proliferation. Cancer Surv. 12:161-195. [PubMed] [Google Scholar]
- 11.Egan, C., T. N. Jelsma, J. A. Howe, S. T. Bayley, B. Ferguson, and P. E. Branton. 1988. Mapping of cellular protein-binding sites on the products of early-region 1A of human adenovirus type 5. Mol. Cell. Biol. 8:3955-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold, M. O., J. P. Tassan, E. A. Nigg, A. P. Rice, and C. H. Herrmann. 1996. Viral transactivators E1A and VP16 interact with a large complex that is associated with CTD kinase activity and contains CDK8. Nucleic Acids Res. 24:3771-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 14.Gu, W., S. Malik, M. Ito, C. X. Yuan, J. D. Fondell, X. Zhang, E. Martinez, J. Qin, and R. G. Roeder. 1999. A novel human SRB/MED-containing cofactor complex, SMCC, involved in transcription regulation. Mol. Cell 3:97-108. [DOI] [PubMed] [Google Scholar]
- 15.Harlow, E., B. R. Franza, Jr., and C. Schley. 1985. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J. Virol. 55:533-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harlow, E., P. Whyte, B. R. Franza, Jr., and C. Schley. 1986. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol. Cell. Biol. 6:1579-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrmann, C. H., M. O. Gold, and A. P. Rice. 1996. Viral transactivators specifically target distinct cellular protein kinases that phosphorylate the RNA polymerase II C-terminal domain. Nucleic Acids Res. 24:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, N., and T. Shenk. 1979. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc. Natl. Acad. Sci. USA 76:3665-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimelman, D., J. S. Miller, D. Porter, and B. E. Roberts. 1985. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J. Virol. 53:399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, T. I., and R. A. Young. 2000. Transcription of eukaryotic protein-coding genes. Annu. Rev. Genet. 34:77-137. [DOI] [PubMed] [Google Scholar]
- 21.Lillie, J. W., and M. R. Green. 1989. Transcription activation by the adenovirus E1a protein. Nature 338:39-44. [DOI] [PubMed] [Google Scholar]
- 22.Liu, F., and M. R. Green. 1994. Promoter targeting by adenovirus E1a through interaction with different cellular DNA-binding domains. Nature 368:520-525. [DOI] [PubMed] [Google Scholar]
- 23.Malik, S., and R. G. Roeder. 2000. Transcriptional regulation through Mediator-like coactivators in yeast and metazoan cells. Trends Biochem. Sci. 25:277-283. [DOI] [PubMed] [Google Scholar]
- 24.Martin, K. J., J. W. Lillie, and M. R. Green. 1990. Evidence for interaction of different eukaryotic transcriptional activators with distinct cellular targets. Nature 346:147-152. [DOI] [PubMed] [Google Scholar]
- 25.Mittler, G., E. Kremmer, H. T. Timmers, and M. Meisterernst. 2001. Novel critical role of a human Mediator complex for basal RNA polymerase II transcription. EMBO Rep. 2:808-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montell, C., G. Courtois, C. Eng, and A. Berk. 1984. Complete transformation by adenovirus 2 requires both E1A proteins. Cell 36:951-961. [DOI] [PubMed] [Google Scholar]
- 27.Montell, C., E. F. Fisher, M. H. Caruthers, and A. J. Berk. 1982. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature 295:380-384. [DOI] [PubMed] [Google Scholar]
- 28.Myers, L. C., and R. D. Kornberg. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69:729-749. [DOI] [PubMed] [Google Scholar]
- 29.Perricaudet, M., G. Akusjarvi, A. Virtanen, and U. Pettersson. 1979. Structure of two spliced mRNAs from the transforming region of human subgroup C adenoviruses. Nature 281:694-696. [DOI] [PubMed] [Google Scholar]
- 30.Rachez, C., and L. P. Freedman. 2001. Mediator complexes and transcription. Curr. Opin. Cell Biol. 13:274-280. [DOI] [PubMed] [Google Scholar]
- 31.Schaeper, U., J. M. Boyd, S. Verma, E. Uhlmann, T. Subramanian, and G. Chinnadurai. 1995. Molecular cloning and characterization of a cellular phosphoprotein that interacts with a conserved C-terminal domain of adenovirus E1A involved in negative modulation of oncogenic transformation. Proc. Natl. Acad. Sci. USA 92:10467-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh, N., and M. Han. 1995. sur-2, a novel gene, functions late in the let-60 ras-mediated signaling pathway during Caenorhabditis elegans vulval induction. Genes Dev. 9:2251-2265. [DOI] [PubMed] [Google Scholar]
- 33.Spindler, K. R., and A. J. Berk. 1984. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J. Virol. 52:706-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stephens, C., and E. Harlow. 1987. Differential splicing yields novel adenovirus 5 E1A mRNAs that encode 30 kd and 35 kd proteins. EMBO J. 6:2027-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens, J. L., G. T. Cantin, G. Wang, A. Shevchenko, A. Shevchenko, and A. J. Berk. 2002. Transcription control by E1A and ERK MAP kinase pathway through Sur2 Mediator subunit. Science 296:755-758. [DOI] [PubMed] [Google Scholar]
- 36.Sun, X., Y. Zhang, H. Cho, P. Rickert, E. Lees, W. Lane, and D. Reinberg. 1998. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol. Cell 2:213-222. [DOI] [PubMed] [Google Scholar]
- 37.Thompson, N. E., D. B. Aronson, and R. R. Burgess. 1990. Purification of eukaryotic RNA polymerase II by immunoaffinity chromatography. Elution of active enzyme with protein stabilizing agents from a polyol-responsive monoclonal antibody. J. Biol. Chem. 265:7069-7077. [PubMed] [Google Scholar]
- 38.Wang, G., G. T. Cantin, J. L. Stevens, and A. J. Berk. 2001. Characterization of mediator complexes from HeLa cell nuclear extract. Mol. Cell. Biol. 21:4604-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Webster, L. C., and R. P. Ricciardi. 1991. trans-Dominant mutants of E1A provide genetic evidence that the zinc finger of the trans-activating domain binds a transcription factor. Mol. Cell. Biol. 11:4287-4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whyte, P., N. M. Williamson, and E. Harlow. 1989. Cellular targets for transformation by the adenovirus E1A proteins. Cell 56:67-75. [DOI] [PubMed] [Google Scholar]
- 41.Winberg, G., and T. Shenk. 1984. Dissection of overlapping functions within the adenovirus type 5 E1A gene. EMBO J. 3:1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yee, S. P., and P. E. Branton. 1985. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology 147:142-153. [DOI] [PubMed] [Google Scholar]