Abstract
Although highly active antiretroviral therapy (HAART) for human immunodeficiency virus type 1 (HIV-1) infection can reduce levels of HIV-1 RNA in plasma to below the limit of detection, replication-competent forms of the virus persist in all infected individuals. One form of persistence involves a stable reservoir of latent but potentially infectious virus that resides in resting memory CD4+ T cells. The mechanisms involved in maintaining this latent reservoir are incompletely understood. In the present study, we examined the dynamic characteristics of this reservoir in a cohort of children who developed drug-resistant HIV-1 as a result of extensive exposure to inadequately suppressive one- or two-drug regimens prior to the advent of HAART. We have previously shown that drug-resistant viruses selected by nonsuppressive pre-HAART regimens can enter and persist in this reservoir. We have extended these findings here by demonstrating that archival wild-type HIV-1 persists in this reservoir despite the fact that in these patients drug-resistant mutants have been favored by the selective conditions for many years. Phylogenetic analysis of replication-competent viruses persisting in resting CD4+ T cells revealed a striking lack of temporal structure in the sense that isolates obtained at later time points did not show greater sequence divergence than isolates from earlier time points. The persistence of drug-sensitive virus and the lack of temporal structure in the latent reservoir provide genetic evidence for the idea that HIV-1 can persist in a latent form free of selective pressure from antiretroviral drugs in long-lived resting memory CD4+ T cells. Although there may be other mechanisms for viral persistence, this stable pool of latently infected cells is of significant concern because of its potential to serve as a lasting source of replication-competent viruses, including the infecting wild-type form and all drug-resistant variants that have arisen subsequently.
Treatment with highly active antiretroviral therapy (HAART) can decrease human immunodeficiency virus type 1 (HIV-1) RNA levels in plasma to below the limit of detection of ultrasensitive clinical assays (15, 17, 24, 28), but several potential mechanisms allow the virus to persist. One mechanism involves the ability of HIV-1 to establish a state of latent infection in resting memory CD4+ T cells (4, 5). In both children and adults treated with HAART, replication-competent HIV-1 persists in this latent reservoir (7, 12, 13, 29, 37). Longitudinal studies suggest that the size of this reservoir remains stable in most patients despite long-term treatment. In some studies, the decay rate of the reservoir appears to be so slow (t1/2 = 44 months) that eradication of the infection would be unlikely (12, 29, 31). Other studies have described a subset of patients in whom a slow but measurable decay appears to be taking place (t1/2 = 6 months) (31, 40).
Two distinct but not mutually exclusive hypotheses have been put forward to explain the stability of the latent reservoir (12). Persistence may be a consequence of the long life span of the memory CD4+ T lymphocytes that comprise the reservoir (12). In addition, a low level of ongoing viral replication in patients on suppressive HAART may replenish the latent compartment (10, 14, 31, 40). In particular, it has been suggested that the stability of the reservoir may be associated with the intermittent episodes of low-level viremia that are common in patients on HAART (31). Although these episodes, known as “blips,” are not necessarily associated with failure of the drug regimen (18, 19), they may reflect a process of ongoing viral replication that replenishes the pool of latently infected cells (31).
To gain insight into the mechanisms that maintain the latent reservoir, we examined the genetic characteristics of infectious biological clones of HIV-1 isolated from the latent reservoir of children on HAART in order to detect sequence patterns reflecting stability or turnover of the reservoir. We reasoned that a stable, long-lived viral reservoir would continue to harbor viruses that were generated at various times throughout the life of perinatally infected children, including wild type, drug-sensitive viruses transmitted from the mother and any drug-resistant viruses arising during nonsuppressive, pre-HAART therapy. In contrast, a reservoir that persists only because of ongoing replenishment would consist primarily of viruses that were more contemporaneous and might include newly emerging drug-resistant viruses.
Children with perinatally acquired HIV-1 infection provide the ideal patient population in which to observe turnover of the latent reservoir for several reasons. First, before HAART became standard of care, infected children frequently received many years of nonsuppressive therapy with one or two nucleoside analogue reverse transcriptase inhibitors (RTIs), leading to substantial resistance to this class of drugs. These resistant viruses can enter and persist in the latent reservoir (29). Second, early combination therapy regimens for children often included the same or related nucleoside analogues, resulting in continued selection for the previously generated resistant viruses. In this situation, many years of selection for nucleoside analogue-resistant viruses would be expected to deplete the original wild-type sequences from the reservoir if the entire latent reservoir was constantly turning over. Third and most importantly, many of these patients have had intermittent low-level viremia, a condition that is thought to allow for replenishment of the reservoir. To analyze turnover of the latent reservoir in this cohort, we have used isolates obtained in an initial study of this cohort (29) and a large number of new isolates in a phylogenetic analysis of reservoir dynamics. Our results provide evidence for both mechanisms of persistence.
MATERIALS AND METHODS
Study population.
Children with perinatally acquired HIV-1 infection who achieved durable suppression of viral replication in HAART were eligible. Informed consent was obtained from the parent or guardian, and assent was obtained from children who were aware of their diagnosis.
HIV-1 plasma RNA.
Plasma HIV-1 RNA assays were carried out by using the standard (detection limit = 200 or 400 copies/ml) and ultrasensitive (detection limit = 50 copies/ml) Roche Amplicor Monitor System (Roche Diagnostic Systems, Nutley, N.J.).
Culture assay for latently infected cells.
Resting CD4+ T cells were isolated as described previously (4). Purities, determined by flow cytometry analysis of sorted cells for the expression of CD4 and the absence of HLA-DR, were typically greater than 97%. For children in whom it became difficult to culture HIV-1 from 2 to 5 million purified resting CD4+ lymphocytes, the cell-sorting step was omitted and bead-depleted cells (purity ∼90%, data not shown) were assayed. Latently infected cells were detected by a previously described (12) limiting dilution culture assay in which purified CD4+ HLA-DR− cells were activated with phytohemagglutinin and irradiated allogeneic peripheral blood mononuclear cells from an HIV-1-seronegative donor in medium containing interleukin-2 and then cocultured with CD8-depleted, CD4+ lymphoblasts from an HIV-1-seronegative donor. Supernatants were collected on day 14 and analyzed for p24 antigen by enzyme-linked immunosorbent assay (ELISA; Coulter Electronics, Ltd., Hialeah, Fla.). Isolates were obtained from p24+ wells in plates seeded with input cell doses at which only fraction of the wells were positive. Subsequent sequence analysis confirmed that most positive wells were monoclonal. Occasionally, wells contained more than one viral clone. In this case, independence of sequences was established as described below.
Sequencing of the HIV-1 pol gene.
HIV-1 pol sequences were amplified by PCR from cell-associated DNA from cultures of CD4+ lymphoblasts infected with biological clones of HIV-1 isolated from latently infected cells. Genomic DNA was isolated by standard methods (Gentra Systems, Inc., Minneapolis, Minn.). The pol gene was amplified by using published (20) primers with PRT O5 primers (nucleotides 2008 to 2031; 5′-GCCCCTAGGAAAAAGGGCTGTTGG-3′) and OUT3′ (nucleotides 4263 to 4295; 5′-CATTGCTCTCCAATTACTGTGATATTTCTCATG-3′) or primers PRT15 (nucleotides 2057 to 2080; 5′-TGAAAGATTGTACTGAGAGACAGG-3′) and IN3 (nucleotides 4212 to 4246; 5′-TCTATTCCATCTAAAAATAGTACTTTCCTGATTCC-3′). The positions of the primers are numbered according to the pol gene of the HXB2R isolate (http://hiv-web.lanl.gov/NUM-HXB2/HXB2.MAIN.html). The DNA samples were heated to 94°C for 3 min, and then 30 amplification cycles were performed (94°C for 30 s, 57°C for 30 s, 68°C for 2 min, and 15 s), followed by an incubation at 68°C for 5 min. Bands were gel purified by using a QIAquick PCR purification kit (Qiagen, Valencia, Calif.) and then cloned into a pCR-Blunt II-Topo vector (Invitrogen Corp., Carlsbad, Calif.). Plasmid DNA purified by using the Qiagen purified plasmid DNA kit was sequenced on a DNA sequencer (ABI PRISM model 377; Perkin-Elmer Applied Biosystems, Foster City, Calif.) by using the following sequencing primers for forward sequencing (DP10 [CAACTCCCTCTCAGAAGCAGGAGCCC] and RT5′ [AAATCCATACAATACTCCAGTATTTGC]) and the following antisense primers for reverse sequencing (DP11 [GCAAATGGAGTATTGTATGGATTT] and 3RT [CTGTATGTCATTGACAGTCCA] or [TCTGTATGTCATTGACAGTCC]). Sequence validation was carried out by recommended methods (23). Algorithms were used to distinguish PCR errors from polymorphisms and resistance mutations and to establish the independence of clones obtained from the same patient (19). Sequence changes at positions associated with drug resistance were considered polymorphisms if the treatment history was negative for drugs that select these changes or if it was inconsistent with reported patterns of ordered accumulation of resistance mutations. Clones obtained from independent wells in the culture assay were considered independent. Clones obtained from the same PCR were only considered independent if they differed by drug resistance mutations, had four or more nonsynonymous differences, or had two or more synonymous differences. The later two criteria were based on the predicted frequencies of PCR-induced mutation (34). Sequencing was performed on three to five clones per positive culture. Basic local alignment search tool (BLAST) searches of GenBank (http://www.ncbi.nlm.nih.gov/GenBank/GenbankOverview.html) revealed that none of the sequences matched laboratory or patient isolates published previously. Phylogenetic trees were inferred from gap-stripped nucleotide sequences by using PAUP* version 4b8a (Sinauer Associates, Inc., Sunderland, Mass). The best-fit evolutionary model and parameters were selected by using ModelTest version 3.06 and ModelBlock version 3.1 (30). The K81uf+I+G model was used for subsequent analyses. Trees were constructed by using the neighbor-joining method (32), and internal node support was verified by using the bootstrap method (11) with 1,000 replicates. Trees were also inferred by using two other models (maximum parsimony and maximum likelihood). The most parsimonious tree was sought by using a heuristic search procedure with 100 random-addition sequence replicates and tree bisection-reconnection branch swapping.
Nucleotide sequence accession numbers. The GenBank accession numbers for the new sequences described here are AY133113 through AY133193.
RESULTS
Analysis of the latent reservoir in HIV-1-infected children with prolonged suppression of viral replication on HAART.
Analysis of turnover of the latent reservoir is complicated by the fact that two forms of latent HIV-1 can reside in resting CD4+ T cells, a labile preintegration form that arises from abortive infection of resting cells (1, 3, 21, 35, 38) and a stable postintegration form in which the provirus is irreversibly integrated into the host cell genome (4, 5). Limiting dilution culture methods detect both forms (1, 4). We have previously shown that, after the initiation of HAART, there is a biphasic decay in the frequency of latently infected resting CD4+ T cells. By 3 months, the unstable forms decay to such an extent that the stable integrated form of latent infection becomes quantitatively dominant (1, 29). Therefore, to characterize the stable, postintegration form of HIV-1 latency, children who had achieved suppression of viral replication for prolonged periods (up to 5.3 years) were studied.
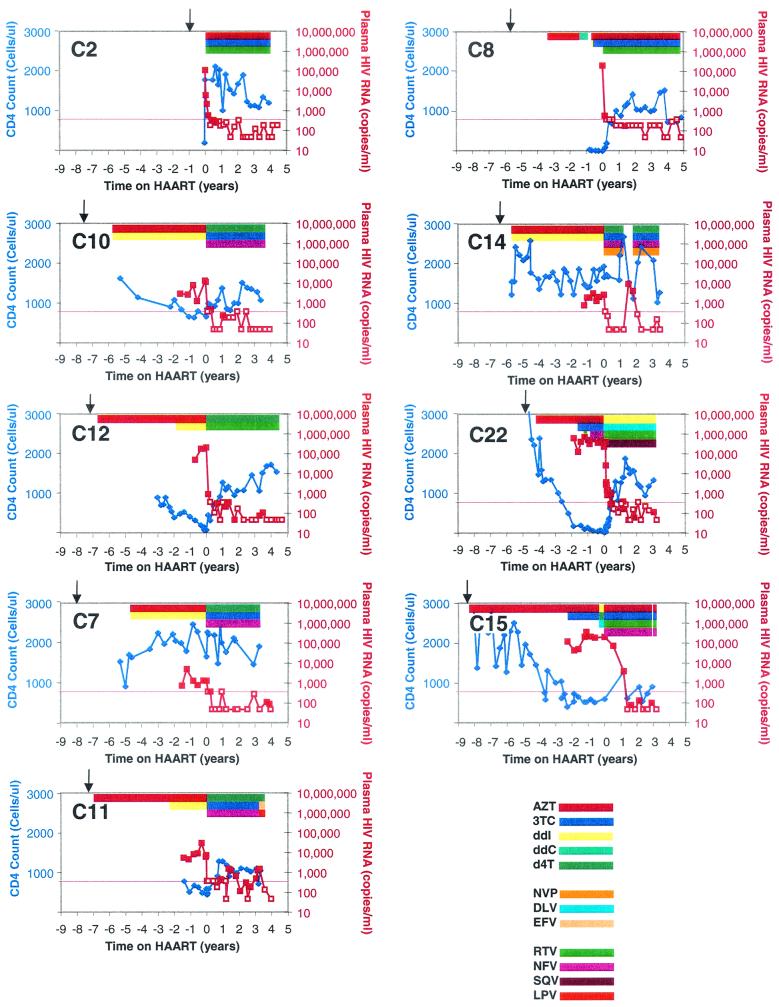
The children studied all acquired HIV-1 infection from their mothers but were diverse with respect to age, sex, race, CD4 levels, treatment histories before HAART, and individual HAART regimens. Patient characteristics are shown in Table 1, with patients categorized based on history of antiretroviral exposure and suppression of viral replication. All children except C2 were born before zidovudine (AZT) therapy for the prevention of mother-to-child transmission of HIV-1 was the standard of care in the United States. As shown in Fig. 1, durable suppression of HIV-1 replication with HAART was achieved for up to 5.3 years (median, 4 years; range, 3.2 to 5.3 years) in eight of the nine children, as determined by using assays for HIV-1 RNA in plasma with increasingly sensitive detection limits (<400, <200, or <50 copies/ml). Importantly, suppression was achieved despite prior extensive and continuous exposure (median, 6.2 years; range, 3.1 to 7.5 years) to nonsuppressive, one or two drug regimens. The efficacy of HAART was also reflected in the stability of CD4+ T cells throughout the study period (Fig. 1). Suppression of viral replication was maintained despite the fact that all children studied except C2 and C14 had isolated episodes of low-level viremia (median, 2.5 episodes; range, 1 to 6 episodes) while on HAART (mean time on HAART, 3.7 years). In two children (C7 and C15), late-onset, low-level viremia was indicative of recent nonadherence. In one child (C14), loss of suppression for 3 months was due to nonadherence, but reinitiation of the same regimen resulted in durable suppression. Although blips were generally isolated events, one child (C11) had less optimal suppression of viral replication after the first year of HAART with several consecutive plasma HIV-1 RNA measurements of between 200 and 1,500 copies/ml.
TABLE 1.
Treatment and virologic and immunologic profiles of children treated with prolonged HAART
| Category and patient identificationa | Sex/raceb | Age (yr) at initiation of antiretroviral therapy | Pre-HAART antiretroviral regimen(s)c,d (duration [yr]) | HAART
|
No. of CD4+ cells/μl (%) at:
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Regimend | Age started (yr) | Duration (yr) of suppression | Duration (yr) of HAART at last sampling | Start of HAART | Last analysis | ||||
| 1 | |||||||||
| C2 | F/AA | 0.9 | None | AZT-3TC-RTV | 0.9 | 4.6 | 3.4 | 196 (10.4) | 1,198 (40) |
| 2 | |||||||||
| C8 | M/C | 2.3 | AZT (2), ddC (0.4), AZT-ddI (0.2), AZT-3TC (0.5) | AZT-3TC-RTV | 5.6 | 5.3 | 4.8 | 9 (0.5) | 836 (49) |
| C10 | F/AA | 3.2 | AZT (1.7), AZT-ddI (5.9) | d4T-3TC-NFV | 7.5 | 4.0 | 3.1 | 859 (36) | 1,284 (42) |
| C14 | F/AA | 0.72 | AZT-ddI (5.7) | d4T-3TC-NFV-NVP | 6.4 | 3.4f | 1.2 | 1,657 (49) | 2,683 (41) |
| 3 | |||||||||
| C12 | F/AA | 0.5 | AZT (4.8), AZT-ddI (1.8) | d4T-RTV | 7.2 | 4.8 | 4.8 | 79 (19) | 1,538 (45) |
| C22e | F/AA | 0.6 | AZT (2.4), AZT-3TC (0.6), AZT-3TC-RTV (0.3), AZT-3TC-NFV (0.8) | DLV-ddI-RTV-SQV | 4.8 | 3.3 | 3.3 | 28 (5) | 1,325 (36) |
| C7 | F/AA | 3.2 | AZT-ddI (4.8) | d4T-3TC-NFV | 7.9 | 4.0 | 3.7 | 1,657 (48) | 942 (54) |
| C15 | M/AA | 0.1 | AZT (6.0), AZT-3TC (2.1), ddI-d4T-DLV (0.2) | AZT-3TC-NFV-RTV | 8.4 | 3.2f | 3.2 | 605 (16) | 919 (28) |
| 4 | |||||||||
| C11 | F/AA | 0.3 | AZT (4.7), AZT-ddI (2.3) | d4T-3TC-NFV | 7.3 | 1.2g | 3 | 453 (24) | 1,120 (28) |
Patient identification numbers are consistent with those used in earlier publications (19, 29). Categories: 1, no pre-HAART and no episodes of detectable viremia during HAART; 2, pre-HAART therapy with RTIs and ≤2 episodes of detectable viremia during HAART; 3, pre-HAART therapy with RTIs and >2 episodes of detectable viremia during HAART; 4, pre-HAART therapy with RTIs and suboptimal suppression on HAART.
M, male; F, female; C, Caucasian; AA, African American.
Pre-HAART therapy refers to treatment with single- and multiple-drug regimens that failed to suppress viremia to below the limit of detection prior to the initiation of suppressive HAART.
Antiretroviral drugs: ddC, dideoxycytidine; ddI, didanosine; 3TC, lamivudine; d4T, stavudine; DLV, delavirdine; EFV, efavirenz; NVP, nevirapine; LPV, lopinavir; NFV, nelfinavir; RTV, ritonavir; SQV, saquinavir.
Patient C22 received two different protease inhibitor-containing regimens, but compliance with the protease inhibitor part of the regimen was poor prior to the four-drug HAART.
Had an episode of treatment interruption during HAART.
Duration of suppressive HAART before onset of treatment failure.
FIG. 1.
Treatment, immunologic and virologic profiles of children in the study. Absolute CD4 counts (blue diamonds) and HIV-1 RNA levels (red squares) in plasma are shown as a function of time, with zero time defined by the initiation of HAART. Arrows (↓) indicate the time of birth. Open symbols for plasma HIV-1 RNA measurements indicate that the level was below the limit of detection; the plotted value is the limit of detection of the assay used (400, 200, or 50 copies/ml). Colored bars indicate denote treatment with the indicated drugs. For full names of antiretroviral drugs, see Table 1, footnote d.
Previous studies have suggested that the frequency of latently infected cells does not decrease in patients on HAART who have blips (31). To examine the stability of the latent reservoir, resting CD4+ T cells were purified from study participants at multiple time points, and negative selection with monoclonal antibodies to CD69, CD25, and HLA-DR was used to remove cells expressing early, intermediate, and later markers to T-cell activation. All cultures performed before viral suppression with HAART was achieved were done on resting CD4+ T cells purified by bead depletion, followed by cell sorting. However, because of limitations in the numbers of purified resting CD4+ T cells obtained from children, the cell-sorting step was excluded when viral suppression was achieved with HAART. The median purity (proportion of CD4+ T cells that were HLA-DR negative) after bead depletion alone was 98% (range, 90 to 99.8%). The contribution of the activated CD4+ T cells to virus recovery in this setting is expected to be minimal since the frequency of infected, activated CD4+ T cells during suppressive HAART is low (ca. 1/50,000) (13). Purified resting CD4+ T cells were subject to limiting dilution culture assays to measure the frequency of cells harboring replication-competent virus. Replication-competent HIV-1 remained recoverable from the resting CD4+ T cells sampled from the peripheral blood of each child despite prolonged suppression of viral replication for up to 5 years on protease inhibitor-containing HAART regimens (data not shown). In each child, the frequency remained roughly stable over the duration of the study (mean frequency, 0.86 infectious units per million resting CD4+ T cells). Thus, in children who have stable suppression of viremia on HAART to below the limit of detection (except for isolated blips), the size of the latent reservoir remains roughly constant, reflecting intrinsic stability, replenishment, or some combination thereof.
Archival, drug-resistant viruses persist in a latent but replication-competent form in resting CD4+ T cells during suppressive HAART in children.
To probe the mechanism underlying the stability of the latent reservoir, we analyzed the genotypic drug resistance patterns in biological clones of replication-competent HIV-1 isolated from resting CD4+ T cells of children on HAART and correlated these findings with antiretroviral treatment histories and patterns of virologic suppression (Table 2). Viruses were isolated at limiting dilution in the absence of drugs. Under these conditions, competition is minimized and both wild-type and drug-resistant viruses grow out. Because these isolates are grown from individual latently infected cells that are activated in vitro, they all have an enormous capacity for in vitro expansion and must be considered potentially pathogenic. Table 2 presents sequences of the RT and protease genes from a total of 81 new isolates. To provide the most complete picture of virus evolution in this cohort, a set of clones (n = 17) identified in our preliminary report on the latent reservoir in children (29) are also included. In this Table, patients are categorized based on pre-HAART histories and the number of documented episodes of detectable viremia during the study period.
TABLE 2.
HIV-1 reverse transcriptase and protease sequences from viral isolates cultured from resting CD4+ T lymphocytesa
Time points marked by asterisks indicate previously published sequences included to provide a complete picture of the evolution of HIV- 1 in the patient.
In one child (C2) who had no prior antiretroviral exposure before starting HAART, clones isolated from the latent reservoir continued to show a complete absence of resistance mutations in either RT or protease, even after 40 months of HAART. All other children in the study had some prior therapy with one or two drug regimens before starting HAART. Generally, this therapy involved nucleoside analogue RT inhibitors. The patients were then started on HAART regimens that included nucleoside analogues and protease inhibitors. Long-term follow-up in these patients confirms a prediction made in our initial analysis, namely, that resistance mutations in the latent reservoir virus were generally those selected by nonsuppressive pre-HAART therapy. AZT-resistant viruses predominated (69% of the clones analyzed; Table 2).
Importantly, in all six children who maintained suppression of viral replication, we did not detect new drug resistance mutations to the protease inhibitors in their current suppressive regimen even after 5 years on continuous HAART and despite intermittently detectable viremia in the range of 50 to 200 copies/ml. However, in the one patient (C11) who developed sustained low-level viremia beginning 14.3 months after the initiation of HAART, we detected in resting CD4+ T cells the accumulation of new drug resistance mutations in reverse transcriptase and protease on a backbone of preexisting AZT mutations. This demonstrates the ability of the culture method used in the present study to detect evolution of drug resistance when the level of free virus in the plasma is in the low detectable range (192 copies/ml at the time of study). Of note, the six clones with genotypic resistance in protease all had changes at D30N and N88D in association with the lamivudine (3TC)-selected M184V mutation and multiple AZT-associated mutations. From the current studies, it is not possible to say whether these sequences are derived from resting CD4+ T cells in pre- or postintegration latency. Interestingly, these new mutations were not seen in limited sampling at a subsequent time point when highly purified cells were cultured. The extent to which these highly resistant clones have become fixed in the latent reservoir during recent treatment failure at low levels of viral replication will require further longitudinal study when viral suppression is achieved on a subsequent antiretroviral regimen. In two additional children, substitutions were detected at the amino acid position 82 in protease. In C7, this mutation was detected in the setting of recent noncompliance, and in patient C15 it was detected after a 5-day treatment interruption. Thus, although most of the viruses isolated from resting CD4+ T cells of patients on suppressive HAART regimens show only archival drug-resistant mutations selected by prior nonsuppressive therapy, the evolution of new drug resistance mutations can be readily detected in the culture system used in the setting of noncompliance or failure. These newly generated resistant viruses therefore have the potential to become stably integrated and stored in the resting CD4+ T-cell latent reservoir.
Long-term persistence of archival wild-type viruses in children on HAART.
The results presented above demonstrate that the latent reservoir for HIV-1 in resting CD4+ T cells can harbor previously selected drug-resistant viruses in a replication-competent form for long periods of time. However, concurrent with the detection of resistant viruses, we also isolated archival wild-type, drug-sensitive HIV-1 (Table 2). Viruses lacking any known drug resistance mutations were isolated from five of the eight children who had documented drug resistance in the latent reservoir (C7, C8, C10, C11, and C15). Wild-type viruses were isolated despite up to 10.2 years (median, 9 years; range, 6.2 to 10.2 years) of continuous exposure to nonsuppressive and suppressive antiretroviral regimens. Of the 89 biological clones cultured from the groups with extensive therapy before HAART, 10 were wild-type and had no drug-resistant mutations. The isolation of wild-type viruses is particularly surprising in light of the fact that these patients had already developed AZT-resistant viruses that should have had a selective advantage during HAART. In C8 and C15, the HAART regimen actually included AZT, giving a selective advantage to the previously generated AZT-resistant viruses. In three other children (C10, C7, and C11) the HAART regimen included stavudine (d4T), which also selects for AZT-resistant virus (8). Even in the one child (C11) who had evidence of treatment failure with persistently low levels of viremia, wild-type HIV-1 was recovered from resting CD4+ T cells at the same time point (33 months) that newly emergent, highly resistant viruses were found (Table 2).
Even in cases where wild-type virus was not seen among the clones sampled, there was evidence of preservation of early, archival forms. In patient C12, the majority of the clones contained a signature insertion sequence at position 69 that is associated with high-level resistance to multiple nucleoside analogues. This insertion was seen on a background of four to five standard AZT resistance mutations. However, two clones contained only the K70R mutation associated with low-level resistance to AZT and d4T. The K70R mutation is one of the earliest substitutions observed with the emergence of genotypic resistance to AZT and d4T (22). In this child, who was extensively treated with nonsuppressive AZT or AZT-didanosine regimens for 7 years, mutants containing only the K70R mutation persisted despite continued drug-selective pressure for further accumulation of RT mutations, strongly suggesting that these clones are archival.
The persistence of wild-type HIV-1 in the latent compartment in the setting of extensive uninterrupted courses of antiretroviral therapy and ongoing viral replication with the evolution of high-level genotypic drug resistance suggests that at least a component this reservoir has remarkable intrinsic stability. Our results also suggest that replenishment of the reservoir can occur, but formal proof will require demonstrating the newly evolved species enter the stable postintegration state in resting CD4+ T cells. An alternative explanation for persistence of wild-type virus involves replenishment of the reservoir from an as yet unidentified “drug-free” sanctuary site.
Phylogenetic structure of HIV-1 quasispecies recovered longitudinally from the resting CD4+ T-cell compartment.
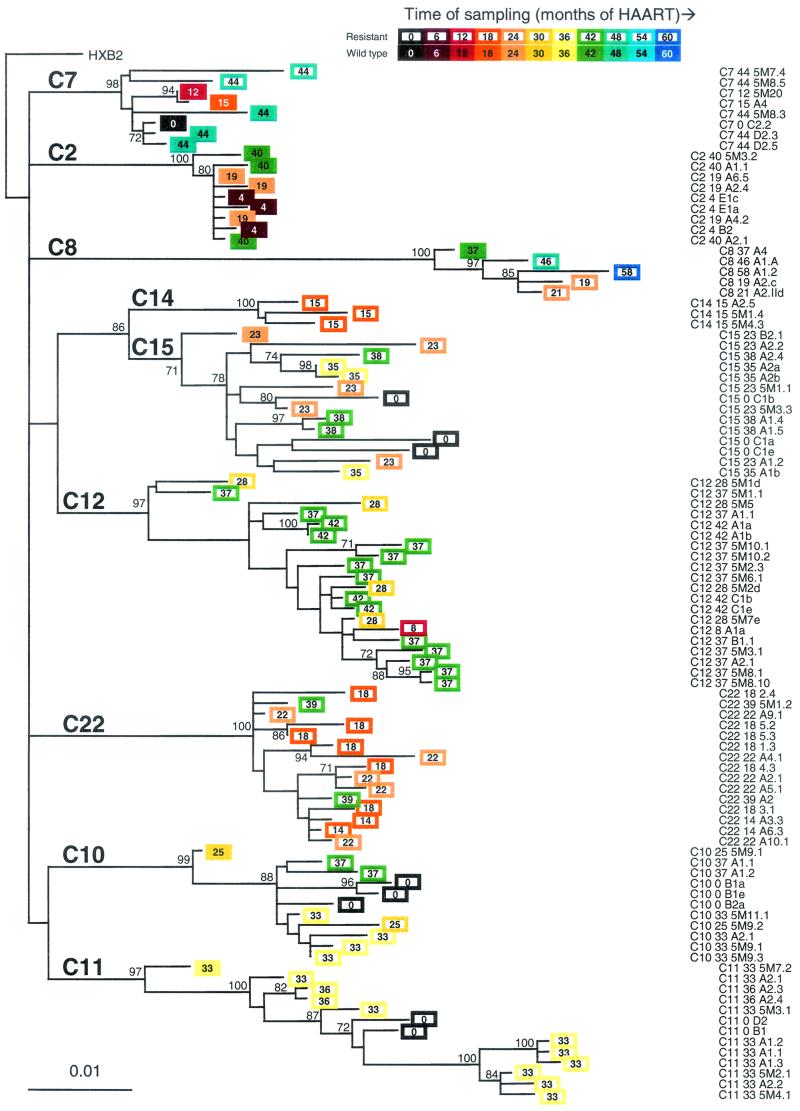
To examine the genetic structure of the replication-competent HIV-1 quasispecies that persist in this reservoir, we performed phylogenetic analyses on a total of 98 pol sequences of latent HIV-1 cultured from the resting CD4+ T-cell compartment (Fig. 2). In all cases, the sequences of viruses cultured from the resting CD4+ T-cell compartment showed the expected patient-specific clustering.
FIG. 2.
Phylogenetic analysis of HIV-1 pol sequences obtained from resting CD4+ T cells of children receiving HAART. Sequences from study subjects are represented by colored boxes with the sampling time indicated in terms of the number of months since initiation of HAART. Lack of temporal structure is indicated by the fact that genetic distances (horizontal scale) do not correlate with sampling time (indicated by a colored bar). In the classic pattern of progressive evolutionary change, viruses obtained at later time points should have diverged further from the most recent common ancestor. Filled boxes indicate wild-type viruses with no antiretroviral resistance mutations. Open boxes indicate viruses with one or more antiretroviral resistance mutations. The numbers at the internal nodes indicate the percentage of 1,000 bootstrap replicates that reproduced the clade. Only values of >70% are indicated; these clades were supported by all three models of phylogenetic inference applied (neighbor-joining, maximum parsimony, and maximum likelihood). The maximum-likelihood tree is shown. The reference sequence HXB2 (GenBank accession no. K03455) was used as an outgroup.
The most striking feature of the phylogenetic analysis is the lack of any temporal structure in the latent reservoir sequences. Studies by Mullins and coworkers have previously shown that, in the absence of HAART, there is progressive evolution the env gene of actively replicating viruses in the plasma, with a time-dependent divergence from ancestral sequences (33). In contrast, analysis of pol gene sequences of the latent viruses described here revealed no such temporal pattern. The viruses isolated at the later time points were often not the most divergent. In some cases, viruses isolated at later time points were ancestral to viruses obtained earlier, whereas viruses isolated at earlier time points showed greater divergence from the most recent common ancestor than viruses isolated subsequently (Fig. 2). In two patients, C10 and C15, viruses isolated at the time of initiation of HAART showed greater divergence from the most recent common ancestor than viruses isolated several years later. These results suggest that most of the observed evolution predates HAART and that the latent reservoir can preserve viruses arising at different time points in the evolutionary history of the virus in a given patient. In all cases, there was phylogenetic intermingling of viruses cultured from earlier and later time points. Strikingly, in the one patient (C11) who had less optimal suppression of viral replication and evidence of viral evolution, viruses cultured at several later time points were actually ancestral to the virus cultured before initiation of HAART (patient C11 0 D2 and C11 0 B1). This remarkable lack of temporal structure is a hallmark of a viral reservoir according to a new genetic definition proposed by Mullins (26). This finding, taken together with ability to simultaneously recover archival wild-type and drug-resistant HIV-1 along with newly generated mutant viruses, suggests that the resting CD4+ T-cell compartment serves as a stable, archival reservoir for replication-competent HIV-1 with the potential to release viruses that could contribute to ongoing viremia when therapy is discontinued or drug levels are suboptimal.
DISCUSSION
Understanding the nature of persistent HIV-1 has important implications for the management of HIV-1 infection and for understanding the evolutionary dynamics of HIV-1 in vivo. We show here that, in infected children, replication-competent ancestral HIV-1 quasispecies are stored in latently infected resting CD4+ T cells despite successful HAART. Analysis of the genotypic mutational patterns of replication-competent viruses isolated from latently infected cells revealed that viruses persisting in this reservoir are diverse and primarily reflect selection by pre-HAART treatment regimens. Most strikingly, wild-type viruses lacking any known drug resistance mutations persisted in this compartment even in patients who had developed drug resistance and who were treated for many years with regimens that strongly favored these drug-resistant mutants. These results suggest that sequences entering the reservoir at early time points in infection are not completely replaced by the dynamic processes that affect the pool of latently infected cells. The persistence of viruses with widely varying levels of genotypic drug resistance is consistent with the idea that the viruses in the latent reservoir are under no active selective pressure from the drugs. Phylogenetic analysis of reservoir sequences showed a striking lack of temporal structure, in sharp contrast to the continuously divergent evolutionary pattern described for env sequences in the main pool of actively replicating virus (33). The time of sampling showed no consistent correlation with the degree of evolutionary divergence. This lack of temporal structure is characteristic of a stable reservoir (26).
It is important to point out that our results do not exclude the possibility that the reservoir may have a very stable component, as well as a more labile component that is maintained by low-level ongoing viral replication. We detected in one patient (C11) with suboptimal suppression the appearance of new resistance mutations in viruses obtained from resting CD4+ T cells. Although additional studies will be required to determine whether these newly emergent viruses have become fixed in the stable pool of integrated virus in the latent reservoir, the results obtained with this patient suggest that the methods used can detect newly emergent resistant virus entering resting CD4+ T cells. What is particularly striking is that, even in this case, wild-type viruses could still be isolated. Thus, at least a component of the reservoir must be extremely stable.
The recovery of archival wild-type virus and of mutants with different levels of genotypic resistance, in concert with the lack of temporal structure on phylogenetic analysis, strongly supports the notion that resting CD4+ T cells provide a long-term reservoir for HIV-1 (2, 26). This reservoir acts as a stable archive for replication-competent viruses in infected individuals and has the potential to serve as a persistent source of infectious HIV-1, including wild-type virus, despite prolonged suppressive HAART, a conclusion that has important implications for the treatment of HIV-1 infection.
Alternative explanations for the persistence of wild-type virus in this compartment include the existence of a drug sanctuary site in which wild-type virus replicates without evolving drug resistance and from which seeding of the reservoir occurs. This sanctuary site would have to have limited permeability to the nucleoside analogues. Although such a site has not yet been identified, we cannot exclude this alternative. Another alternative explanation is that the presence of wild-type virus reflects poor compliance. This explanation is not consistent with the prolonged suppression of viremia that most of these patients have experienced. A final consideration is whether the wild-type viruses persist because they are more fit than resistant viruses even in the presence of drug selection. This alternative is not consistent with the dramatic effects of HAART on virus levels in plasma and the well-accepted observation that treatment failure generally involves resistant viruses.
It is important to emphasize that, in contrast to most genetic studies of viral persistence, only replication-competent viruses were analyzed here. This allowed us to avoid the analysis of defective viruses that can be detected in PCR-based studies of viral persistence. Thus, the persistence of wild-type virus does not reflect the survival of defective sequences not capable of mediating viral gene expression or virus production. In addition, the fact that all of the viruses analyzed have demonstrated potential for robust in vitro replication highlights the potential pathogenic significance of this archival reservoir.
The mechanism involved in the long-term maintenance of infectious archival HIV-1 in resting CD4+ T cells is a matter of great interest. One possibility is that the stability of the reservoir is a reflection of the establishment of HIV-1 latency in a cell type that is designed to survive individually or in the form of clonal progeny for an individual's lifespan. This mode of persistence is consistent with the fundamental biology of memory T cells (27, 36). Another factor contributing to the stability of the latent reservoir may be replenishment by ongoing virus release from other sources (10, 31, 40). Previous studies of the evolution of HIV-1 in the resting CD4+ T-cell compartment in infected adults treated with suppressive HAART for 2 years have revealed two patterns of viral persistence. Sequence evolution in the C2 to V3 domains of HIV-1 env gene suggestive of ongoing viral replication could be detected in some patients, whereas a striking arrest in the evolution was detected in others (16, 40). Similarly, studies of the evolutionary patterns of the pol gene in infected adults treated with up to 2.3 years of HAART have largely demonstrated the lack of detectable evolution of genotypic drug resistance to the new drugs in the HAART regimen (13, 37). The occurrence of new drug resistance mutations in patients on HAART has been associated with suboptimal suppression of viral replication (25). In the present study, we provide further longitudinal evidence that, in HIV-1-infected children who achieve suppression of viral replication, a stable pattern of archival wild-type and drug-resistant virus predominates in the resting CD4+ T-cell compartment despite up to 5 years of suppressive HAART and intermittent viremia. In patients with suboptimal suppression of viremia, newly arising drug-resistant viruses can enter the resting CD4+ T-cell compartment, but even in this situation the persistence of wild-type, drug-sensitive viruses is observed. Some investigators have suggested that the latent reservoir may turnover with a half-life as short as 6 months (31, 40). If this were the case, >98% of the original latent reservoir sequences should be replaced in 3 years. The finding that wild-type viruses persist despite many years of nonsuppressive antiretroviral therapy suggests that while replenishment of the latent reservoir may occur, at least a substantial portion of the reservoir behaves as an extremely stable archive that is not undergoing active selection by the drugs being used.
The contribution of the latent reservoir to the low level of virus production that continues in patients on HAART (10, 19) is a matter of great interest. In a recent study of the genotypic resistance profiles of virus present in plasma at low levels in children and adults who had prolonged suppression of viremia to <50 copies/ml on HAART, we found a similar archival pattern of viral persistence and demonstrated the presence in the plasma of sequences that were indistinguishable from sequences isolated from the latent reservoir at earlier time points (19). The present study demonstrates that one potential source of archival virus found at low levels in the plasma of patients on HAART is release from cells in the latent reservoir that become activated. When antiretroviral therapy is discontinued or drug concentrations are lowered by nonadherence or compromised bioavailability, archival HIV-1 with the greatest replicative capacity can become the predominate species in plasma. Indeed, recent studies by Deeks et al. have shown that, in patients failing combination antiretroviral therapy with highly resistant virus, wild-type virus comes to predominate in plasma several weeks after therapy is discontinued (9). This observation supports the notion of reemergence of archival HIV-1 from a reservoir that has an extremely long half-life or is not under drug selective pressure. Other groups have shown that following interruption of HAART, the viruses that appear in the circulation in some cases resemble viruses detected in the latent reservoir, while in other cases sequences not identified in the reservoir were seen (6, 39). In the present study, we demonstrated that archival wild-type HIV-1 persists in a replication-competent form in resting CD4+ T cells despite up to 10 years of continuous antiretroviral exposure. This replication-competent wild-type HIV-1 has the potential to reemerge when therapy is discontinued. Our results imply that the most likely mechanism for the reappearance of the wild-type virus after the interruption of therapy in patients with multidrug-resistant HIV-1 infection is not genetic reversion of multiple drug resistance mutations but rather the reemergence of archived wild-type virus from the latent reservoir in resting CD4+ T cells or some similarly stable reservoir. These findings support the idea that memory T cells or their progeny can survive for years and likely decades. Therapeutic decisions regarding changes in regimens, “recycling drugs” or treatment interruption must be made with the knowledge that all previously circulating wild-type and drug-resistant forms of the virus in a given patient can be archived in this reservoir and may emerge when conditions are favorable.
Acknowledgments
We thank Susan Marvin and Mary Joyner for help with patient enrollment.
The work was supported by grants from the Elisabeth Glaser Pediatric AIDS Foundation (D.P.) and the Doris Duke Charitable Foundation (D.P. and R.F.S.) and by NIH grant AI43222 to R.F.S.
REFERENCES
- 1.Blankson, J. N., D. Finzi, T. C. Pierson, B. P. Sabundayo, K. Chadwich, J. B. Margolick, T. C. Quinn, and R. F. Siliciano. 2000. Biphasic decay of latently infected CD4+ T cells in acute HIV-1 infection. J. Infect. Dis. 182:1636-1642. [DOI] [PubMed] [Google Scholar]
- 2.Blankson, J. N., D. Persaud, and R. F. Siliciano. 2002. The challenge of viral reservoirs in HIV-1 infection. Annu. Rev. Med. 53:557-593. [DOI] [PubMed] [Google Scholar]
- 3.Bukrinsky, M. I., T. L. Stanwick, M. P. Dempsey, and M. Stevenson. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun, T.-W., L. Carruth, D. Finzi, X. Shen, J. A. Digiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y.-H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantitation of latent tissue reservoirs and total body load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 5.Chun, T.-W., D. Finzi, J. Margolick, K. Chadwich, D. Schwartz, and R. F. Siliciano. 1995. Fate of HIV-1-infected T cells in vivo: rates of transition to stable latency. Nat. Med. 1:1284-1290. [DOI] [PubMed] [Google Scholar]
- 6.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. J. Shawn, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between pre-existing viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 7.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. M. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Mendoza, C., V. Soriano, C. Briones, O. Gallego, P. Barreiro, A. Alvarez, and J. Gonzalez-Lahoz. 2000. Emergence of zidovudine resistance in HIV-infected patients receiving stavudine. J. Acquir. Immune Defic. Syndr. 23:279-281. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 10.Dornadula, G., H. Zhang, B. VanUitert, J. Stern, L. Livornese, Jr., M. J. Ingerman, J. Witek, R. J. Kedanis, J. Natkin, J. DeSimone, and R. J. Pomerantz. 1999. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA 282:1627-1632. [DOI] [PubMed] [Google Scholar]
- 11.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 12.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 13.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 14.Furtaldo, M. R., D. S. Callaway, J. P. Phair, B. S. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 15.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 16.Gunthard, H. F., S. D. W. Frost, A. J. Leigh-Brown, C. C. Ignacio, K. Kee, A. S. Perelson, C. A. Spina, D. V. Havlir, M. Hezareh, D. J. Looney, D. D. Richman, and J. K. Wong. 1999. Evolution of envelope sequences of human immunodeficiency virus type 1 in cellular reservoirs in the setting of potent antiviral therapy. J. Virol. 73:9404-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, and M. A. Fischl. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 18.Havlir, D. V., R. Bassett, D. Levitan, P. Gilbert, P. Tebas, A. C. Collier, M. S. Hirsch, C. Ignacio, J. Condra, H. F. Gunthard, D. D. Richman, and J. K. Wong. 2001. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA 286:171-179. [DOI] [PubMed] [Google Scholar]
- 19.Hermankova, M., S. C. Ray, C. Ruff, M. Powell-Davis, R. Ingersoll, R. T. D'Aquila, T. C. Quinn, J. D. Siliciano, R. F. Siliciano, and D. Persaud. 2001. HIV-1 drug resistance profiles in children and adults with viral load <50 copies/ml receiving combination therapy. JAMA 286:196-207. [DOI] [PubMed] [Google Scholar]
- 20.Hertogs, K., M. P. de Bethune, V. Miller, T. Ivens, P. Schel, A. van Cauwenberge, E. C. Van den, G. Van, V., H. Azijn, M. van Houtte, F. Peeters, S. Staszewski, M. Conant, S. Bloor, S. Kemp, B. Larder, and R. Pauwels. 1998. A rapid method for simultaneous detection of phenotypic resistance to inhibitors of protease and reverse transcriptase in recombinant human immunodeficiency virus type 1 isolates from patients treated with antiretroviral drugs. Antimicrob. Agents Chemother. 42:269-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larder, B. A., and S. D. Kemp. 1989. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science 246:1155-1158. [DOI] [PubMed] [Google Scholar]
- 23.Learn, G. H., Jr., B. T. Korber, B. Foley, B. H. Hahn, S. M. Wolinsky, and J. I. Mullins. 1996. Maintaining the integrity of human immunodeficiency virus sequence databases. J. Virol. 70:5720-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luzuriaga, K., Y. Bryson, P. Krogstad, J. Robinson, B. Stechenberg, M. Lamson, S. Cort, and J. L. Sullivan. 1997. Combination treatment with zidovudine, didanosine, and nevirapine in infants with human immunodeficiency virus type I infection. N. Engl. J. Med. 336:1343-1349. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Picado, J., N. Kartsonis, G. J. Hanna, J. Wong, D. Finzi, E. Rosenberg, H. F. Gunthard, L. Sutton, A. Savara, C. Petropoulos, N. Hellmann, B. D. Walker, D. D. Richman, R. Siliciano, and R. T. D'Aquila. 2000. Antiretroviral resistance during successful therapy of human immunodeficiency virus type 1 infection. Proc. Natl. Acad. Sci. USA 97:10948-10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mullins, J. I. 2001. Compartments and reservoirs of HIV infection in vivo. Keystone Symposium on AIDS Pathogenesis 2001. Keystone Symposia, Silverthorne, Colo.
- 27.Murali-Krishna, K., L. L. Lau, S. Sambhara, F. Lemonnier, J. D. Altman, and R. Ahmed. 1999. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science 286:1377-1381. [DOI] [PubMed] [Google Scholar]
- 28.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 29.Persaud, D., T. Pierson, C. Ruff, D. Finzi, K. R. Chadwick, J. B. Margolick, A. Ruff, N. Hutton, S. Ray, and R. F. Siliciano. 2000. A stable latent reservoir for HIV-1 in resting CD4+ T lymphocytes in infected children. J. Clin. Investig. 105:995-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posada, D., and K. A. Crandall. 2001. Selecting models of nucleotide substitution: an application to human immunodeficiency virus 1 (HIV-1). Mol. Biol. Evol. 18:897-906. [DOI] [PubMed] [Google Scholar]
- 31.Ramratnam, B., J. E. Mittler, L. Zhang, D. Boden, A. Hurley, F. Fang, C. A. Macken, A. S. Perelson, M. Markowitz, and D. D. Ho. 2000. The decay of the latent reservoir of replication-competent HIV-1 is inversely correlated with the extent of residual viral replication during prolonged antitretroviral therapy. Nat. Med. 6:82-85. [DOI] [PubMed] [Google Scholar]
- 32.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 33.Shankarappa, R., J. B. Margolick, S. J. Gange, A. G. Rodrigo, D. Upchurch, H. Farzadegan, P. Gupta, C. R. Rinaldo, G. H. Learn, X. He, X. L. Huang, and J. I. Mullins. 1999. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J. Virol. 73:10489-10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith, D. B., J. McAllister, C. Casino, and P. Simmonds. 1997. Virus “quasispecies”: making a mountain out of a molehill? J. Gen. Virol. 78(Pt. 7):1511-1519. [DOI] [PubMed] [Google Scholar]
- 35.Spina, C. A., J. C. Guatelli, and D. D. Richman. 1995. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J. Virol. 69:2977-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6:578-582. [DOI] [PubMed] [Google Scholar]
- 37.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 38.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Y. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, L., C. Chung, B.-S. Hu, T. He, Y. Guo, A. J. Kim, E. Skulsky, X. Jin, A. Hurley, B. Ramratnam, M. Markowitz, and D. D. Ho. 2000. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J. Clin. Investig. 106:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]