Abstract
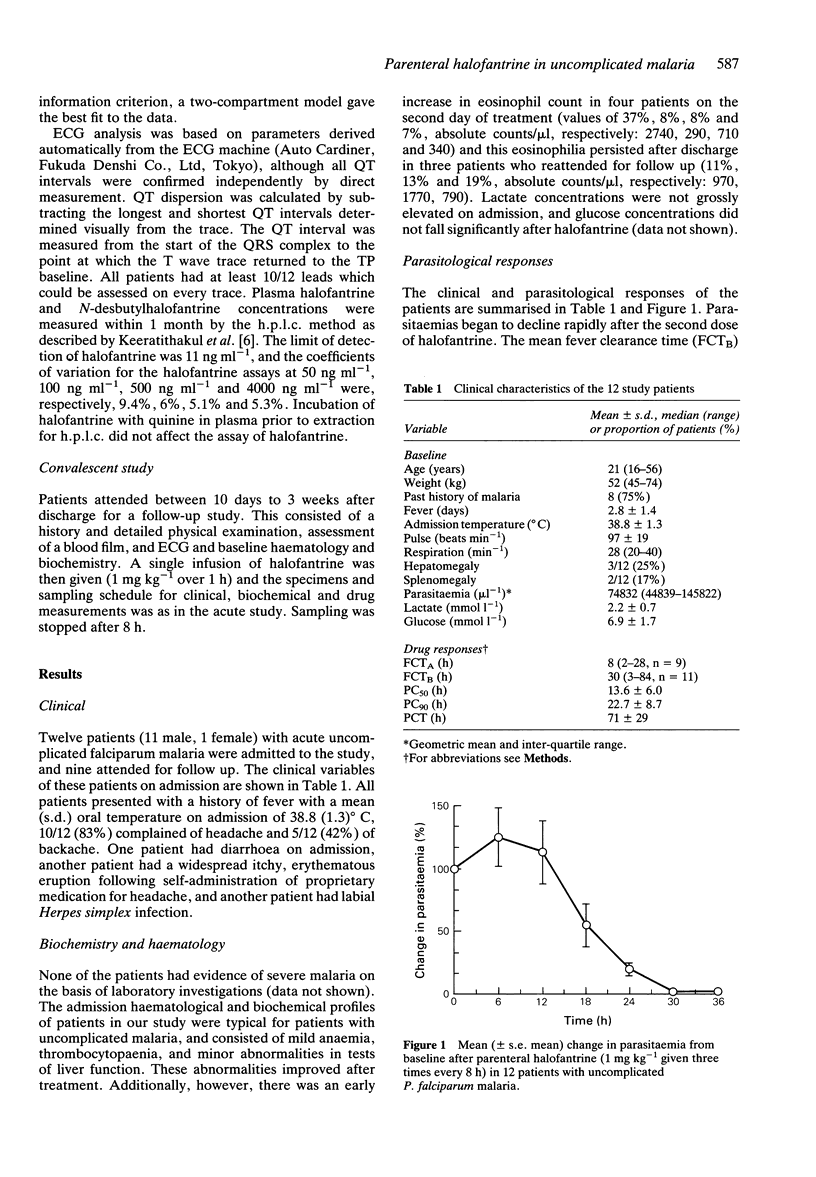
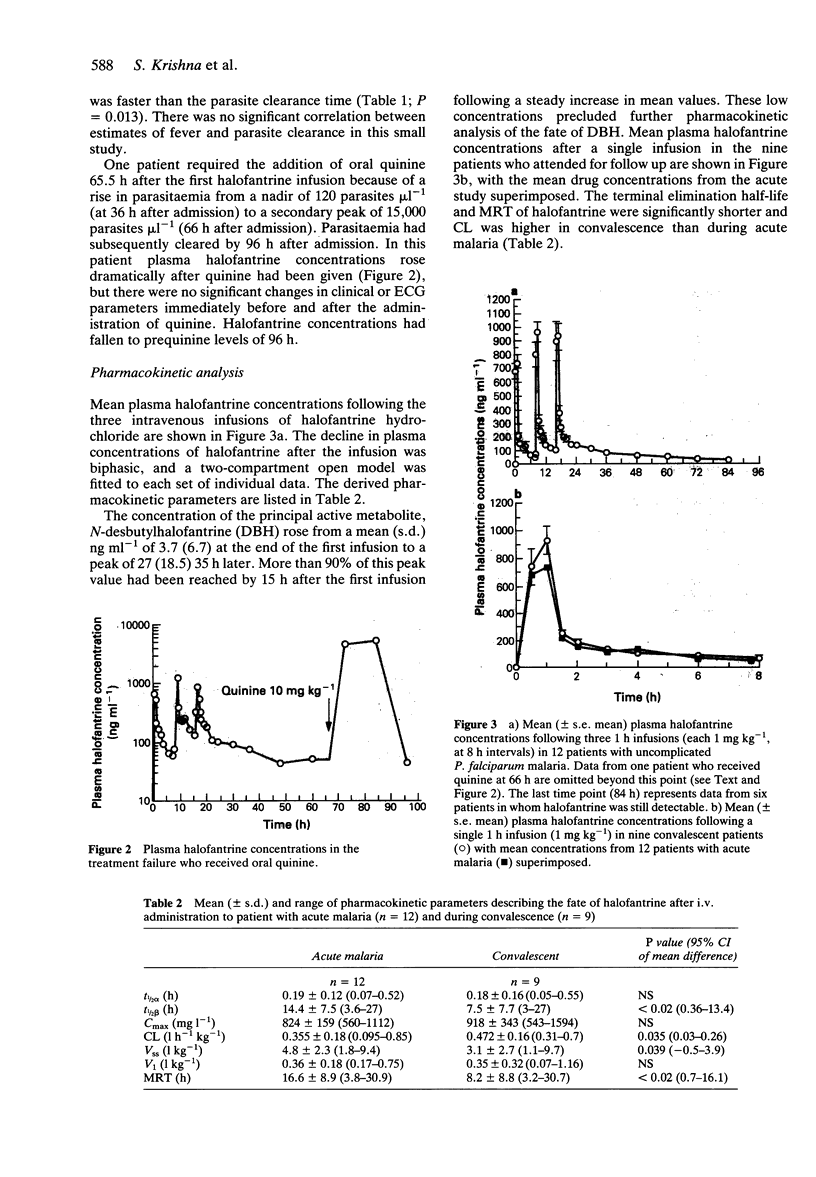
1 The pharmacokinetics, efficacy and toxicity of a new parenteral formulation of halofantrine hydrochloride were evaluated in 12 adults with acute uncomplicated falciparum malaria and nine adults who attended in convalescence. 2 Intravenous halofantrine (1 mg kg(-1) infused in 1 h) was given every 8 h for a total of three doses in the acute study. Halofantrine cleared parasitaemia rapidly in all but one patient, with a mean (s.d.) parasite clearance time of 71 (29) h. Convalescent patients received a single infusion (1 mg kg(-1) in 1 h). 3 An open two-compartment model with the following parameters described the pharmacokinetics of halofantrine in acute malaria (mean (s.d)): V1 = 0.36 (0.18) l kg(-1); CL = 0.355 (0.18) l h(-1) kg(-1); t1/2alpha = 0.19 (0.12) h; t1/2beta = 14.4 (7.5) h. 4 Intravenous halofantrine in acute malaria produced significant prolongations of the QT and QTc intervals (mean (s.d.)) of 20 (15%) and 8.2 (5.6)%, respectively (P < 0.001) after the third dose, but no clinically significant cardiotoxcity. Eight patients experienced mild to moderate thrombophlebitis at the halofantrine infusion site which had resolved in six by the time of follow-up. In the single treatment failure who received oral quinine, there was a large rise in plasma halofantrine concentration but this did not result in detectable toxicity. 5 These data provide the basis for the design of improved dosing regimens for the use of parenteral halofantrine in malaria.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryson H. M., Goa K. L. Halofantrine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1992 Feb;43(2):236–258. doi: 10.2165/00003495-199243020-00009. [DOI] [PubMed] [Google Scholar]
- Cowan J. C., Yusoff K., Moore M., Amos P. A., Gold A. E., Bourke J. P., Tansuphaswadikul S., Campbell R. W. Importance of lead selection in QT interval measurement. Am J Cardiol. 1988 Jan 1;61(1):83–87. doi: 10.1016/0002-9149(88)91309-4. [DOI] [PubMed] [Google Scholar]
- Davis T. M., Ho M., Supanaranond W., Looareesuwan S., Pukrittayakamee S., White N. J. Changes in the peripheral blood eosinophil count in falciparum malaria. Acta Trop. 1991 Jan;48(3):243–246. doi: 10.1016/0001-706x(91)90052-l. [DOI] [PubMed] [Google Scholar]
- Day C. P., McComb J. M., Campbell R. W. QT dispersion in sinus beats and ventricular extrasystoles in normal hearts. Br Heart J. 1992 Jan;67(1):39–41. doi: 10.1136/hrt.67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeratithakul D., Teja-Isavadharm P., Shanks G. D., Webster H. K., Edstein M. D. An improved high-performance liquid chromatographic method for the simultaneous measurement of halofantrine and desbutylhalofantrine in human serum. Ther Drug Monit. 1991 Jan;13(1):64–68. doi: 10.1097/00007691-199101000-00009. [DOI] [PubMed] [Google Scholar]
- Nosten F., ter Kuile F. O., Luxemburger C., Woodrow C., Kyle D. E., Chongsuphajaisiddhi T., White N. J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993 Apr 24;341(8852):1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- Veenendaal J. R., Parkinson A. D., Kere N., Rieckmann K. H., Edstein M. D. Pharmacokinetics of halofantrine and n-desbutylhalofantrine in patients with falciparum malaria following a multiple dose regimen of halofantrine. Eur J Clin Pharmacol. 1991;41(2):161–164. doi: 10.1007/BF00265910. [DOI] [PubMed] [Google Scholar]
- Watkins W. M., Woodrow C., Marsh K. Falciparum malaria: differential effects of antimalarial drugs on ex vivo parasite viability during the critical early phase of therapy. Am J Trop Med Hyg. 1993 Jul;49(1):106–112. doi: 10.4269/ajtmh.1993.49.106. [DOI] [PubMed] [Google Scholar]
- Weinke T., Loscher T., Fleischer K., Kretschmer H., Pohle H. D., Kohler B., Schlunk T., Clemens R., Bock H. L. The efficacy of halofantrine in the treatment of acute malaria in nonimmune travelers. Am J Trop Med Hyg. 1992 Jul;47(1):1–5. [PubMed] [Google Scholar]
- White N. J., Krishna S. Treatment of malaria: some considerations and limitations of the current methods of assessment. Trans R Soc Trop Med Hyg. 1989 Nov-Dec;83(6):767–777. doi: 10.1016/0035-9203(89)90322-2. [DOI] [PubMed] [Google Scholar]
- White N. J., Looareesuwan S., Warrell D. A. Quinine and quinidine: a comparison of EKG effects during the treatment of malaria. J Cardiovasc Pharmacol. 1983 Mar-Apr;5(2):173–175. doi: 10.1097/00005344-198303000-00001. [DOI] [PubMed] [Google Scholar]
- ter Kuile F. O., Dolan G., Nosten F., Edstein M. D., Luxemburger C., Phaipun L., Chongsuphajaisiddhi T., Webster H. K., White N. J. Halofantrine versus mefloquine in treatment of multidrug-resistant falciparum malaria. Lancet. 1993 Apr 24;341(8852):1044–1049. doi: 10.1016/0140-6736(93)92409-m. [DOI] [PubMed] [Google Scholar]


