Abstract
Herpes simplex virus type 1 (HSV-1) encodes a complement-interacting glycoprotein, gC, and an immunoglobulin G (IgG) Fc binding glycoprotein, gE, that mediate immune evasion by affecting multiple aspects of innate and acquired immunity, including interfering with complement components C1q, C3, C5, and properdin and blocking antibody-dependent cellular cytotoxicity. Previous studies evaluated the individual contributions of gC and gE to immune evasion. Experiments in a murine model that examines the combined effects of gC and gE immune evasion on pathogenesis are now reported. Virulence of wild-type HSV-1 is compared with mutant viruses defective in gC-mediated C3 binding, gE-mediated IgG Fc binding, or both immune evasion activities. Eliminating both activities greatly increased susceptibility of HSV-1 to antibody and complement neutralization in vitro and markedly reduced virulence in vivo as measured by disease scores, virus titers, and mortality. Studies with C3 knockout mice indicated that other activities attributed to these glycoproteins, such as gC-mediated virus attachment to heparan sulfate or gE-mediated cell-to-cell spread, do not account for the reduced virulence of mutant viruses. The results support the importance of gC and gE immune evasion in vivo and suggest potential new targets for prevention and treatment of HSV disease.
Herpes simplex virus type 1 (HSV-1) and HSV-2 are common human pathogens that cause herpes labialis (cold sores or fever blisters), encephalitis, keratitis, and genitalis. HSV encephalitis is the most common form of sporadic encephalitis in the United States, while herpes keratitis affects approximately 400,000 individuals and is the leading infectious cause of corneal blindness in the United States (31, 48). Herpes genitalis is a common sexually transmitted disease, with HSV-2 accounting for approximately two-thirds and HSV-1 accounting for one-third of new cases in the United States (46). Prevention of HSV-1 and -2 is an important public health goal which will likely only be achieved by developing effective vaccines.
Chiron Biocine evaluated an HSV-2 glycoprotein subunit vaccine containing gB-2 and gD-2, which failed to prevent HSV infection but reduced duration and severity of recurrences (4, 29, 47). Results of two trials for prevention of genital herpes were reported by SmithKline Beecham (now GlaxoSmithKline) using a gD-2 subunit vaccine and a adjuvant different from that used in the Chiron study (S. L. Spruance and the SmithKline Beecham Herpes Vaccine Efficacy Study Group, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother, abstr. L-6, p. 18, 2000). Vaccine efficacy against genital herpes disease (lesion formation) was observed in HSV-1-seronegative, HSV-2-seronegative women (73% in trial 1 [P = 0.01]; 74% in trial 2 [P = 0.02]). However, the vaccine offered no protection for male subjects or for HSV-1-seropositive, HSV-2-seronegative females. Vaccine efficacy failed to achieve statistical significance when evaluated for infection rather than disease, where infection included asymptomatic seroconversion to nonvaccine HSV antigens. These results show promise for subunit HSV vaccines but indicate that improvements on the current formulation may be required.
Epidemiological studies of humans indicate that prior HSV-1 infection protects against subsequent symptomatic HSV-2 infection and vice versa, supporting the concept that immunity to HSV can be protective (3, 30). However, HSV is capable of evading immune attack by interfering with major histocompatibility complex class I antigen presentation, inhibiting activities mediated by complement (C) components C3, C5, and properdin (P) and interfering with activities mediated by the Fc domain of immunoglobulin G (IgG) antibodies (7, 12, 13, 16, 17, 19, 28, 34, 38, 50). Some HSV-1 immune evasion molecules are surface glycoproteins expressed on the virion envelope and at the infected cell surface; therefore, these molecules are potential targets for antibodies that may bind to critical domains and block their functions. Blocking immune evasion may improve the effectiveness of innate and vaccine-induced immune responses (H. M. Friedman, Letter, JAMA 283:746-747, 2000).
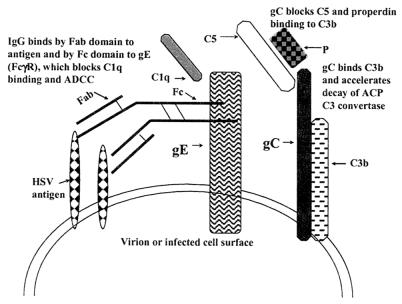
HSV-1 glycoprotein gE binds the IgG Fc domain and interferes with C1q binding and antibody-dependent cellular cytotoxicity (ADCC) (Fig. 1) (7). HSV-1 glycoprotein gC binds C component C3 and its activation products, C3b, iC3b, and C3c, and accelerates the decay of the alternative C pathway C3 convertase (16, 28). HSV-1 gC also interferes with C5 and P binding to gC (16, 28) (Fig. 1). HSV-1 gC and gE interfere with different aspects of host immunity, including blocking C activation at different stages of the cascade; therefore, these glycoproteins may be additive or synergistic in mediating immune evasion.
FIG. 1.
Model showing gC- and gE-mediated protection against antibody and C. gC binds C3b, blocks C5 and P binding to C3b, and accelerates decay of the alternative C pathway (ACP) C3 convertase, while gE binds the IgG Fc domain, which blocks C1q binding and ADCC. gC and gE affect different stages of the C cascade, suggesting that in combination the effects may be greater than those mediated by either glycoprotein alone.
Viral genes that encode IgG Fcγ receptors (FcγRs) are widely expressed by herpesviruses, including HSV-1 and -2, cytomegalovirus, varicella-zoster virus, and pseudorabies virus, while C-interacting proteins are also expressed by many herpesviruses, including HSV-1 and -2, pseudorabies virus, equine herpesvirus type 1, bovine herpesvirus type 1, herpesvirus saimiri, and murine gammaherpesvirus (1, 8, 9, 11, 13, 20-22, 27, 32, 33, 35, 39-41). We previously reported that mutant HSV-1 viruses defective in gC binding to C3b or gE binding to IgG Fc are approximately 50- to 100-fold more susceptible to C-enhanced antibody neutralization in vitro (34, 38). We showed that human HSV antibody is more active in vivo against gE mutant than wild-type (WT) virus because WT virus binds the IgG Fc domain and blocks activities mediated by this region and that gC mutant virus is more susceptible than WT virus to C-mediated attack in vivo (34, 38). The present experiments were performed to extend our previous studies by comparing the pathogenic properties of a gC/gE double-mutant virus with those of gC and gE single-mutant and WT viruses for susceptibility to C-enhanced antibody neutralization in vitro and for virulence in vivo.
MATERIALS AND METHODS
Construction of gC/gE double-mutant virus.
HSV-1 strain NS, a low-passage-number clinical isolate, was the parental strain for the gC/gE double-mutant virus and the gC and gE single-mutant viruses previously described (14, 34, 38). The gC single-mutant virus NS-gCΔC3 has a deletion of 93 amino acids (gC amino acids 275 to 367), which eliminates the ability of gC to bind C3b (Fig. 2) (34). The gE single-mutant virus NS-gE339 has four amino acids inserted after gE position 339, which eliminates the ability of gE to bind the Fc domain of human IgG (38). The gC/gE double-mutant virus NS-gCΔC3,gE339 was prepared by cotransfecting Vero cells with NS-gE339 DNA and the plasmid used to construct NS-gCΔC3 (34). Recombinant viruses were screened by immunoperoxidase assay using monoclonal antibody (MAb) 1C8, which detects gC sequences between amino acids 275 to 367. White plaques were picked and subjected to three rounds of plaque purification. The location of the gCΔC3 mutation in NS-gCΔC3,gE339 virus was verified by PCR amplification of the gC gene using DNA primers situated 5′ (5′-CAG CCC CAC GGG AAG ATC TT-3′) and 3′ (5′-GGC CGT GCA GAC CAC ATG-3′) of the gC deletion. The viruses used in this study were NS, NS-gCΔC3, NS-gE339, and NS-gCΔC3,gE339. NS is the parental WT virus. NS-gCΔC3 is a gC single-mutant virus that has a deletion of C3b binding regions II and III to eliminate C3b binding (34). NS-gE339 is a gE single-mutant virus that has an insertion of 4 amino acids at gE339 to eliminate IgG Fc binding (38). NS-gCΔC3,gE339 is a gC/gE double-mutant virus that combines features of both gC and gE single-mutant viruses.
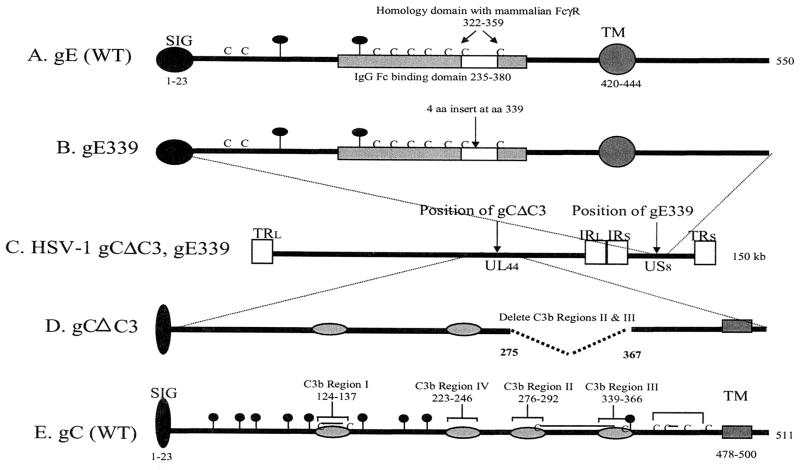
FIG. 2.
Features of WT and mutant gC and gE genes. (A) gE from WT virus. The IgG Fc binding domain extends from amino acids 235 to 380. (B) gE with a mutation at amino acid 339. The arrow indicates the position of a 4-amino-acid insertion after amino acid 339. (C) Features of the gC/gE double-mutant virus HSV-1 gCΔC3,gE339. Diagram of HSV-1 genome showing recombination sites of gC and gE mutant genes. (D) gC with a mutation in the C3b binding domain. The position of a gC deletion from amino acids 275 to 367 that eliminates C3b binding regions II and III is shown. (E) gC from WT virus. The positions of four C3b binding regions are shown. Abbreviations and symbols: SIG, signal sequence; TM, transmembrane domain; C, cysteine; lines joining Cs indicate the disulfide binding pattern of gC; balloons, predicted N-linked glycosylation sites; TRL, terminal repeat long; IRL, internal repeat long; IRS, internal repeat short; TRS, terminal repeat short; UL44, unique long gene 44; US8, unique short gene 8; gray shaded box, gE IgG Fc binding domain; white box, gE domain of homology with mammalian FcγR; gray ovals on gC, C3b binding regions I to IV.
Sucrose gradient purification of viruses.
Vero cells were infected at a ratio of five infectious virus particles per cell (multiplicity of infection [MOI] = 5) for 20 h at 37°C, and then virus was purified from supernatant fluids on 5 ml of 5 to 65% sucrose gradient (15). Fractions (0.5 ml) were collected, and the virus in fractions 6 to 8 was dialyzed against phosphate-buffered saline (PBS) at 4°C and stored at −70°C. Virus titers were determined by plaque assay on Vero cells.
One-step growth curves.
Vero cells were inoculated at an MOI of 5, and at 1, 4, 8, 12, 20, and 24 h postinfection, cells and supernatant fluids were harvested, cells were lysed by sonication, and virus titers were determined by plaque assay on Vero cells.
Western blots.
Purified viruses were run on sodium dodecyl sulfate (SDS)-10% polyacrylamide gels and transferred to Immobilon-P membranes (Millipore, Bedford, Mass.), and gC protein was visualized using R47, a polyclonal rabbit anti-gC antibody, while gE protein was visualized using R575, a polyclonal rabbit anti-gE antibody (38). Horseradish peroxidase-conjugated goat anti-rabbit IgG and enhanced chemiluminescence (Amersham Pharmacia, Piscataway, N.J.) were used to visualize the gC and gE antibodies.
Antibody and C neutralization assays.
Antibody and C neutralization assays were performed by incubating 105 to 106 PFU of purified virus with pooled human IgG as a source of antibody and HSV-1- and -2-seronegative human serum as a source of C. Pooled human IgG was purchased from the Michigan State Health Laboratories and represents IgG pooled from sera of thousands of healthy donors; therefore, this reagent reflects antibody responses in the general population (38). The concentration of antibody against WT virus (NS) at which 50% of NS was neutralized in the absence of C was 50 μg/ml, which is approximately equivalent to the IgG content in a 1:200 dilution of serum. Pooled human IgG was used at concentrations of 50 and 100 μg/ml, which were selected because antibody binds to virus at these concentrations yet the neutralizing activity is modest enough that if C adds to the effect it can be measured in the assay (C-enhanced antibody neutralization assay) (38). HSV-seronegative human serum was diluted to a concentration 1 or 10%, which had little neutralizing activity against the viruses in the absence of antibody. Virus was incubated with antibody alone, C alone, or antibody and C for 1 h at 37°C, and titers were determined by plaque assay on Vero cells.
C3b and IgG Fc rosetting assays.
Vero cells were infected at an MOI of 2 for 20 h, and then cells were removed using cell dissociation buffer (Invitrogen Corp., Carlsbad, Calif.) incubated with C3b-coated erythrocytes or IgG-coated erythrocytes for 1 h at 37°C and examined by microscopy for rosettes. Cells with four or more erythrocytes bound were considered positive (15, 38).
Flow cytometry forC3b and IgG Fc binding.
Vero cells were infected at an MOI of 2 for 16 h, and cells were dissociated using cell dissociation buffer. gE expression at the cell surface was detected using anti-gE MAb 1BA10, while IgG Fc binding was measured using biotinylated nonimmune IgG and phycoerythrin-streptavidin (44). gC expression was detected using MAb 1C8, while C3b binding was measured using fluorescein isothiocyanate-labeled C3b (28). Cells were fixed in 2% paraformaldehyde, and fluorescence intensity was measured by flow cytometry (FACScan, Becton Dickinson, San Jose, Calif.).
Murine flank model.
For some experiments, 16 h prior to infection mice were passively immunized intraperitoneally with 200 μg of pooled human IgG (HSV antibody) or 200 μg of human nonimmune IgG as a control (38). Human HSV antibody is used to assess the importance of gE in immune evasion in vivo because the Fc domain of human IgG binds to the HSV FcγR while the Fc domain of murine IgG does not (25). Murine C3b binds to gC; therefore, C-sufficient (C-intact) mice serve as the source of C for these studies (21, 34). The flanks of 6- to 7-week-old female BALB/c, C56BL/6, or C3 knockout mice were shaved and chemically denuded with Nair, and purified virus was inoculated by scratching the skin using a 30-gauge needle (34, 45). Disease at the inoculation site was scored as follows. Erythema or swelling with no vesicles was assigned 0.5 points, and individual vesicles were scored as 1 point each, with a total maximum daily score of 5. If lesions coalesced, up to 5 points were assigned based on the size of the lesions. Zosteriform disease was scored similarly except that the maximum daily score was 10, because more lesions could be counted over the larger skin area involved. Use of animals in these studies complies with all relevant federal guidelines and with policies of the University of Pennsylvania Office of Regulatory Affairs.
Virus titers from skin inoculation sites.
At 1 h and 1, 2, 3, or 4 days postinfection, mice were euthanized and a 0.5-cm2 area of skin was excised from the inoculation site, Dounce homogenized, and stored at −70°C (38). Later, the homogenates were thawed and aliquots were taken for measuring virus titers by plaque assay on Vero cells.
Statistical analysis.
Student's t test was used to calculate differences between viruses in neutralization assays and disease scores. The lower limit of detection of virus in the neutralization assay is 1.3 log10. Virus titers that were undetectable were assigned a value of 1.3 log10 for statistical analyses and when plotted in Fig. 5 and 9.
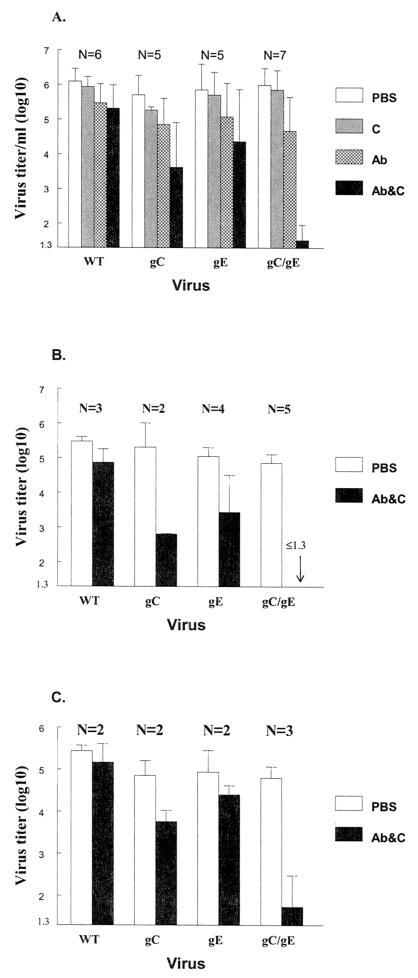
FIG. 5.
Complement-enhanced antibody neutralization of WT and gC and gE mutant viruses. (A) WT virus NS, gC single-mutant virus NS-gCΔC3 (gC), gE single-mutant virus NS-gE339 (gE), or gC/gE double-mutant virus NS-gCΔC3,gE339 (gC/gE) (each at approximately 106 PFU/ml) was incubated with PBS, 10% seronegative human serum as source of C, pooled human IgG (HSV antibody) (100 μg/ml), or pooled human IgG (100 μg/ml) and 10% C. Statistical analyses compared differences in titer when viruses were incubated with antibody alone or antibody and C (for gC versus WT, P = 0.002; for gE versus WT, P = 0.03; for gC/gE versus WT, P = 0.00001; for gC/gE versus gC, P = 0.001; for gC/gE versus gE, P = 0.0001). (B) Neutralization using less virus and lower concentrations of C. Virus (1 × 105 to 5 × 105 PFU/ml) was incubated with antibody (100 μg/ml) and 1% C. The gC/gE mutant virus titer was undetectable (≤1.3 log10) when incubated with antibody and C (indicated by arrow). Statistical analyses compared PBS with antibody and C (for gC/gE versus WT, P = 0.00001; for gC/gE versus gC, P = 0.02; for gC/gE versus gE, P = 0.007). (C) Neutralization using less virus and lower concentrations of antibody. Virus (1 × 105 to 5 × 105 PFU/ml) was incubated with antibody (50 μg/ml) and 10% C. Statistical analyses compared PBS with antibody and C (for gC/gE versus WT, P = 0.03; for gC/gE versus gC, P = 0.08 [not significant]; for gC/gE versus gE, P = 0.045). Error bars represent standard deviations. The number of replicate experiments is shown above the bars. Abbreviations: Ab, HSV IgG antibody alone; Ab&C, antibody and C.
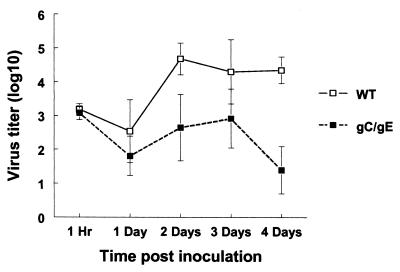
FIG. 9.
Viral titers at the inoculation site in C-sufficient mice passively immunized with pooled human IgG (HSV antibody) and infected with WT (NS) or gC/gE mutant virus NS-gCΔC3,gE339 at 5 × 105 PFU. Skin at the inoculation site was excised at 1 h and 1 to 4 days postinoculation, and titers were determined. Three mice were evaluated at each time point. Results are plotted as means ± standard errors of the means (error bars). For comparison of WT and gC/gE mutant virus titers on day 4, P = 0.02.
RESULTS
Characterization of gC/gE mutant virus.
We previously reported the in vitro and in vivo phenotypes of HSV-1 gE mutant virus NS-gE339 that lacks the ability to bind the IgG Fc domain, and HSV-1 gC mutant virus NS-gCΔC3 that fails to bind C components C3, C3b, iC3b, and C3c (34, 38). We now describe the phenotype of an HSV-1 mutant virus impaired in both IgG Fc and C3 binding. Features of WT and mutant gE and gC glycoproteins are shown in Fig. 2. WT gE has an IgG Fc binding domain extending from amino acids 235 to 380 (Fig. 2A). Located within this region is a domain of homology with mammalian IgG Fc binding proteins from amino acids 322 to 359. A 4-amino-acid insert after gE position 339 (Fig. 2B) eliminates IgG Fc binding (6). WT gC (Fig. 2E) has four C3b binding regions (light gray), each of which is required to bind C3b (23). The gCΔC3 construct deletes C3b binding regions II and III extending from gC amino acids 275 to 367. This deletion removes two cysteines linked by a disulfide-bond, which should minimize the effects of the deletion on gC conformation (Fig. 2D) (43). The gCΔC3 gene was used to replace WT gC by homologous recombination with HSV-1 gE339 DNA to create NS-gCΔC3,gE339 virus (Fig. 2C).
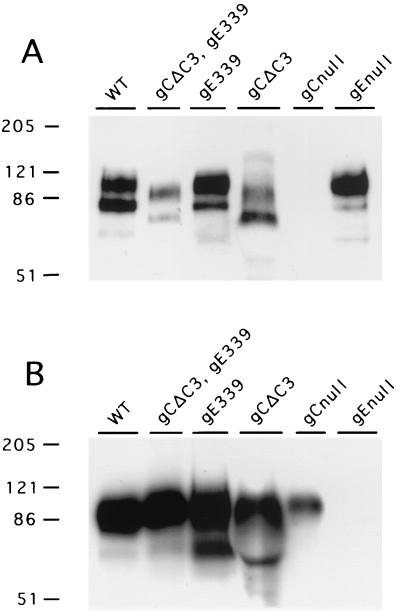
The accuracy of the gCΔC3 recombination in NS-gE339 was verified by sequencing across the gC gene locus, using PCR to amplify the gene. The kinetics of virus replication and peak titers achieved for NS-gCΔC3,gE339 (referred to as gC/gE) mutant virus were similar to those for WT virus when evaluated by single-step growth curves (results not shown). The expression of gC and gE in purified viruses was determined by SDS-polyacrylamide gel electrophoresis and Western blotting (Fig. 3B) (15, 38). Each mutant virus expressed gC and gE. The mutant gC proteins in NS-gCΔC3 and NS-gCΔC3,gE339 were of the expected size based on deleting amino acids 275 to 367, while the gE mutant virus that contains four additional amino acids had a size indistinguishable from WT gE.
FIG. 3.
Detection of gC and gE in purified HSV-1 viruses. Approximately 106 PFU of each virus was electrophoresed on SDS-polyacrylamide gels, and Western blots were probed for gC (A) and gE (B). Molecular mass markers (in kilodaltons) are shown on the left. WT is the parental strain NS; gCΔC3,gE339 is the gC/gE double-mutant virus NS-gCΔC3,gE339; gE339 is the gE single-mutant virus NS-gE339; gCΔC3 is the gC single-mutant virus NS-gCΔC3; gCnull is a gC mutant virus (NS-gCnull) that has the entire gC protein deleted; and gEnull is a gE mutant virus (NS-gEnull) that deletes the gE protein (15, 38).
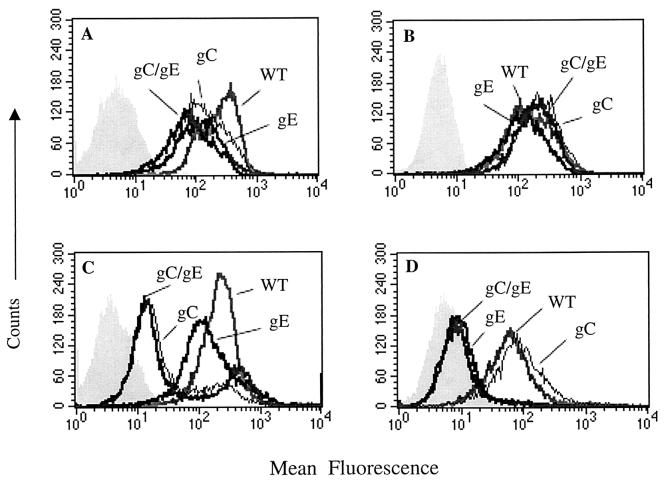
Flow cytometry assays were performed to assess gC and gE expression at the infected cell surface and to measure C3b or IgG Fc binding (Fig. 4). Cells infected with each mutant virus expressed comparable levels of gC and gE (Fig. 4A and B), suggesting that any differences in gC or gE band intensity among the three mutant viruses shown in Fig. 3A and B are likely related to unequal loading. C3b binding was greatly reduced when cells were infected with gC single or gC/gE double-mutant virus compared with WT or gE single-mutant virus (Fig. 4C). IgG Fc failed to bind when cells were infected with gE single or gC/gE double-mutant virus (Fig. 4D). Rosetting assays were preformed as an additional method to assess C3b and IgG Fc binding. Seventy-eight percent (78 of 100 cells) of WT-virus-infected cells formed C3b rosettes, while no gC/gE double-mutant-virus-infected cells formed rosettes (0 of 100 cells). Similarly, 72% (72 of 100) of WT-virus-infected cells formed rosettes with IgG-coated erythrocytes, compared with 4% (4 of 97) of gC/gE double-mutant-virus-infected cells. The flow cytometry and rosetting assays demonstrate that little or no C3b or IgG Fc binds to gC or gE mutant viruses.
FIG. 4.
Flow cytometry probing for gC or gE expression on infected cells and for C3b or IgG Fc binding. WT refers to parental strain NS, gC refers to mutant virus NS-gCΔC3, gE refers to mutant virus NS-gE339, and gC/gE refers to mutant virus NS-gCΔC3,gE339. Shaded areas show mean fluorescence of unstained cells or isotype controls. (A) gC expression; (B) gE expression; (C) C3b binding; (D) IgG Fc binding.
The gC/gE double-mutant virus is more susceptible to C-enhanced antibody neutralization than either gC or gE single-mutant virus.
Approximately 106 PFU/ml of WT or each mutant virus was incubated for 1 h at 37°C with PBS, C alone (10% serum), antibody alone (IgG, 100 μg/ml), or antibody (100 μg/ml) and C (10% serum), and titers were measured by plaque assay (Fig. 5A). C alone had little neutralizing activity against any virus. IgG at 100 μg/ml neutralized each virus by approximately 1 log10 (range, 0.6 to 1.3 log10 [calculated as the difference in titer comparing PBS with antibody]). Neutralizing activity of antibody and C was greater against gC and gE single-mutant viruses than WT virus, which is consistent with our previous results (34, 38). However, the most significant finding was the marked effect of antibody and C on the gC/gE double-mutant virus compared with WT or gC or gE single-mutant virus. Therefore, the combined mutations in the gC/gE virus markedly increase its susceptibility to C-enhanced antibody neutralization.
Additional experiments were performed comparing antibody and C neutralization using lower virus titers than those depicted in Fig. 5A (1 × 105 to 5 × 105 PFU/ml), reducing antibody concentrations twofold or C concentrations 10-fold. Figure 5B shows neutralization results when virus was incubated with antibody at 100 μg/ml and 1% C, while Fig. 5C shows neutralization using antibody at 50 μg/ml and 10% C. In each case, the gC/gE double-mutant virus was more susceptible to antibody and C neutralization than WT or gC or gE single-mutant virus.
Comparisons of WT and mutant viruses in vivo.
We evaluated the ability of WT and mutant viruses to cause disease in the murine flank model. This model involves passive transfer of human immune IgG (HSV antibody), or nonimmune IgG as a control, 16 h prior to infection. Disease scores are determined at both the inoculation and zosteriform sites from days 3 to 7 postinoculation, which is the peak phase of infection. Zosteriform disease develops as virus spreads from the inoculation site to spinal ganglia and returns along nerves to produce skin lesions in a dermatomal (zosteriform) distribution (38, 45).
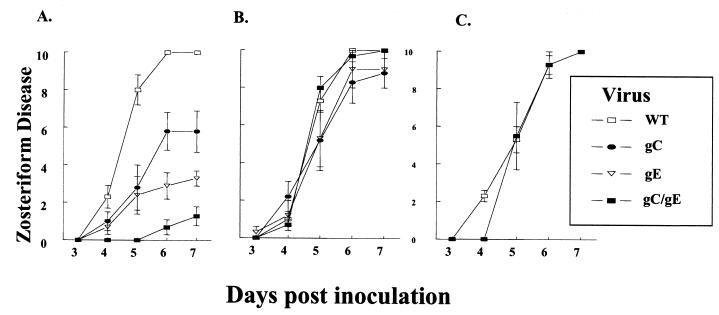
Previously we reported that gC mutant virus at 5 × 104 PFU produced little disease in C-sufficient mice (34) and that gE mutant virus at 5 × 105 PFU caused little disease in the presence of HSV antibody (38). Therefore, higher titers (5 × 106 PFU) were inoculated with the goal of producing more disease with single-mutant viruses so that if the gC/gE double-mutant virus were less virulent we could observe an effect. At 5 × 106 PFU, each virus, except gC/gE mutant virus, produced near-maximum disease scores of 25 at the inoculation site (based on maximum daily scores of 5 points for days 3 to 7). The WT virus score (scores are presented as means ± standard errors of the means) was 24.8 ± 0.2, the gC single-mutant virus score was 24.8 ± 0.1, the gE single-mutant virus score was 24 ± 0.4, and the gC/gE double-mutant virus score was 21 ± 1.4 (for comparison of gC/gE versus WT, P = 0.016; for comparison of gC/gE versus gC, P = 0.014; for comparison of gC/gE versus gE, P = 0.07 [not significant]). Differences among the four viruses were greater at the zosteriform site (Fig. 6A). Each single-mutant virus produced lower disease scores than the WT virus; however, the most striking finding was the reduction in zosteriform disease caused by the gC/gE double-mutant virus.
FIG. 6.
Disease scores at the zosteriform site in mice infected with WT or gC and gE mutant viruses at 5 × 106 PFU. (A) C-sufficient mice were passively immunized with 200 μg of pooled human IgG (HSV antibody). The next day, mice were scratch inoculated on the denuded flank with 5 × 106 PFU and scored for zosteriform disease (maximum daily score of 10). Eight animals are in the NS and gC virus groups, and seven are in the gE and gC/gE virus groups. Cumulative disease scores on days 3 to 7 were compared (for gC/gE mutant versus WT virus, P = 0.000001; for gC/gE versus gC, P = 0.001; for gC/gE versus gE, P = 0.005). (B) C-sufficient mice were passively immunized with human nonimmune IgG, infected with each of the four viruses, and scored for zosteriform disease. Six mice were in each group. (C) C3 knockout mice were passively immunized with pooled human IgG (HSV antibody), infected with WT or gC/gE mutant virus, and scored for zosteriform disease. Three mice were infected with WT virus, and four were infected with gC/gE mutant virus. Abbreviations: WT, wild-type virus NS; gC, gC single-mutant virus NS-gCΔC3; gE, gE single-mutant virus NS-gE339; gC/gE, gC/gE double-mutant virus NS-gCΔC3,gE339. Results are plotted as means ± standard errors of the means (error bars).
In the above experiments, both antibody and C were present since animals were passively immunized with HSV antibody and the mice had intact C systems. To determine if both were required to modify disease scores, C-sufficient mice were passively immunized with nonimmune IgG (C present but no antibody) (Fig. 6B), and C3 knockout mice were passively immunized with pooled human IgG (antibody present but no C) (Fig. 6C). Under these conditions, disease scores of gC/gE mutant virus were similar to disease scores of WT virus, indicating that both antibody and C contribute to viral pathogenesis.
Defining the magnitude of the effect of gC and gE mutations on disease severity.
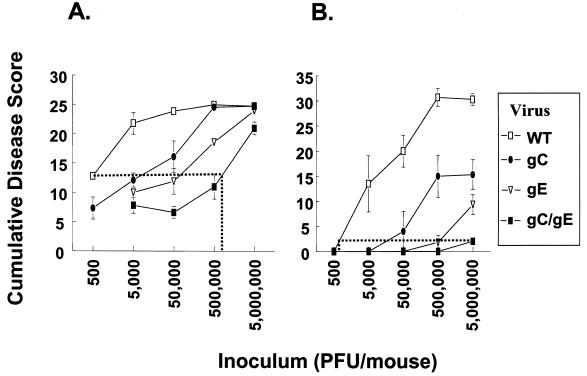
We compared disease scores of mice infected with WT or mutant viruses over a 3- to 4-log10 range of inoculation titers. All animals were passively immunized with 200 μg of pooled human IgG (HSV antibody) prior to infection (Fig. 7). The results demonstrate that the gC and gE single-mutant viruses caused less disease than WT virus and that the gC/gE double-mutant virus was less virulent than either single-mutant virus. At the inoculation site, approximately 1,000-fold more gC/gE mutant virus was required to produce disease comparable to that produced by WT virus, as shown by the dotted lines in Fig. 7A.
FIG. 7.
Disease scores at the inoculation and zosteriform sites in mice infected with WT or gC and gE mutant viruses at doses ranging from 5 × 102 to 5 × 106 PFU. Mice were passively immunized with 200 μg of pooled immune IgG (HSV antibody). The next day, mice were inoculated with 5 × 102 to 5 × 106 (labeled as 500 to 5,000,000) PFU and scored for disease at the inoculation (A) and zosteriform (B) sites. Disease scores are plotted as the average cumulative scores ± standard errors of the means (error bars) for days 3 to 7 postinfection. Four to eight animals were in each group. Abbreviations: WT, wild-type virus NS; gC, gC single-mutant virus NS-gCΔC3; gE, gE single-mutant virus NS-gE339; gC/gE, gC/gE double-mutant virus NS-gCΔC3,gE339. (A) The horizontal dotted line connects WT virus disease score after infection with 500 PFU with the disease score for gC/gE mutant virus. The line intersects the gC/gE mutant virus curve at a disease score produced when slightly more than 500,000 PFU was inoculated (vertical dotted line). (B) The dotted line connects the disease score produced after infection with 5,000,000 PFU of gC/gE mutant virus with the curve for WT virus. Inoculation with 5,000,000 PFU of gC/gE mutant virus produces a zosteriform disease score similar to that produced by inoculation with approximately 500 PFU of WT virus. At the inoculation site, statistical differences between WT and gC/gE mutant viruses are highly significant (P ≤ 0.001 at 5 × 103 to 5 × 105 PFU; P = 0.016 at 5 × 106 PFU). Differences between gC/gE and gC mutant viruses are also significant at each dose tested (P = 0.035 at 5 × 103 PFU, P = 0.03 at 5 × 104 PFU, P < 0.001 at 5 × 105 PFU, and P = 0.014 at 5 × 106 PFU). Differences between gC/gE and gE mutant viruses are significant at 5 × 105 PFU (P < 0.001), while differences between gC and gE mutant viruses are significant at the two highest inoculation titers (P < 0.001 at 5 × 105 PFU; P = 0.04 at 5 × 106 PFU). At the zosteriform site, statistical differences between WT and gC/gE mutant viruses are significant (P ≤ 0.001 at 5 × 104 to 5 × 106 PFU; P = 0.05 at 5 × 103 PFU). Differences between gC/gE and gC mutant viruses are significant at 5 × 105 and 5 × 106 PFU (P ≤ 0.01), while differences between gC/gE and gE mutant viruses are significant at 5 × 106 PFU (P = 0.006). Differences between gC and gE mutant viruses are significant at 5 × 105 PFU (P = 0.03).
The differences in virulence among the four viruses at the zosteriform site are shown in Fig. 7B. Between 1,000- and 10,000-fold more gC/gE mutant virus is required to produce disease scores comparable to those produced by WT virus. Mortality results confirmed large differences in virulence between WT and mutant viruses. Seventeen of 19 mice inoculated with WT virus at titers of ≥5 × 104 PFU died between 8 and 12 days postinfection, whereas only 1 of 19 mice inoculated with gE single-mutant virus died and none inoculated with gC single-mutant or gC/gE double-mutant virus died.
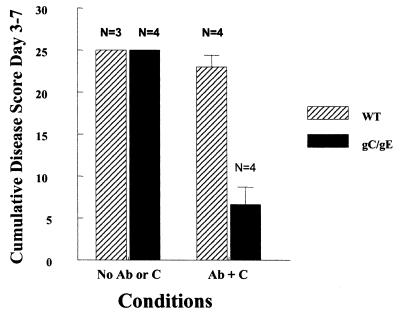
gC mediates virus attachment to heparan sulfate proteoglycans on cells, while gE promotes virus spread from cell to cell (5, 10, 18, 26, 37, 42, 44, 49). Additional experiments were performed to help distinguish gC- and gE-mediated immune evasion from these other activities mediated by gC and gE. WT and gC/gE mutant viruses were inoculated at 5 × 104 PFU, and disease scores were compared in C3 knockout mice that received no passive transfer of human HSV antibody (no antibody or C) (Fig. 8). Under these conditions, we postulated that the gC/gE mutant virus should be as virulent as WT virus if gC and gE domains involved in immune evasion are modified without affecting regions involved in other activities. As controls, C-sufficient mice were passively immunized with pooled human IgG (antibody and C present) and infected with WT or gC/gE mutant virus. We focused on inoculation site disease based on our previous study that showed a possible zosteriform spread defect in gE single-mutant virus when inoculated at this titer (38). No differences in disease scores at the inoculation site were detected in C3 knockout mice (no antibody and C) (Fig. 8). In contrast, large differences were detected when antibody and C were present (Fig. 8). Therefore, differences between disease scores of WT and gC/gE mutant viruses at the inoculation site cannot be attributed to defects in gC-mediated virus attachment or gE-mediated virus spread.
FIG. 8.
Comparison of inoculation site disease scores for WT HSV-1 (NS) and gC/gE mutant virus NS-gCΔC3,gE339 at 5 × 104 PFU in the presence or absence of antibody and C. C3 knockout mice were mock immunized (No Ab or C), or C-sufficient mice were passively immunized with pooled human IgG (HSV antibody) (Ab + C). Results are plotted as the mean cumulative disease score from days 3 to 7 ± standard errors of the means (error bars). The number of replicate experiments is shown above the bars. Differences between the effects of antibody and C on WT versus gC/gE mutant virus, P = 0.001.
We measured virus titers as an additional marker for differences between WT and gC/gE mutant viruses. C-sufficient mice were passively immunized with pooled human IgG (HSV antibody) and infected (5 × 105 PFU), and skin was excised at the inoculation site at 1 h and 1 to 4 days postinoculation. At 1 h, titers of WT and gC/gE mutant viruses were similar; however, by 1 day gC/gE mutant virus titers were 0.7 log10 lower than WT virus titers, and by 4 days gC/gE mutant virus titers were 3 log10 lower (Fig. 9).
DISCUSSION
Our results demonstrate that gC and gE provide significant protection against C and IgG Fc-mediated activities. The gC/gE mutant virus lacks the C3-interacting domain but contains the region that blocks the interaction of C5 and P with C3b located at the gC amino terminus (28, 34). Therefore, it is possible that a mutant virus lacking this additional immune evasion domain would be even more attenuated than the gC/gE mutant virus used in this study. However, the gC C5-P interacting domain has been implicated in mediating virus attachment to heparan sulfate; therefore, by leaving this region intact we attempted to modify C interaction without affecting virus attachment (42). A second region of gC that is involved in virus attachment to heparan sulfate is located between gC amino acids 129 and 160 (36). This region is also intact in the gC/gE mutant virus. Our results indicate that attachment of gC/gE mutant virus is not impaired, since at the inoculation site the gC/gE mutant virus is as virulent as WT virus when antibody and C are absent. If the gC/gE mutant virus were impaired in attachment, we would not expect this result.
HSV-1 gE is involved in IgG Fc binding and virus spread from cell to cell (5, 12, 26, 37, 39, 44). The gE domains involved in spread have not been completely defined but include portions of the carboxyl terminus intracytoplasmic tail and amino acids around gE 210 and gE 380 (26, 44). Murine IgG Fc does not bind to the HSV-1 FcγR; therefore, virulence of gE IgG Fc mutant viruses should be similar to WT virus in mice (25). Passive transfer of IgG antibody that is capable of binding to the HSV-1 FcγR is required to reveal differences between WT and gE mutant viruses (38). We previously reported that the gE single-mutant virus NS-gE339 eliminated IgG Fc binding to gE without impairing cell-to-cell spread in epidermal cells in vitro or modifying virulence at the inoculation site in mice passively immunized with nonimmune human IgG (38). However, zosteriform disease was reduced in these mice, suggesting that gE single-mutant virus spread may be impaired as virus travels from the inoculation site to the ganglion or returns from the ganglion to skin to produce zosteriform lesions. Therefore, in our previous report and in this study we emphasized disease scores and virus titers at the inoculation site to avoid confounding interpretation of results because of spread defects. Here, we found that disease scores at the inoculation site were similar for WT and gC/gE mutant viruses in the absence of antibody and C. If gE-mediated cell-to-cell spread were impaired, the gC/gE mutant virus should have scored lower than WT virus under these conditions.
Somewhat unexpectedly, with high-titer inoculations (5 × 106 PFU), no defect was detected in zosteriform spread for gE single-mutant or gC/gE double-mutant virus, since disease scores were comparable for WT and mutant viruses when antibody or C was absent. This result suggests that at high doses the spread phenotype of gE single or gC/gE double-mutant virus is similar to that of WT virus. One thousand-fold more gC/gE mutant virus was required to cause disease comparable to WT virus at the inoculation site, while at the zosteriform site close to 10,000-fold more gC/gE mutant virus was required to cause disease comparable to WT virus. This 10-fold difference between inoculation and zosteriform sites is likely related to spread defects in the gE single- or gC/gE double-mutant virus that become apparent at inoculation titers below 5 × 106 PFU. Further evidence of a gE spread defect at lower inoculation titers became apparent when C3 knockout mice were infected at 5 × 104 PFU, since zosteriform disease scores were reduced after infection with gC/gE mutant virus compared with WT virus (result not shown). Based on our present and previous reports, we conclude that the gE339 mutation leads to reduced zosteriform disease but does not affect inoculation site disease (38).
The in vitro neutralization studies were designed to measure the ability of C to enhance antibody-neutralizing activity, since antibody was used at a concentration that had relatively little neutralizing activity against the four viruses. Addition of C had a significant effect on antibody neutralization of gC or gE single-mutant virus compared with WT virus; however, the greatest effect was noted comparing gC/gE double mutant with WT virus. Antibody and C also had a much greater effect on gC/gE double mutant than WT virus in vivo, suggesting that the concentrations of antibody and C selected for in vitro studies were biologically relevant. gE blocks C activation by inhibiting C1q binding to IgG, while gC inhibits C activation by binding C3b and blocking C5 and properdin interaction with C3b (Fig. 1) (7, 16, 28). The results indicate that by acting at multiple steps in the C cascade, the aggregate effects of gC and gE are much greater than either alone, suggesting cooperation between gC and gE in mediating immune evasion.
The magnitude of the gC and gE effects on immune evasion as measured by neutralization assays in vitro is similar to results in vivo; however, this does not imply that virus neutralization is the critical step affected by antibody and C in vivo. If neutralization were the critical determinant, we would expect differences in titers between WT and gC/gE mutant viruses to be apparent by 1 h postinoculation, which did not occur. By inhibiting C activation, gC and gE could affect virus infection by many mechanisms, including reducing generation of C5a, a potent chemoattractant for granulocytes; reducing C3b and iC3b deposition on cells, which serve as ligands for monocytes, granulocytes, and lymphocytes that target infected cells; and preventing generation of the membrane attack complex that may lead to lysis of infected cells (2, 24). In vivo, gE could mediate immune evasion by inhibiting ADCC, since gE blocks ADCC in vitro (7). C3 knockout mice are not defective in ADCC, yet C3 knockout mice were as susceptible to disease caused by gC/gE mutant as WT virus, suggesting that gE-mediated evasion from C attack is an important component of gE-mediated immune evasion.
Disease scores are one marker of virulence; others include virus titers and mortality. Only one animal died in the gC, gE, and gC/gE virus groups at any inoculation titer, while the majority of mice (89%) infected with WT virus at titers of ≥5 × 104 PFU died. Titers in skin correlate with disease scores, which supports the finding that the gC/gE mutant virus is less virulent than WT virus.
HSV-1 gC and gE are expressed on the virion envelope and at the surface of infected cells; therefore, these glycoproteins are potential targets for vaccines that induce antibodies that block immune evasion domains. Although attenuated, the gC/gE mutant virus produced disease at the inoculation site even at the lowest dose studied. Therefore, it is unlikely that blocking gC and gE immune evasion will prevent infection unless additional correlates of immunity are present, such as potent T-cell responses. However, no zosteriform disease developed at doses below 5 × 106 PFU. Whether this finding correlates with diminished infection of spinal ganglia, which is the site of HSV latency, is currently under evaluation. If so, preventing immune evasion may reduce viral load in ganglia and diminish the frequency of recurrent infections. Therefore, including immunogens that induce blocking antibodies to immune evasion domains as components of an HSV subunit vaccine may be a useful strategy to improve vaccine efficacy.
Acknowledgments
This work was supported by grants R01 HL 28220, R01 Al 33063, and R01 DE14152 from the United States Public Health Service.
We thank Ronald Collman for help with the graphics in Fig. 3.
REFERENCES
- 1.Albrecht, J. C., and B. Fleckenstein. 1992. New member of the multigene family of complement control proteins in herpesvirus saimiri. J. Virol. 66:3937-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Binder, R., A. Kress, G. Kan, K. Herrmann, and M. Kirschfink. 1999. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol. Immunol. 36:885-892. [DOI] [PubMed] [Google Scholar]
- 3.Brown, Z. A., S. Selke, J. Zeh, J. Kopelman, A. Maslow, R. L. Ashley, D. H. Watts, S. Berry, M. Herd, and L. Corey. 1997. The acquisition of herpes simplex virus during pregnancy. N. Engl. J. Med. 337:509-515. [DOI] [PubMed] [Google Scholar]
- 4.Corey, L., A. G. Langenberg, R. Ashley, R. E. Sekulovich, A. E. Izu, J. M. Douglas, Jr., H. H. Handsfield, T. Warren, L. Marr, S. Tyring, R. DiCarlo, A. A. Adimora, P. Leone, C. L. Dekker, R. L. Burke, W. P. Leong, S. E. Straus, et al. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331-340. [DOI] [PubMed] [Google Scholar]
- 5.Dingwell, K. S., and D. C. Johnson. 1998. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubin, G., S. Basu, D. L. Mallory, M. Basu, R. Tal-Singer, and H. M. Friedman. 1994. Characterization of domains of herpes simplex virus type 1 glycoprotein E involved in Fc binding activity for immunoglobulin G aggregates. J. Virol. 68:2478-2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubin, G., E. Socolof, I. Frank, and H. M. Friedman. 1991. Herpes simplex virus type 1 Fc receptor protects infected cells from antibody-dependent cellular cytotoxicity. J. Virol. 65:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 9.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J. Virol. 71:8254-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feyzi, E., E. Trybala, T. Bergstrom, U. Lindahl, and D. Spillmann. 1997. Structural requirement of heparan sulfate for interaction with herpes simplex virus type 1 virions and isolated glycoprotein C. J. Biol. Chem. 272:24850-24857. [DOI] [PubMed] [Google Scholar]
- 11.Fodor, W. L., S. A. Rollins, S. Bianco-Caron, R. P. Rother, E. R. Guilmette, W. V. Burton, J. C. Albrecht, B. Fleckenstein, and S. P. Squinto. 1995. The complement control protein homolog of herpesvirus saimiri regulates serum complement by inhibiting C3 convertase activity. J. Virol. 69:3889-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, I., and H. M. Friedman. 1989. A novel function of the herpes simplex virus type 1 Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 14.Friedman, H. M., E. J. Macarak, R. R. MacGregor, J. Wolfe, and N. A. Kefalides. 1981. Virus infection of endothelial cells. J. Infect. Dis. 143:266-273. [DOI] [PubMed] [Google Scholar]
- 15.Friedman, H. M., L. Wang, N. O. Fishman, J. D. Lambris, R. J. Eisenberg, G. H. Cohen, and J. Lubinski. 1996. Immune evasion properties of herpes simplex virus type 1 glycoprotein gC. J. Virol. 70:4253-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fries, L. F., H. M. Friedman, G. H. Cohen, R. J. Eisenberg, C. H. Hammer, and M. M. Frank. 1986. Glycoprotein C of herpes simplex virus 1 is an inhibitor of the complement cascade. J. Immunol. 137:1636-1641. [PubMed] [Google Scholar]
- 17.Fruh, K., K. Ahn, H. Djaballah, P. Sempe, P. M. van Endert, R. Tampe, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 18.Herold, B. C., D. WuDunn, N. Soltys, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 20.Huemer, H. P., C. Larcher, and N. E. Coe. 1992. Pseudorabies virus glycoprotein III derived from virions and infected cells binds to the third component of complement. Virus Res. 23:271-280. [DOI] [PubMed] [Google Scholar]
- 21.Huemer, H. P., C. Larcher, S. van Drunen Littel-van den Hurk, and L. A. Babiuk. 1993. Species selective interaction of Alphaherpesvirinae with the “unspecific” immune system of the host. Arch. Virol. 130:353-364. [DOI] [PubMed] [Google Scholar]
- 22.Huemer, H. P., N. Nowotny, B. S. Crabb, H. Meyer, and P. H. Hubert. 1995. gp13 (EHV-gC): a complement receptor induced by equine herpesviruses. Virus Res. 37:113-126. [DOI] [PubMed] [Google Scholar]
- 23.Hung, S. L., S. Srinivasan, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 1992. Structural basis of C3b binding by glycoprotein C of herpes simplex virus. J. Virol. 66:4013-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janeway, C. A., P. Travers, M. Walport, and M. Shlomchik. 2001. Innate immunity, p. 35-91. In Immunobiology. The immune system in health and disease, 5th ed. Churchill Livingstone, Inc., Camden Town, Great Britain.
- 25.Johansson, P. J., E. B. Myhre, and J. Blomberg. 1985. Specificity of Fc receptors induced by herpes simplex virus type 1: comparison of immunoglobulin G from different animal species. J. Virol. 56:489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapadia, S. B., H. Molina, V. van Berkel, S. H. Speck, and H. W. Virgin IV. 1999. Murine gammaherpesvirus 68 encodes a functional regulator of complement activation. J. Virol. 73:7658-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostavasili, I., A. Sahu, H. M. Friedman, R. J. Eisenberg, G. H. Cohen, and J. D. Lambris. 1997. Mechanism of complement inactivation by glycoprotein C of herpes simplex virus. J. Immunol. 158:1763-1771. [PubMed] [Google Scholar]
- 29.Langenberg, A. G., R. L. Burke, S. F. Adair, R. Sekulovich, M. Tigges, C. L. Dekker, and L. Corey. 1995. A recombinant glycoprotein vaccine for herpes simplex virus type 2: safety and immunogenicity. Ann. Intern. Med. 122:889-898. [DOI] [PubMed] [Google Scholar]
- 30.Langenberg, A. G., L. Corey, R. L. Ashley, W. P. Leong, S. E. Straus, et al. 1999. A prospective study of new infections with herpes simplex virus type 1 and type 2. N. Engl. J. Med. 341:1432-1438. [DOI] [PubMed] [Google Scholar]
- 31.Liesegang, T. J., L. J. Melton III, P. J. Daly, and D. M. Ilstrup. 1989. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn., 1950 through 1982. Arch. Ophthalmol. 107:1155-1159. [DOI] [PubMed] [Google Scholar]
- 32.Lilley, B. N., H. L. Ploegh, and R. S. Tirabassi. 2001. Human cytomegalovirus open reading frame TRL11/IRL11 encodes an immunoglobulin G Fc-binding protein. J. Virol. 75:11218-11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litwin, V., W. Jackson, and C. Grose. 1992. Receptor properties of two varicella-zoster virus glycoproteins, gpI and gpIV, homologous to herpes simplex virus gE and gI. J. Virol. 66:3643-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubinski, J., L. Wang, D. Mastellos, A. Sahu, J. D. Lambris, and H. M. Friedman. 1999. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J. Exp. Med. 190:1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacCormac, L. P., and J. E. Grundy. 1996. Human cytomegalovirus induces an Fc gamma receptor (Fc γR) in endothelial cells and fibroblasts that is distinct from the human cellular Fc γRs. J. Infect. Dis. 174:1151-1161. [DOI] [PubMed] [Google Scholar]
- 36.Mardberg, K., E. Trybala, J. C. Glorioso, and T. Bergstrom. 2001. Mutational analysis of the major heparan sulfate-binding domain of herpes simplex virus type 1 glycoprotein C. J. Gen. Virol. 82:1941-1950. [DOI] [PubMed] [Google Scholar]
- 37.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagashunmugam, T., J. Lubinski, L. Wang, L. T. Goldstein, B. S. Weeks, P. Sundaresan, E. H. Kang, G. Dubin, and H. M. Friedman. 1998. In vivo immune evasion mediated by the herpes simplex virus type 1 immunoglobulin G Fc receptor. J. Virol. 72:5351-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Para, M. F., R. B. Baucke, and P. G. Spear. 1982. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced Fc-binding receptors. J. Virol. 41:129-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Para, M. F., L. Goldstein, and P. G. Spear. 1982. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J. Virol. 41:137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rother, R. P., S. A. Rollins, W. L. Fodor, J. C. Albrecht, E. Setter, B. Fleckenstein, and S. P. Squinto. 1994. Inhibition of complement-mediated cytolysis by the terminal complement inhibitor of herpesvirus saimiri. J. Virol. 68:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 43.Rux, A. H., W. T. Moore, J. D. Lambris, W. R. Abrams, C. Peng, H. M. Friedman, G. H. Cohen, and R. J. Eisenberg. 1996. Disulfide bond structure determination and biochemical analysis of glycoprotein C from herpes simplex virus. J. Virol. 70:5455-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saldanha, C. E., J. Lubinski, C. Martin, T. Nagashunmugam, L. Wang, H. van Der Keyl, R. Tal-Singer, and H. M. Friedman. 2000. Herpes simplex virus type 1 glycoprotein E domains involved in virus spread and disease. J. Virol. 74:6712-6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons, A., and A. A. Nash. 1984. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J. Virol. 52:816-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanberry, L., A. Cunningham, G. Mertz, A. Mindel, B. Peters, M. Reitano, S. Sacks, A. Wald, S. Wassilew, and P. Woolley. 1999. New developments in the epidemiology, natural history and management of genital herpes. Antivir. Res. 42:1-14. [DOI] [PubMed] [Google Scholar]
- 47.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virus type 2 glycoproteins D and B: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 48.Whitley, R. J. 1990. Viral encephalitis. N. Engl. J. Med. 323:242-250. [DOI] [PubMed] [Google Scholar]
- 49.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]