Abstract
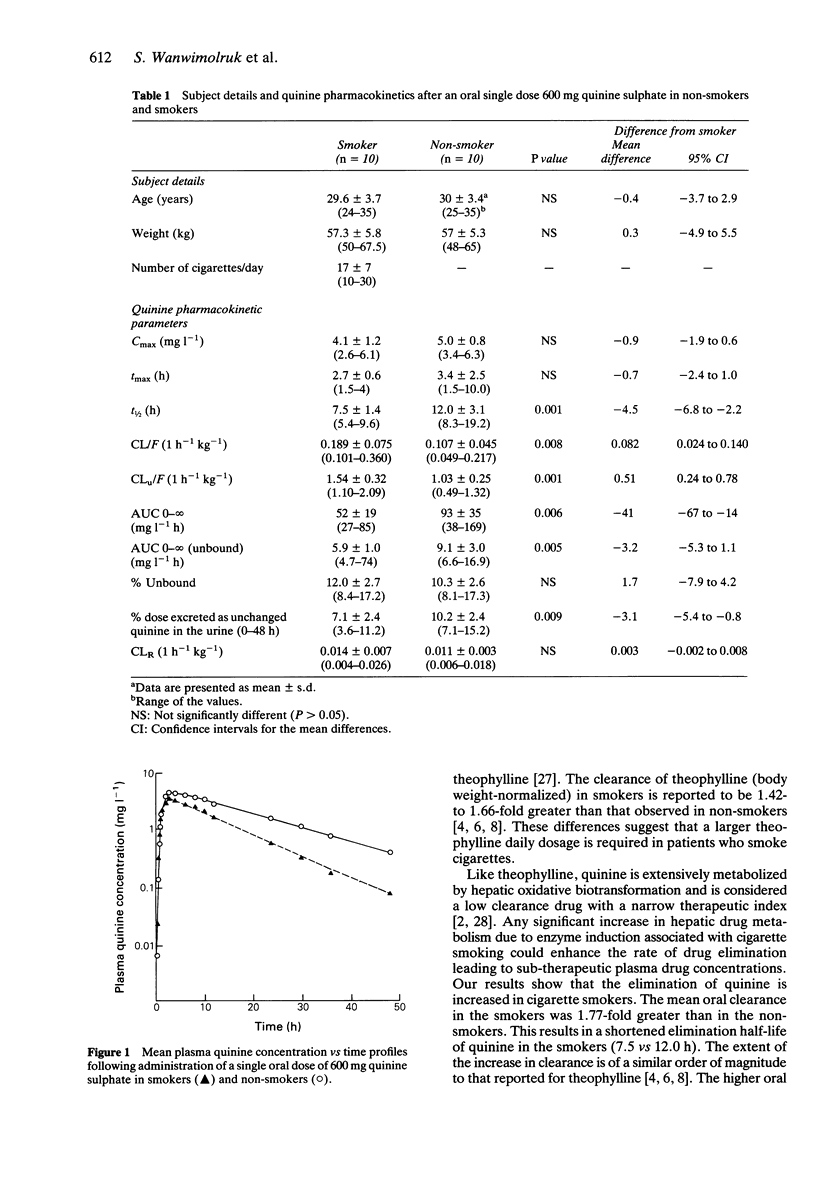
The pharmacokinetics of a single dose (600 mg) of quinine sulphate were examined in a group of non-smokers (n = 10) and in heavy cigarette smokers (n = 10). The mean (+/- s.d.) oral clearance of quinine in smokers (0.189 +/- 0.075 1 h(-1) kg(-1)) was significantly greater than in non-smokers (0.107 +/- 0.045 1 h(-1) kg(-1) , P < 0.01). The unbound clearance of quinine which reflects activity of the drug-metabolizing enzyme, was considerably greater (1.5-fold) in the smokers than in the non-smoker subjects. The mean elimination half-life of quinine in smokers was 7.5 +/- 1.4 (s.d.) h, significantly shorter (P < 0.005) than the mean value in non-smokers (12.0 +/- 3.1 h). These results suggest that cigarette smoking enhances the elimination of quinine. The clinical significance of these findings is unknown but they indicate the need for caution in the administration of quinine to patients who are heavy cigarette smokers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann K. A., Nunlee M., Martin M., Schwartz J., Jauregui L., Forney R. B., Jr The use of single sample clearance estimates to probe hepatic drug metabolism: handprinting the influence of cigarette smoking on human hepatic drug metabolism. Xenobiotica. 1990 May;20(5):537–547. doi: 10.3109/00498259009046868. [DOI] [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Grant D. M., Inaba T., Kalow W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome(s) P-450 in human liver microsomes. Drug Metab Dispos. 1987 Mar-Apr;15(2):237–249. [PubMed] [Google Scholar]
- Combalbert J., Fabre I., Fabre G., Dalet I., Derancourt J., Cano J. P., Maurel P. Metabolism of cyclosporin A. IV. Purification and identification of the rifampicin-inducible human liver cytochrome P-450 (cyclosporin A oxidase) as a product of P450IIIA gene subfamily. Drug Metab Dispos. 1989 Mar-Apr;17(2):197–207. [PubMed] [Google Scholar]
- Deanfield J., Jonathan A., Selwyn A., Fox K. M. Treatment of angina pectoris with propranolol: the harmful effects of cigarette smoking. Cardiology. 1981;68 (Suppl 2):186–189. doi: 10.1159/000173335. [DOI] [PubMed] [Google Scholar]
- Distlerath L. M., Reilly P. E., Martin M. V., Davis G. G., Wilkinson G. R., Guengerich F. P. Purification and characterization of the human liver cytochromes P-450 involved in debrisoquine 4-hydroxylation and phenacetin O-deethylation, two prototypes for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1985 Jul 25;260(15):9057–9067. [PubMed] [Google Scholar]
- Edwards D. J., Axelson J. E., Visco J. P., vanEvery S., Slaughter R. L., Lalka D. Lack of effect of smoking on the metabolism and pharmacokinetics of quinidine in patients. Br J Clin Pharmacol. 1987 Mar;23(3):351–354. doi: 10.1111/j.1365-2125.1987.tb03057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ged C., Rouillon J. M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989 Oct;28(4):373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel J. J., Birkett D. J. Cigarette smoking and theophylline clearance and metabolism. Clin Pharmacol Ther. 1981 Oct;30(4):491–496. doi: 10.1038/clpt.1981.193. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Martin M. V., Beaune P. H., Kremers P., Wolff T., Waxman D. J. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986 Apr 15;261(11):5051–5060. [PubMed] [Google Scholar]
- Guengerich F. P., Müller-Enoch D., Blair I. A. Oxidation of quinidine by human liver cytochrome P-450. Mol Pharmacol. 1986 Sep;30(3):287–295. [PubMed] [Google Scholar]
- Hart P., Farrell G. C., Cooksley W. G., Powell L. W. Enhanced drug metabolism in cigarette smokers. Br Med J. 1976 Jul 17;2(6028):147–149. doi: 10.1136/bmj.2.6028.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. N., Jusko W. J., Yurchak A. M. Effect of smoking on theophylline disposition. Clin Pharmacol Ther. 1976 May;19(5 Pt 1):546–551. doi: 10.1002/cpt1976195part1546. [DOI] [PubMed] [Google Scholar]
- Jusko W. J. Role of tobacco smoking in pharmacokinetics. J Pharmacokinet Biopharm. 1978 Feb;6(1):7–39. doi: 10.1007/BF01066061. [DOI] [PubMed] [Google Scholar]
- Jusko W. J., Schentag J. J., Clark J. H., Gardner M., Yurchak A. M. Enhanced biotransformation of theophylline in marihuana and tobacco smokers. Clin Pharmacol Ther. 1978 Oct;24(4):405–410. [PubMed] [Google Scholar]
- Kuntzman R., Pantuck E. J., Kaplan S. A., Conney A. H. Phenacetin metabolism: effect of hydrocarbons and cigarette smoking. Clin Pharmacol Ther. 1977 Nov;22(5 Pt 2):757–764. doi: 10.1002/cpt1977225part2757. [DOI] [PubMed] [Google Scholar]
- Küpfer A., Branch R. A. Stereoselective mephobarbital hydroxylation cosegregates with mephenytoin hydroxylation. Clin Pharmacol Ther. 1985 Oct;38(4):414–418. doi: 10.1038/clpt.1985.196. [DOI] [PubMed] [Google Scholar]
- Küpfer A., Patwardhan R., Ward S., Schenker S., Preisig R., Branch R. A. Stereoselective metabolism and pharmacogenetic control of 5-phenyl-5-ethylhydantoin (nirvanol) in humans. J Pharmacol Exp Ther. 1984 Jul;230(1):28–33. [PubMed] [Google Scholar]
- Pantuck E. J., Kuntzman R., Conney A. H. Decreased concentration of phenacetin in plasma of cigarette smokers. Science. 1972 Mar 17;175(4027):1248–1250. doi: 10.1126/science.175.4027.1248. [DOI] [PubMed] [Google Scholar]
- Powell J. R., Thiercelin J. F., Vozeh S., Sansom L., Riegelman S. The influence of cigarette smoking and sex on theophylline disposition. Am Rev Respir Dis. 1977 Jul;116(1):17–23. doi: 10.1164/arrd.1977.116.1.17. [DOI] [PubMed] [Google Scholar]
- Robson R. A., Miners J. O., Matthews A. P., Stupans I., Meller D., McManus M. E., Birkett D. J. Characterisation of theophylline metabolism by human liver microsomes. Inhibition and immunochemical studies. Biochem Pharmacol. 1988 May 1;37(9):1651–1659. doi: 10.1016/0006-2952(88)90423-6. [DOI] [PubMed] [Google Scholar]
- Sarkar M., Polk R. E., Guzelian P. S., Hunt C., Karnes H. T. In vitro effect of fluoroquinolones on theophylline metabolism in human liver microsomes. Antimicrob Agents Chemother. 1990 Apr;34(4):594–599. doi: 10.1128/aac.34.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Edwards R. J., Davies D. S. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1988 Oct;26(4):363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shader R. I., Greenblatt D. J., Harmatz J. S., Franke K., Koch-Weser J. Absorption and disposition of chlordiazepoxide in young and elderly male volunteers. J Clin Pharmacol. 1977 Nov-Dec;17(11-12):709–718. doi: 10.1002/j.1552-4604.1977.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Vestal R. E., Norris A. H., Tobin J. D., Cohen B. H., Shock N. W., Andres R. Antipyrine metabolism in man: influence of age, alcohol, caffeine, and smoking. Clin Pharmacol Ther. 1975 Oct;18(4):425–432. doi: 10.1002/cpt1975184425. [DOI] [PubMed] [Google Scholar]
- Wanwimolruk S., Birkett D. J., Brooks P. M. Protein binding of some non-steroidal anti-inflammatory drugs in rheumatoid arthritis. Clin Pharmacokinet. 1982 Jan-Feb;7(1):85–92. doi: 10.2165/00003088-198207010-00005. [DOI] [PubMed] [Google Scholar]
- Wanwimolruk S., Chalcroft S., Coville P. F., Campbell A. J. Pharmacokinetics of quinine in young and elderly subjects. Trans R Soc Trop Med Hyg. 1991 Nov-Dec;85(6):714–717. doi: 10.1016/0035-9203(91)90423-v. [DOI] [PubMed] [Google Scholar]
- Wanwimolruk S., Chalcroft S. Lack of relationship between debrisoquine oxidation phenotype and the pharmacokinetics of quinine. Br J Clin Pharmacol. 1991 Nov;32(5):617–620. doi: 10.1111/j.1365-2125.1991.tb03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanwimolruk S., Kaewvichit S., Tanthayaphinant O., Suwannarach C., Oranratnachai A. Lack of effect of oral contraceptive use on the pharmacokinetics of quinine. Br J Clin Pharmacol. 1991 Feb;31(2):179–181. doi: 10.1111/j.1365-2125.1991.tb05509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J., Chanthavanich P., Krishna S., Bunch C., Silamut K. Quinine disposition kinetics. Br J Clin Pharmacol. 1983 Oct;16(4):399–403. doi: 10.1111/j.1365-2125.1983.tb02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J. Clinical pharmacokinetics of antimalarial drugs. Clin Pharmacokinet. 1985 May-Jun;10(3):187–215. doi: 10.2165/00003088-198510030-00001. [DOI] [PubMed] [Google Scholar]


