Abstract
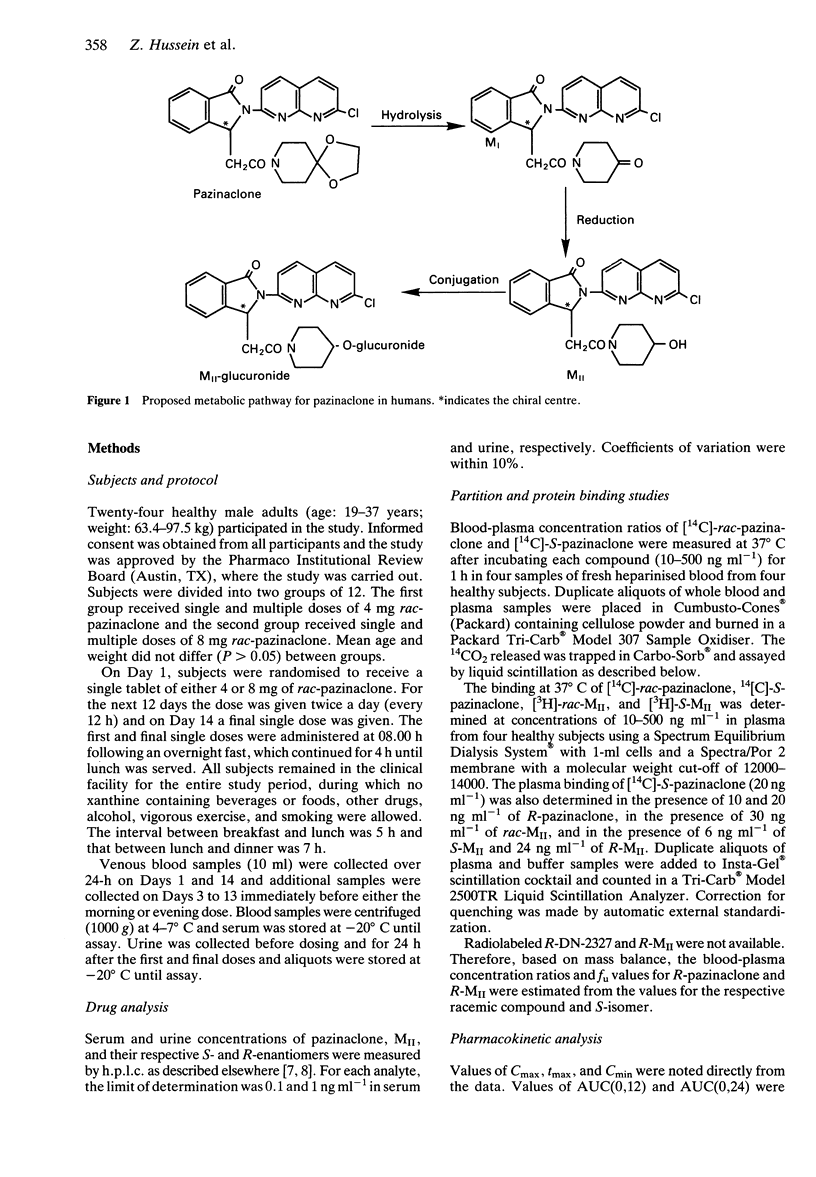
1. Serum and urine concentrations of enantiomers of pazinaclone (DN-2327) and an active metabolite MII, were measured after single and twice daily oral doses of 4 and 8 mg racemic drug to healthy subjects. 2. The kinetics of rac-pazinaclone and rac-MII were dose-independent and no unchanged drug was recovered in urine. 3. The terminal elimination half-lives of the drug isomers were similar (about 10.5 h), but mean steady-state values of AUC were twofold higher for the S-isomer than those of the antipode (e.g., 8 mg dose: 127 vs 69 ng ml(-1) h). However, the corresponding AUC values based upon unbound drug were similar (5.71 vs 5.73 ng ml(-1) h) indicating no stereoselectivity in intrinsic metabolic clearance. 4. The terminal elimination half-lives of S- and R-MII were similar to those of parent compound indicating that the elimination of these metabolites is formation rate-limited. 5. The R:S-ratio for the AUCs of MII was 4:1. Both enantiomers were excreted in the urine mainly as glucuronide conjugates, with stereoselectivity toward S-MII. 6. Since only the S-enantiomers of DN-2327 and MII bind to the benzodiazepine receptor, further measurements of drug effect in patients should be related to combine serum concentrations of the S-enantiomers of both parent drug and MII.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goo R. H., Moore J. G., Greenberg E., Alazraki N. P. Circadian variation in gastric emptying of meals in humans. Gastroenterology. 1987 Sep;93(3):515–518. doi: 10.1016/0016-5085(87)90913-9. [DOI] [PubMed] [Google Scholar]
- Hussein Z., Chu S. Y., Granneman G. R. Enantioselective determination of DN-2327, a novel non-benzodiazepine anxiolytic, and/or its active metabolite in human plasma and urine using high-performance liquid chromatography. J Chromatogr. 1993 Mar 5;613(1):113–120. doi: 10.1016/0378-4347(93)80203-g. [DOI] [PubMed] [Google Scholar]
- Hussein Z., Chu S. Y., Granneman G. R. High-performance liquid chromatographic method for determination of DN-2327, a novel non-benzodiazepine anxiolytic, and/or its active metabolite in human plasma and urine. J Chromatogr. 1993 Mar 5;613(1):105–112. doi: 10.1016/0378-4347(93)80202-f. [DOI] [PubMed] [Google Scholar]
- Wada T., Fukuda N. Effect of a new anxiolytic, DN-2327, on learning and memory in rats. Pharmacol Biochem Behav. 1992 Mar;41(3):573–579. doi: 10.1016/0091-3057(92)90375-p. [DOI] [PubMed] [Google Scholar]
- Wada T., Fukuda N. Effects of DN-2327, a new anxiolytic, diazepam and buspirone on exploratory activity of the rat in an elevated plus-maze. Psychopharmacology (Berl) 1991;104(4):444–450. doi: 10.1007/BF02245647. [DOI] [PubMed] [Google Scholar]
- Wada T., Fukuda N. Pharmacologic profile of a new anxiolytic, DN-2327: effect of Ro15-1788 and interaction with diazepam in rodents. Psychopharmacology (Berl) 1991;103(3):314–322. doi: 10.1007/BF02244284. [DOI] [PubMed] [Google Scholar]
- Wada T., Nakajima R., Kurihara E., Narumi S., Masuoka Y., Goto G., Saji Y., Fukuda N. Pharmacologic characterization of a novel non-benzodiazepine selective anxiolytic, DN-2327. Jpn J Pharmacol. 1989 Mar;49(3):337–349. doi: 10.1254/jjp.49.337. [DOI] [PubMed] [Google Scholar]


