Abstract
1. The effect of carbamazepine (CBZ), sodium valproate (VPA) and phenytoin (PHT) on the pharmacokinetics of oxcarbazepine (OXC) was explored in three groups of 12 epileptic patients taking one of these drug as monotherapy. 2. Each patient took a single 600 mg dose of OXC followed 7 days later by 3 weeks' treatment with OXC 300 mg thrice daily and matched placebo in random order. 3. Seven untreated patients, acting as controls, were prescribed the single OXC dose and 3 weeks' active treatment only. 4. In those patients completing the study, the area under the concentration-time curve (AUC) at steady-state for hydroxycarbazepine (OHCZ), the active metabolite of OXC, was significantly lower in the CBZ-treated group than in controls (P < 0.05). 5. No other differences in AUCs or elimination half-lives for OHCZ were found between treated and untreated patients following single or multiple OXC dosing. 6. Median AUCs of CBZ, VPA and PHT during a dosage interval did not differ significantly after treatment with OXC and placebo. 7. Ten patients completing the study complained of side-effects during treatment with OXC compared with one taking placebo (P < 0.01). 8. There were no important changes in cognitive function testing during administration of OXC compared with placebo. 9. Standard doses of OXC can be given as add-on therapy in epileptic patients receiving CBZ, VPA or PHT without producing a clinically relevant pharmacokinetic interaction.
Full text
PDF

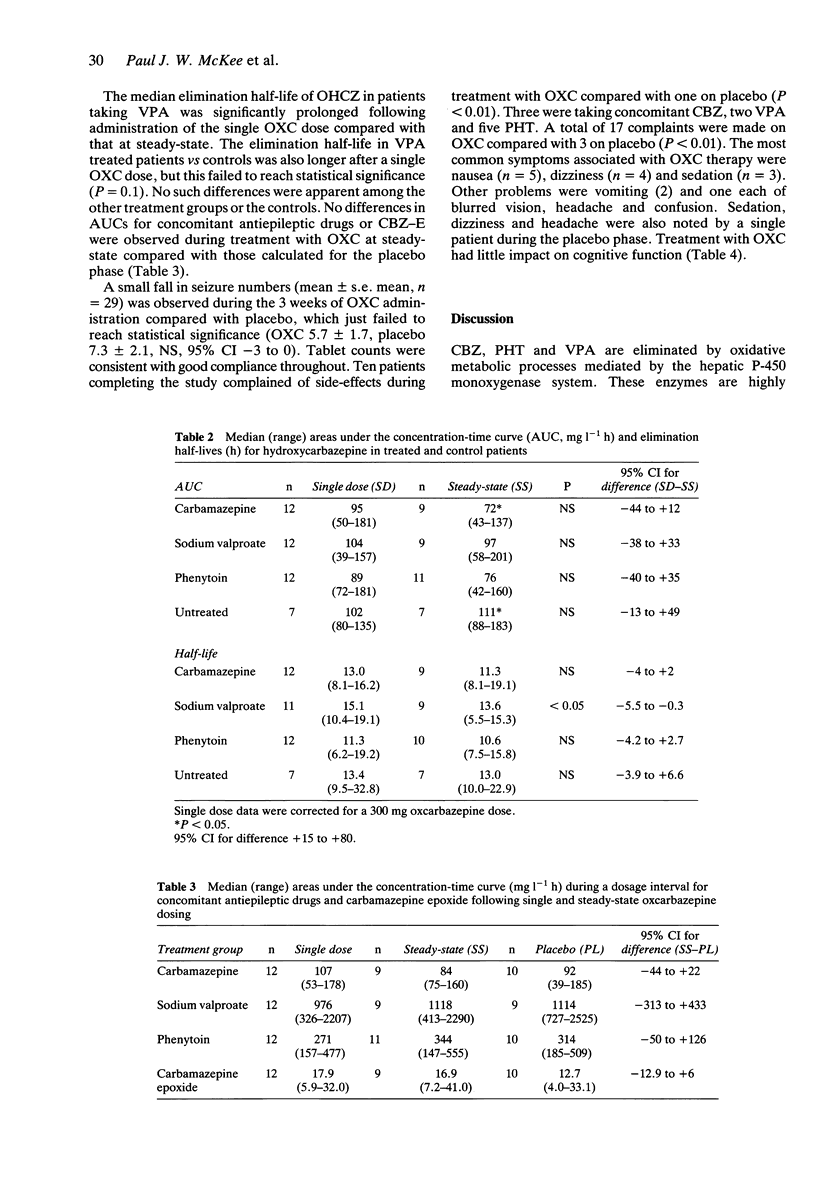


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aikiä M., Kälviäinen R., Sivenius J., Halonen T., Riekkinen P. J. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow-up. Epilepsy Res. 1992 May;11(3):199–203. doi: 10.1016/0920-1211(92)90099-f. [DOI] [PubMed] [Google Scholar]
- Bachur N. R. Cytoplasmic aldo-keto reductases: a class of drug metabolizing enzymes. Science. 1976 Aug 13;193(4253):595–597. doi: 10.1126/science.959821. [DOI] [PubMed] [Google Scholar]
- Baciewicz A. M. Carbamazepine drug interactions. Ther Drug Monit. 1986;8(3):305–317. doi: 10.1097/00007691-198609000-00012. [DOI] [PubMed] [Google Scholar]
- Bock K. W., Bock-Hennig B. S. Differential induction of human liver UDP-glucuronosyltransferase activities by phenobarbital-type inducers. Biochem Pharmacol. 1987 Dec 1;36(23):4137–4143. doi: 10.1016/0006-2952(87)90572-7. [DOI] [PubMed] [Google Scholar]
- Brodie M. J. Drug interactions in epilepsy. Epilepsia. 1992;33 (Suppl 1):S13–S22. doi: 10.1111/j.1528-1157.1992.tb05896.x. [DOI] [PubMed] [Google Scholar]
- Capewell S., Freestone S., Critchley J. A., Pottage A., Prescott L. F. Reduced felodipine bioavailability in patients taking anticonvulsants. Lancet. 1988 Aug 27;2(8609):480–482. doi: 10.1016/s0140-6736(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Dam M., Ekberg R., Løyning Y., Waltimo O., Jakobsen K. A double-blind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res. 1989 Jan-Feb;3(1):70–76. doi: 10.1016/0920-1211(89)90070-3. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Köthe K. W., Hoffmann F., von Unruh G. E. Use of stable labelled carbamazepine to study its kinetics during chronic carbamazepine treatment. Eur J Clin Pharmacol. 1982;23(3):241–244. doi: 10.1007/BF00547561. [DOI] [PubMed] [Google Scholar]
- Gillham R. A., Williams N., Wiedmann K. D., Butler E., Larkin J. G., Brodie M. J. Cognitive function in adult epileptic patients established on anticonvulsant monotherapy. Epilepsy Res. 1990 Dec;7(3):219–225. doi: 10.1016/0920-1211(90)90018-q. [DOI] [PubMed] [Google Scholar]
- Gillham R. A., Williams N., Wiedmann K., Butler E., Larkin J. G., Brodie M. J. Concentration-effect relationships with carbamazepine and its epoxide on psychomotor and cognitive function in epileptic patients. J Neurol Neurosurg Psychiatry. 1988 Jul;51(7):929–933. doi: 10.1136/jnnp.51.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S. M., Faulds D. Oxcarbazepine. A review of its pharmacology and therapeutic potential in epilepsy, trigeminal neuralgia and affective disorders. Drugs. 1992 Jun;43(6):873–888. doi: 10.2165/00003495-199243060-00007. [DOI] [PubMed] [Google Scholar]
- Houtkooper M. A., Lammertsma A., Meyer J. W., Goedhart D. M., Meinardi H., van Oorschot C. A., Blom G. F., Höppener R. J., Hulsman J. A. Oxcarbazepine (GP 47.680): a possible alternative to carbamazepine? Epilepsia. 1987 Nov-Dec;28(6):693–698. doi: 10.1111/j.1528-1157.1987.tb03702.x. [DOI] [PubMed] [Google Scholar]
- Keränen T., Jolkkonen J., Klosterskov-Jensen P., Menge G. P. Oxcarbazepine does not interact with cimetidine in healthy volunteers. Acta Neurol Scand. 1992 Apr;85(4):239–242. doi: 10.1111/j.1600-0404.1992.tb04038.x. [DOI] [PubMed] [Google Scholar]
- Klosterskov Jensen P., Saano V., Haring P., Svenstrup B., Menge G. P. Possible interaction between oxcarbazepine and an oral contraceptive. Epilepsia. 1992 Nov-Dec;33(6):1149–1152. doi: 10.1111/j.1528-1157.1992.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Krämer G., Tettenborn B., Klosterskov Jensen P., Menge G. P., Stoll K. D. Oxcarbazepine does not affect the anticoagulant activity of warfarin. Epilepsia. 1992 Nov-Dec;33(6):1145–1148. doi: 10.1111/j.1528-1157.1992.tb01772.x. [DOI] [PubMed] [Google Scholar]
- Larkin J. G., McKee P. J., Forrest G., Beastall G. H., Park B. K., Lowrie J. I., Lloyd P., Brodie M. J. Lack of enzyme induction with oxcarbazepine (600 mg daily) in healthy subjects. Br J Clin Pharmacol. 1991 Jan;31(1):65–71. doi: 10.1111/j.1365-2125.1991.tb03858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. H., Koch K. M. Drug interactions with valproic acid. Drugs. 1982 Dec;24(6):543–556. doi: 10.2165/00003495-198224060-00004. [DOI] [PubMed] [Google Scholar]
- Macphee G. J., Butler E., Brodie M. J. Intradose and circadian variation in circulating carbamazepine and its epoxide in epileptic patients: a consequence of autoinduction of metabolism. Epilepsia. 1987 May-Jun;28(3):286–294. doi: 10.1111/j.1528-1157.1987.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Macphee G. J., Thompson G. G., Scobie G., Agnew E., Park B. K., Murray T., McColl K. E., Brodie M. J. Effects of cimetidine on carbamazepine auto- and hetero-induction in man. Br J Clin Pharmacol. 1984 Sep;18(3):411–419. doi: 10.1111/j.1365-2125.1984.tb02483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee P. J., Blacklaw J., Butler E., Gillham R. A., Brodie M. J. Variability and clinical relevance of the interaction between sodium valproate and carbamazepine in epileptic patients. Epilepsy Res. 1992 May;11(3):193–198. doi: 10.1016/0920-1211(92)90098-e. [DOI] [PubMed] [Google Scholar]
- Mogensen P. H., Jórgensen L., Boas J., Dam M., Vesterager A., Flesch G., Jensen P. K. Effects of dextropropoxyphene on the steady-state kinetics of oxcarbazepine and its metabolites. Acta Neurol Scand. 1992 Jan;85(1):14–17. [PubMed] [Google Scholar]
- Patsalos P. N., Zakrzewska J. M., Elyas A. A. Dose dependent enzyme induction by oxcarbazepine? Eur J Clin Pharmacol. 1990;39(2):187–188. doi: 10.1007/BF00280057. [DOI] [PubMed] [Google Scholar]
- Reinikainen K. J., Keränen T., Halonen T., Komulainen H., Riekkinen P. J. Comparison of oxcarbazepine and carbamazepine: a double-blind study. Epilepsy Res. 1987 Sep;1(5):284–289. doi: 10.1016/0920-1211(87)90003-9. [DOI] [PubMed] [Google Scholar]
- Schütz H., Feldmann K. F., Faigle J. W., Kriemler H. P., Winkler T. The metabolism of 14C-oxcarbazepine in man. Xenobiotica. 1986 Aug;16(8):769–778. doi: 10.3109/00498258609043567. [DOI] [PubMed] [Google Scholar]
- Theisohn M., Heimann G. Disposition of the antiepileptic oxcarbazepine and its metabolites in healthy volunteers. Eur J Clin Pharmacol. 1982;22(6):545–551. doi: 10.1007/BF00609629. [DOI] [PubMed] [Google Scholar]
- Zaccara G., Gangemi P. F., Bendoni L., Menge G. P., Schwabe S., Monza G. C. Influence of single and repeated doses of oxcarbazepine on the pharmacokinetic profile of felodipine. Ther Drug Monit. 1993 Feb;15(1):39–42. doi: 10.1097/00007691-199302000-00007. [DOI] [PubMed] [Google Scholar]



