Abstract
The glycosylphosphatidylinositol (GPI)-anchored complement regulatory protein decay-accelerating factor (DAF) is used by a number of enteroviruses as a receptor during infection. DAF and other GPI-anchored proteins can be found in cholesterol-rich ordered domains within the plasma membrane that are known as “lipid rafts.” We have shown, by using drugs to specifically inhibit various endocytosis routes, that infection by a DAF-using strain of echovirus 11 (EV11) is dependent upon cholesterol and an intact cytoskeleton, whereas a non-DAF-using mutant derived from it was unaffected by these drugs. Using RNA transfection and virus-binding assays, we have shown that this requirement for cholesterol, the actin cytoskeleton, and the microtubule network occurs postbinding of the virus but prior to uncoating of the RNA, indicating a role during virus entry. Confocal microscopy of virus infection supported the role of cholesterol and the cytoskeleton during entry. In addition, [35S]methionine-labeled DAF-using EV11, but not the non-DAF-using EV11, could be copurified with lipid raft components during infection after Triton X-100 extraction. These data indicate that DAF usage by EV11 enables the virus to associate with lipid rafts and enter cells through this novel route.
The early stages of viral infection involve the attachment of virions to the cell surface by binding to a cellular receptor followed by entry into the cell. Enveloped viruses have two options during entry: receptor-mediated endocytosis or direct fusion of the viral envelope with the plasma membrane to deliver nucleocapsid to cytoplasm. Examples of viruses using the endocytic route include Semliki forest virus (31) and influenza A virus (51). Examples of viruses using direct fusion include Sendai virus (23) and some retroviruses (47). Most nonenveloped viruses bind to a receptor and are internalized through endocytosis pathways. Many nonenveloped viruses have been shown to use the clathrin-mediated endocytic route to infect cells; examples of this include canine parvovirus (57, 83), adenovirus (84), and certain picornaviruses (20, 28). Simian virus 40 (SV40), a polyomavirus, binds to class I HLA on the cell surface (81). The virus is then translocated to noncoated membrane invaginations known as caveolae (1). This process is slow, since virus cannot be detected in caveolar preparations until 2 h postbinding (14). The virus then dissociates from class I HLA and enters cells through the caveolae after initiating a signal transduction cascade. Recent work by Marjomäki et al. (48) has shown that echovirus 1 (EV1) also enters the cells via caveolae.
Two other polyomaviruses, the human polyomavirus JC virus and murine polyomavirus, enter cells through clathrin-mediated endocytosis (64) and an unknown non-clathrin-mediated, noncaveolar, dynamin-independent route (24), respectively. These viruses use glycoproteins expressing terminal sialic acid residues as receptors demonstrating the important role of the virus receptor in selection of the entry route.
The early stages of infection by picornaviruses have been intensively studied and much is known of the initial interactions between the virus and cell surface receptors (3-5, 30, 88, 90). The events following receptor interactions, during entry, are less well understood. There have been a number of studies suggesting that poliovirus, a member of the enterovirus genus of the family Picornaviridae, infects cells via receptor-mediated endocytosis (40, 91). There are conflicting data on the requirements for acidification in endosomes during poliovirus entry (45, 62). It has been reported more recently, however, that poliovirus entry is independent of a dominant-negative form of dynamin, a GTPase required for the pinching off of vesicles at the cell surface (20). This might suggest that poliovirus does not enter via any of the known endocytic routes.
A number of other enteroviruses, including hemagglutinating echoviruses (7, 67, 86), enterovirus 70 (36), coxsackie B viruses (8, 50, 60), and coxsackievirus A21 (CAV21) (74), use decay-accelerating factor (DAF; CD55) as a receptor for infection of host cells. DAF is a 70-kDa glycosylphosphatidylinositol (GPI)-anchored protein involved in the protection of cells from complement-mediated lysis (44). The different viruses bind to DAF at one of three sites within the four short consensus repeat (SCR) regions. CAV21 (74) and enterovirus 70 (37) bind to a region in SCR1, the coxsackie B viruses (50) interact with a region between SCR2 and SCR3, and the hemagglutinating echoviruses bind to a region within SCR3 (15, 42, 68).
However, many of these viruses—and possibly all of them—require additional proteins to infect cells, as demonstrated by the nonpermissivity of CHO-DAF transfectants to infection (8, 86) and the inability of sDAF to promote conformational changes resulting in 135S particle formation (66). CAV21 and coxsackievirus B3 have both been shown to use proteins of the immunoglobulin superfamily (ICAM-1 and coxsackie- and adenovirus receptor [CAR]), respectively, as receptors (9, 10, 60, 75). These proteins can promote 135S particle formation and infection in the absence of DAF, although infection appears to be enhanced by its presence in the case of coxsackievirus B3 (60, 75, 78).
The situation with hemagglutinating echoviruses is less clear. Conformational changes associated with 135S particle formation are observed upon infection of cells. However, these changes appear to be associated mainly with internalized virus rather than occurring at the cell surface, as is observed for poliovirus and coxsackievirus B3 (17, 35, 66). Two additional proteins have been studied as entry cofactors for echoviruses, β2-microglobulin (or its complex with HLA class I) and the GPI-anchored protein CD59 (26, 87). These proteins appear to act after attachment to DAF but prior to 135S particle formation, suggesting a role in the entry process. The entry process thus appears to be distinctly different from those described previously for enteroviruses.
In an effort to examine the entry route(s) for DAF-using enteroviruses, we chose to study virus entry in the context of the normal entry route for internalization of DAF and other GPI-anchored proteins. Many GPI-anchored proteins are found associated with ordered domains within the outer leaflet of the plasma membrane described as “lipid rafts” (53, 80). These domains are composed of saturated glycosphingolipids and sphingomyelin and are enriched for cholesterol, leading to their more organized structure (for reviews, see references 13 and 29). The nature of these domains has led to the suggestion that they are able to sequester certain proteins while excluding others and therefore act as platforms for particular cellular functions including endocytosis, signal transduction, and cholesterol homeostasis (for reviews, see references 12, 33, and 63). Endocytosis of GPI-anchored proteins such as CD59 and CD14 occurs after cross-linking with antibodies or ligand in a cholesterol-dependent manner (19, 38). This requirement for cholesterol suggests a role for lipid rafts in the endocytosis of GPI anchored proteins such as DAF. The multiple DAF-binding sites on the enterovirus virion should enable the virus to cross-link DAF and infect cells through this route.
In relation to other infectious agents, lipid rafts have been shown to be the site of entry for certain DAF-binding enteric bacteria expressing Dr adhesin (73). A proposed subgroup of the lipid rafts is the noncoated membrane invaginations known as caveolae (41, 59). Caveolae have previously been demonstrated to act as the entry route for a number of pathogens. SV40 (1) binds to HLA class I (81) and is translocated to caveolin-rich domains. Cholera toxin associates with the ganglioside GM1 (56) and enters through caveolae. Bacteria such as FimH adhesin expressing Escherichia coli, through their interaction with GPI-linked CD48 (79), and Campylobacter jejuni (89) also invade cells through caveolae. Lipid rafts have also been implicated as sites of virion assembly for poliovirus (49) and sites of virus egress for a number of enveloped viruses. These include influenza A virus (71), measles virus (46), human immunodeficiency virus type 1 (54), and herpesviruses (65).
We examined the role of lipid rafts and/or caveolae in EV11 infection by using a DAF-binding strain, EV11-207, and a tissue culture-derived mutant EV11-207R that does not use DAF. Previous work with EV11-207 has shown that this virus is able to infect cells in a DAF-dependent manner since antibodies to DAF could inhibit infection, whereas EV11-207R infection of cells was unaffected by the same antibodies. EV11-207 was also able to hemagglutinate human red blood cells (RBC), and this interaction was shown to be dependent upon DAF since Pischia expressed soluble DAF could inhibit the hemagglutination. EV11-207R was unable to hemagglutinate RBC (42; Stuart et al., unpublished data).
The entry routes of these viruses were determined by infection of a caveolin-expressing cell line and a non-caveolin-expressing cell line in the presence of drugs (listed in Table 1) shown to disrupt endocytosis. We have shown that infection by the DAF-using strain requires the presence of cholesterol and an intact cytoskeleton, whereas infection by the non-DAF-using virus is partially blocked by inhibitors of the clathrin-mediated endocytic route. The DAF-using virus can be isolated, along with raft components, during Triton X-100 extraction of cells, unlike the non-DAF-using virus. These data suggest strongly that the association with lipid rafts is necessary for the entry of DAF-using enteroviruses.
TABLE 1.
Drugs used to disrupt endocytosis
| Drug | Effect | Reference(s) |
|---|---|---|
| Nystatin | Cholesterol-sequestering agent, disrupts lipid raft formation and internalization | 1, 19 |
| Chlorpromazine | Prevents assembly and/or disassembly of clathrin lattices at the cell surface and on endosomes, decreases receptor recycling, and inhibits clathrin-mediated endocytosis | 85 |
| Nocodazole | Disrupts microtubules and inhibits transport of endosomes and recycling of some lipid raft components | 2, 16 |
| Cytochalasin D | Prevents actin polymerization, disrupts actin cytoskeleton, and inhibits internalization through lipid rafts | 58 |
| Brefeldin A | Inhibits vesicle transport, disrupts Golgi, inhibits enterovirus RNA replication | 21, 52 |
MATERIALS AND METHODS
Virus and cells.
The EV11 isolate EV11-207 and the mutant EV11-207R derived from it have been described elsewhere (61; Stuart et al., unpublished data). HT29 and RD cells were obtained from the European Collection of Cell Cultures and maintained in RPMI 1640 medium (Sigma) containing 10% fetal calf serum and penicillin-streptomycin at 37°C and 5% CO2.
Reagents and antibodies.
Nystatin (Calbiochem), cytochalasin D (Sigma), nocodazole (Sigma), and brefeldin A (Sigma) were dissolved in dimethyl sulfoxide and as stock solutions of 100, 4, 20, and 200 mM, respectively. Chlorpromazine (Sigma), methyl-β-cyclodextrin (Sigma), and chloroquine (Sigma) were dissolved in water as stock solutions of 70, 200, and 200 mM, respectively.
Primary antibodies included anti-DAF rabbit polyclonal antibodies (kindly donated by Paul Morgan, Cardiff), goat anti-CD55 (Santa Cruz), mouse anti-EV11 (Chemicon), mouse anti-CAR (kindly donated by Jeffrey Bergelson, Philadelphia), goat anti-clathrin heavy chain (Santa Cruz), and rabbit anti-caveolin-1 (Santa Cruz). The secondary antibody for immunofluorescence, Alexa 594-conjugated rabbit anti-mouse antibody, was obtained from Molecular Probes. Secondary antibodies conjugated to horseradish peroxidase (HRP) for Western blot were obtained from Dako, Ltd., and included anti-rabbit, anti-mouse, and anti-goat HRP conjugates.
Time course of EV11 infection.
A total of 105 HT29 or RD cells were seeded onto 13-mm-diameter glass coverslips in a 24-well plate. The cells were infected with either EV11-207 or EV11-207R at a multiplicity of infection (MOI) of 10. The infected cells were incubated at 4°C for 30 min to allow attachment of the virus and then washed three times with phosphate-buffered saline (PBS) before incubation at 37°C for 0, 2, 4, 6, or 8 h. At each time point the cells were washed twice with PBS containing 1% newborn calf serum (PBS-NCS) and fixed with PBS containing 4% formaldehyde (PBS-F). The cells were then prepared for immunofluorescence as described below.
Effect of drugs on EV11 infection.
A total of 105 HT29 or RD cells were seeded onto 13-mm-diameter glass coverslips in a 24-well plate. The following day cells were pretreated with drugs (at various concentrations as shown in the figure legends) for 30 min and then infected with EV11-207 or EV11-207R at an MOI of 10 in medium containing the appropriate concentration of each drug for a further 30 min. Supernatant containing virus was removed, and the cells were washed three times with PBS. Fresh medium containing the drugs was added, and the cells were incubated for a further 6 to 8 h. Cells were washed twice with PBS-NCS and then fixed with PBS-F prior to immunofluorescence staining.
Transfection of viral RNA.
EV11 RNA was prepared by proteinase K digestion of concentrated virus stocks. Briefly, 100 μl of virus (at 1010 PFU/ml) was incubated with 200 μl of proteinase K buffer (0.3 M Tris-HCl [pH 8.0], 37.5 mM EDTA, 0.45 M NaCl, 3% SDS) containing 6 μg of proteinase K/ml for 2 h at 37°C. Samples were cleared by phenol-chloroform extraction, and RNA was ethanol precipitated overnight.
Prior to the transfections, 105 cells were seeded into a 24-well plate containing 13-mm-diameter coverslips and pretreated with drugs as described above. For the transfections, 1 μg of viral RNA was mixed with 10 μl of Escort (Sigma) and 240 μl of serum-free medium and incubated at room temperature for 15 min. Cells were washed twice with PBS and overlaid with fresh medium containing drugs and 250 μl of the transfection mix. The cells were incubated overnight at 37°C and then fixed as described above for immunofluorescence staining.
Immunofluorescence staining.
Fixed cells were permeabilized by adding 0.2% Triton X-100, followed by incubation at room temperature for 5 min. Cells were then washed twice with PBS-NCS. Anti-EV11 antibodies were added at the required concentration of 1/1,000, followed by incubation at room temperature for 30 min. Cells were then washed twice with PBS-NCS, and the secondary antibody (also diluted to 1/1,000) and DAPI (4′,6′-diamidino-2-phenylindole) were added; this step was followed by incubation for a further 30 min. Samples were then washed three times with PBS-NCS, and coverslips were removed and mounted onto glass slides by using ProLong antifade mountant (Molecular Probes). Samples were examined by using Leica SP confocal microscope and TCS NT software. Laser and microscope settings were according to the manufacturer's instructions.
Virus-binding assays.
RD or HT29 cells were seeded into a 24-well plate and allowed to grow to confluence. Before use, the cells were pretreated with drugs for 30 min at 37°C. The plates were then washed twice in serum-free RPMI medium, and purified [35S]methionine-labeled virus was then added (30,000 cpm) in 100 μl of medium containing the appropriate drug. The plates were incubated at 4°C for 45 min. Cells were washed three times with serum-free RPMI and lysed with 100 μl of 3 M NaOH. Scintillation counting was used to assess virus binding.
Isolation of lipid rafts and/or caveolae.
Purified [35S]methionine-labeled EV11-207 or EV11-207R (100,000 cpm) was added to 107 cells, followed by incubation at 4°C for 30 min. The cells were then washed with ice-cold PBS and incubated at 37°C for 60 min or for a time course of 0, 10, 20, and 40 min. The infected cells were then washed twice with PBS and lysed with 1 ml of ice-cold TNE (Tris-HCl [pH 8.0], NaCl, EDTA) containing 0.1%Triton X-100 for 30 min at 4°C. An equal volume of 80% sucrose in TNE was added to produce a final sucrose concentration of 40%. This was subsequently overlaid with 2 ml of a 30% sucrose solution in TNE and 1 ml of 4% sucrose in TNE. The samples were then spun in a Beckman SW55 rotor at 100,000 × g for 16 h at 4°C. Fractions were collected starting at the base of the tubes and analyzed by scintillation counting for the presence of virus or by Western blot of marker proteins such as caveolin-1, CAR, clathrin heavy chain, or DAF with HRP-conjugated secondary antibodies. Western blots were detected by using an enhanced chemiluminescence kit from Amersham Pharmacia as described in the manufacturer's instructions.
RESULTS
Infection of RD and HT29 cells by EV11-207 and EV11-207R.
During the experiments to investigate a role for lipid rafts or caveolae in infection by EV11, we used two cell lines: HT29 and RD. The HT29 cell line is a human gut epithelial cell line that was used for the initial isolation and subsequently for the passage of EV11-207 and EV11-207R (61; Stuart et al., unpublished). The RD cell line is a rhabdomyosarcoma line that has been used in many previous studies of enterovirus entry and infection (6, 8, 18, 74, 75, 87).
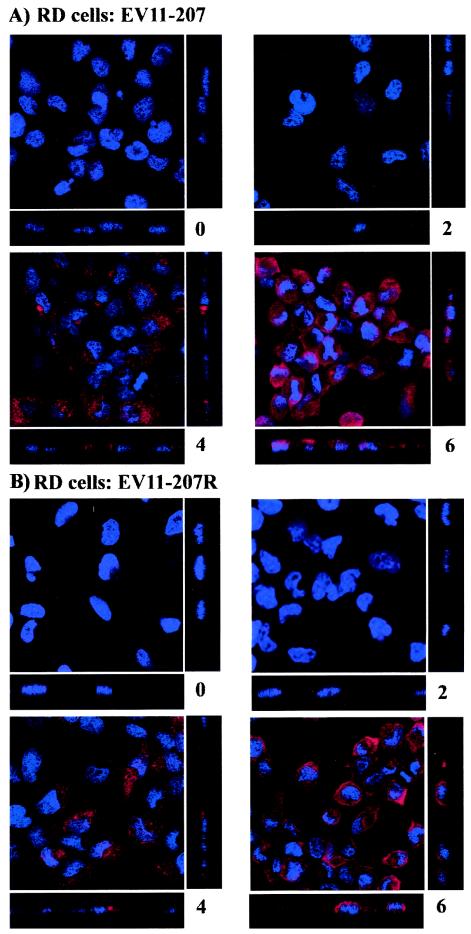
In order to examine the effects of the drugs on the infection by EV11-207 and EV11-207R, we carried out initial experiments to study the time course of infection of these two viruses in RD cells and HT29 cells. As shown in Fig. 1A (EV11-207) and B (EV11-207R), when RD cells were infected at an MOI of 10 no virus can be detected at 0 or 2 h postinfection (hpi) for either virus. Virus replication was detected in the majority of cells at 4 hpi, and by 6 hpi all of the cells were infected for EV11-207 and EV11-207R.
FIG. 1.
Time course of EV11-207 and EV11-207R infection of RD cells and HT29 cells. (A and B) RD cells were infected with EV11-207 (A) or EV11-207R (B). (C and D) HT29 cells were infected with EV11-207 (C) and EV11-207R (D). Cells were fixed at 0, 2, 4, 6, and 8 hpi, and virus replication was detected by indirect immunofluorescence with a monoclonal antibody to EV11 Vp3 and an Alexa 594-conjugated anti-mouse antibody. Nuclei were stained with DAPI.
Infection of HT29 cells with the two viruses at an MOI of 10 is shown in Fig. 1C (EV11-207) and D (EV11-207R). EV11-207 could be detected at 0 and 2 hpi, binding to the surface of HT29 cells (this has been confirmed by immunofluorescence of nonpermeabilized cells [data not shown]); at these time points no EV11-207R was detected. As with RD cells, replicating virus was detected 4 hpi for both EV11-207 and EV11-207R, and by 8 h all of the cells were infected. Subsequent experiments to study the effects of the drugs on the infection by these two viruses were therefore carried out at 6 hpi for RD cells and at 8 hpi for HT29 cells to ensure that detection of all infected cells could be studied.
Dose-dependent effect of drugs on EV11 infection.
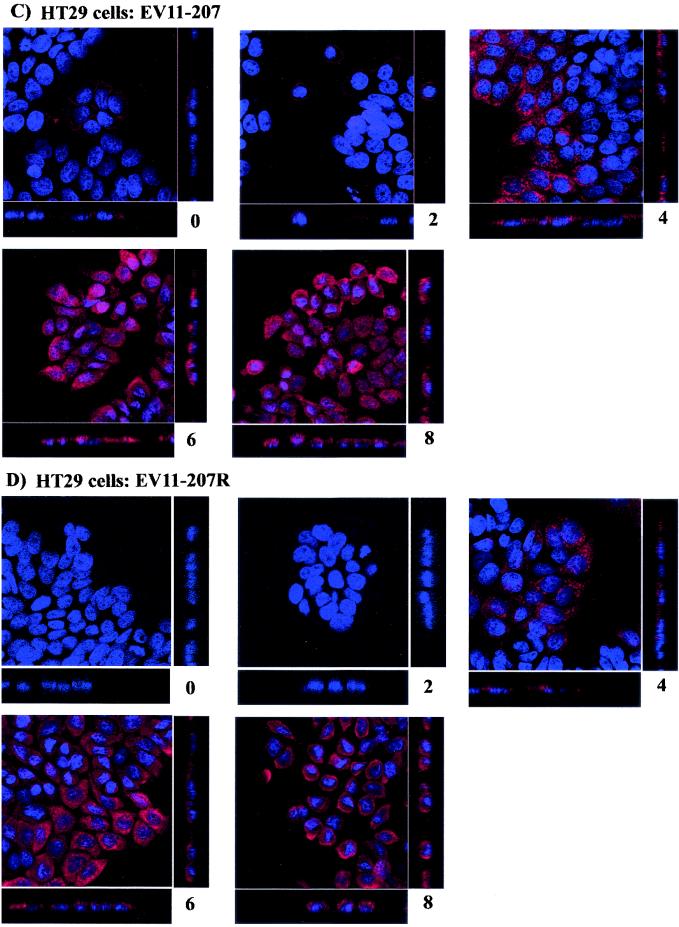
In order to study the effects of the different drugs on infection of RD and HT29 cells by EV11-207 and EV11-207R, initial dose dependence experiments were carried out with virus at an MOI of 10 to discover to concentration of each drug required. The results from a representative assay are shown in Fig. 2. Inhibition of EV11-207 infection by nystatin was optimal at 25 μM (Fig. 2A). There was further inhibition of EV11-207 infection at the higher dose of 50 μM, but some inhibition of EV11-207R infection was also evident. This inhibition of EV11-207R infection at high doses of nystatin most likely arose from excess cholesterol depletion, leading to inhibition of other endocytic pathways as has been reported previously (69, 82). Figure 2B shows the effect of chlorpromazine dose dependence. It can be seen that at 14 μM, chlorpromazine inhibits both EV11-207 and EV11-207R reducing the detected infection by twofold. At this concentration of chlorpromazine infection of RD cells and HT29 cells by avian influenza A virus (strain PR8), a virus known to enter via clathrin-coated pits, was completely inhibited (data not shown). Pretreatment of cells with 28 μM chlorpromazine completely inhibited infection by both EV11-207 and EV11-207R. However, at this concentration DAPI staining of the nuclei of both cells revealed large numbers of apoptotic cells, suggesting that the lack of infection was more likely due to cell death induced by the high levels of drug.
FIG. 2.
Dose response curves showing the effect of the various drugs on EV11 infection. HT29 cells were pretreated with nystatin (0 to 50 μM), chlorpromazine (0 to 28 μM), nocodazole (0 to 20 μM), or cytochalasin D (0 to 8 μM). Drug-treated cells were then infected with EV11-207 or EV11-207R at an MOI of 10. At 8 hpi cells were fixed with 4% formaldehyde and prepared for immunofluorescence. Virus infection was detected by indirect immunofluorescence with a monoclonal antibody to EV11 Vp3 and an Alexa 488-conjugated anti-mouse antibody. Nuclei were stained with DAPI. The percentage of positive cells was calculated as the number of virus infected cells per 200 DAPI-stained nuclei. The assays shown are representative of four repeat assays.
The effect of increasing amounts of nocodazole on EV11 infection is shown in Fig. 2C. The effect of this drug could also be monitored by immunofluorescence staining of the microtubule network with antibodies to alpha-tubulin (data not shown). The optimal dose of nocodazole for disrupting microtubules was 20 μM, at which a complete collapse of the network could be observed. This dose of nocodazole was also optimal for inhibiting the infection of cells by EV11-207. A similar study was carried out for cytochalasin D, whereby the actin cytoskeleton could also be monitored by immunofluorescence staining with fluorescein isothiocyanate-phalloidin (data not shown). The effects of increasing amounts of cytochalasin D are shown in Fig. 2D. The optimal concentration of cytochalasin D required to disrupt the actin cytoskeleton was 4 μM. Further inhibition of EV11-207 infection could be detected at 8 μM but, as with chlorpromazine, this was associated with increased evidence of apoptosis in the target cells, suggesting that the reduction in infection was due to increased cell death.
Subsequent experiments used the drugs at the optimal concentrations of nystatin (25 μM), chlorpromazine (14 μM), nocodazole (20 μM), and cytochalasin D (4 μM).
Effect of MOI on response of EV11 to drugs.
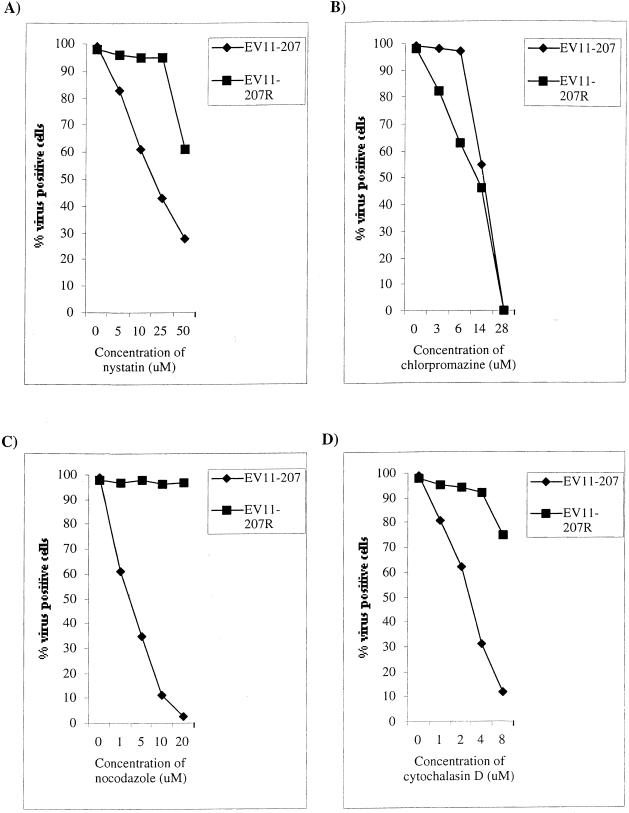
We also investigated the effect of the various drugs on cells infected at decreasing amounts of virus. We infected each cell line with virus at three different MOIs: 10, 1, and 0.1. It has been suggested (20) that infection by viruses at an MOI of <1 reflects the use of a more specific infection route. The effect of the drugs was assessed by using indirect immunofluorescence to quantify the level of infection in each cell line at each MOI. These data were then presented as a percentage of the number of virus-positive cells over the number of DAPI-stained nuclei within a given field. The results from a representative assay (of four repeat assays) are shown in Fig. 3.
FIG. 3.
Effect of various inhibitors of endocytosis on infection of RD and HT29 cells by EV11-207 and EV11-207R. Cells were pretreated with drugs for 30 min before infection with EV11-207 or EV11-207R at an MOI of 10, 1, or 0.1. Infection was detected by immunofluorescence of formaldehyde-fixed RD cells at 6 hpi or HT29 cells at 8 hpi. Data were plotted as the percentage of virus-positive cells over the number of DAPI-stained nuclei. (A) RD cells infected with EV11-207. (B) RD cells infected with EV11-207R. (C) HT29 cells infected with EV11-207. (D) HT29 cells infected with EV11-207R. The assays shown are representative of four repeat assays.
Figure 3A shows the results of the various drug treatments on infection of RD cells by EV11-207 at MOIs of 10, 1, and 0.1. These data show that, at all three MOIs, EV11-207 infection of RD cells is inhibited by the cholesterol-sequestering drug nystatin and the microtubule-depolymerizing drug nocodazole. Nocodazole is a more potent inhibitor of EV11-207 infection, reducing it by between 37 to 97%, whereas nystatin reduced infection by 35 to 50% depending on the MOI used. This suggests that cholesterol and an intact microtubule network are necessary for efficient infection by EV11-207. Chlorpromazine, which inhibits clathrin-mediated endocytosis, reduced EV11-207 infection only at the MOI of 10 and then only by 50%. This indicates that the clathrin-mediated endocytic pathway is only used by EV11-207 at higher concentrations of virus, possibly reflecting a less-specific infection route for the virus. The actin-depolymerizing drug cytochalasin D inhibited the infection of RD cells by EV11-207 only at the lowest MOI. These data suggest that the actin cytoskeleton plays a minimal role in infection of RD cells by EV11-207 but that any role for actin is greatest at low levels of virus when an entry route with greater affinity for the virus may be used.
Figure 3B demonstrates the effect of the various drugs on EV11-207R infection of RD cells at the three different MOIs. These data show that at all three MOIs tested EV11-207R infection was unaffected by nystatin, nocodazole, or cytochalasin D but that it was partially inhibited by chlorpromazine. Chlorpromazine reduced the infection by ca. 50% at all three MOIs. This suggests that EV11-207R uses the clathrin-mediated endocytic route, but the low level of inhibition suggests that other entry routes may also be available to this virus.
The effect of the various drugs on infection of HT29 cells by EV11-207 is shown in Fig. 3C. This shows that EV11-207 infection of HT29 cells is also dependent upon cholesterol and microtubules since nystatin and nocodazole both inhibit infection. Chlorpromazine is effective against EV11-207 infection of HT29 cells only at an MOI of 10, again suggesting that the clathrin-dependent endocytosis route is used when greater amounts of virus are present. In HT29 cells, cytochalasin D inhibits infection by EV11-207 at MOIs of 1 and 0.1, reducing infection by between 37 and 52%. This suggests that the actin cytoskeleton plays a greater role in infection of this cell line by EV11-207. Infection of HT29 cells by EV11-207R was inhibited in exactly the same pattern as were RD cells. Figure 3D shows that EV11-207R infection was only sensitive to chlorpromazine, reducing infection by ca. 50% at all three MOIs.
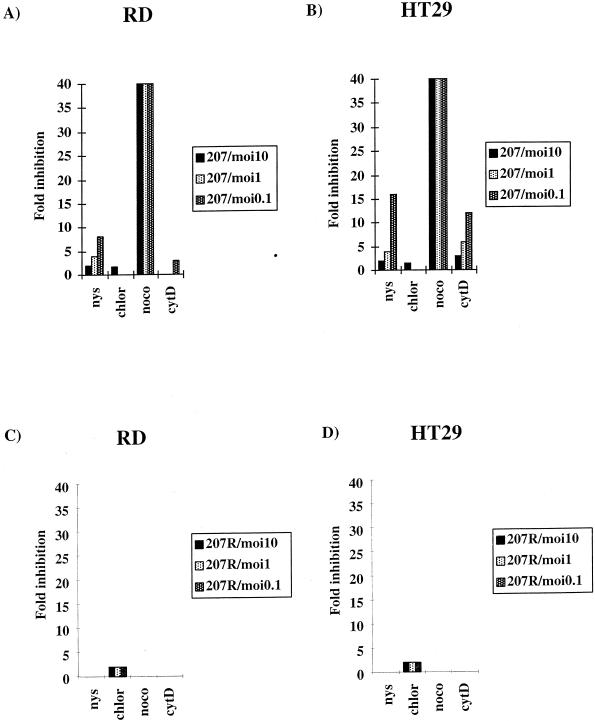
Relative inhibition of EV11-207 infection depends on the MOI used.
As stated above, infection of cells with virus at a lower MOI may reflect the use of a more specific, physiologically relevant route, whereas at a higher MOI the virus may spill over into normally less productive routes. We examined the efficacy of the various drugs on virus infection resulting from the decreasing MOI by comparing the relative inhibition of each drug compared to untreated cells for each MOI. These results are shown in Fig. 4. The results showed that with decreasing MOI the relative inhibition of EV11-207 infection resulting from nystatin increased from 2- to 8-fold in RD cells (Fig. 4A) and from 2- to 16-fold in HT29 (Fig. 4B) cells. Cytochalasin D treatment increased the inhibition of EV11-207 from 0- to 2.5-fold in RD and 3-fold to 13-fold in HT29 cells. Treatment with nocodazole, however, resulted in a constant reduction of 40-fold regardless of MOI (the figure actually ranged from 39.8 to 40.3). Figure 4C and D show the relative inhibition of EV11-207R infection of RD and HT29 cells, respectively. These panels show that EV11-207R infection was inhibited twofold in both cell lines by chlorpromazine only and at all MOIs of virus tested.
FIG. 4.
Relative inhibition resulting from individual drugs. The relative inhibition was calculated for each drug compared to untreated cells for EV11-207 (A and B) and EV11-207R (C and D) at MOIs of 10, 1, and 0.1. Panels A and C show the results for RD cells, and panels B and D show the results for HT29 cells.
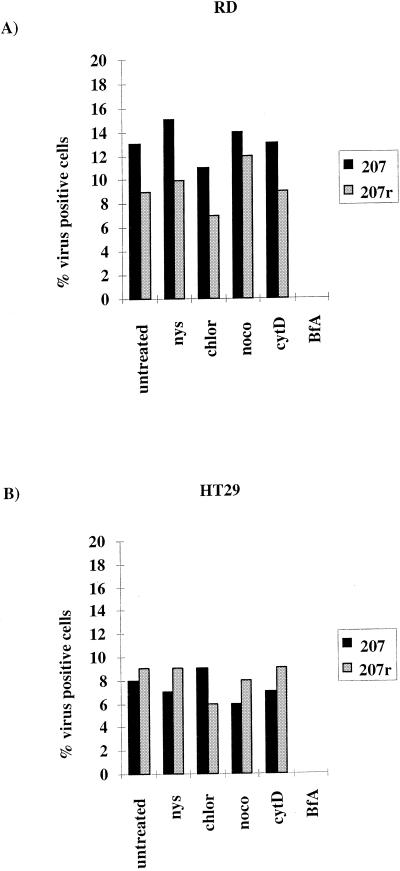
Replication of virus from viral RNA is unaffected by drug treatment.
The assays described above measure both entry and replication of virus. In order to confirm that the drugs were acting at the level of entry, we utilized the fact that enterovirus RNA is infectious to circumvent virus entry mechanisms and allow a direct evaluation of the effect of the drugs on viral replication. The results shown in Fig. 5 represent the percentage of cells staining positive for virus over the DAPI-stained nuclei from a representative assay of four repeat assays. Panel A shows the transfection of RD cells, and panel B shows the transfection of HT29 cells. In both cell lines, the level of transfection ranged from 6 to 15% and, of the drugs tested, only brefeldin A inhibited virus replication. This result strongly suggests that nystatin, chlorpromazine, nocodazole, and cytochalasin D act on a stage prior to viral RNA uncoating and release into the cytoplasm for virus replication.
FIG. 5.
Effect of drugs on the replication of transfected enteroviral RNA. RD and HT29 cells were pretreated with drugs for 30 min. A total of 1 μg of enteroviral RNA was transfected with Escort lipofection reagent. Virus replication was assessed 8 h posttransfection by immunofluorescence and is expressed as the percentage of virus-positive cells divided by the number of DAPI-stained nuclei. Panel A shows the results for RD cells, and panel B shows the results for HT29 cells. The assays shown are representative of four repeat assays.
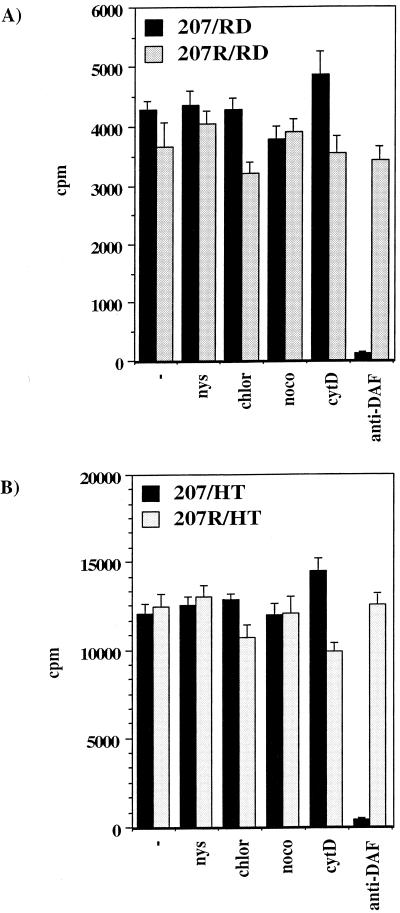
EV11 binding to cells is unaffected by drug treatment.
Further studies were undertaken to discriminate between drug effects on virus binding and entry or uncoating. A potential effect of the drugs could be to alter virus binding by downregulation of receptor and accessory proteins (a known action of chlorpromazine is to downregulate receptor recycling [85]). This effect was examined by using highly purified, [35S]methionine-labeled EV11-207 and EV11-207R in binding assays on drug-treated RD and HT29 cells. Figure 6 shows the results from representative binding assays. The pretreatment of cells with nystatin, chlorpromazine, nocodazole, or cytochalasin D had no effect on the ability of either cell line to bind the virus. Preincubation of cells with anti-DAF polyclonal sera, however, could inhibit binding by EV11-207 but not binding by EV11-207R, thus highlighting the capacity of this virus to bind DAF.
FIG. 6.
Effect of drugs on virus binding. RD and HT29 cells were pretreated with drugs or anti-DAF antibody for 30 min. A total of 30,000 cpm [35S]methionine-labeled virus was added, followed by incubation at 4°C for 45 min. Cells were washed twice and lysed with 3 M NaOH. Virus binding was assessed by scintillation counting. The results for RD cells are shown in panel A, and the results for HT29 cells are shown in panel B.
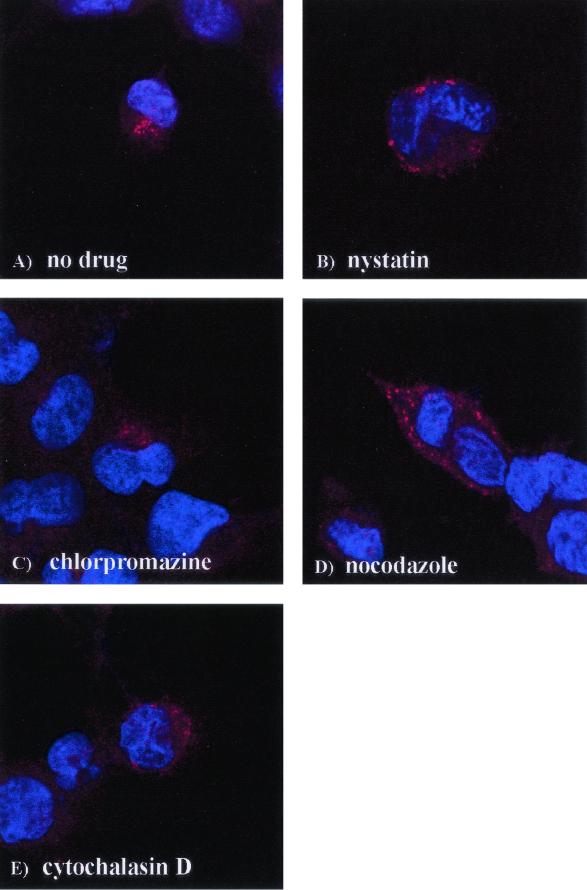
Drug treatment alters the intracellular location of internalized EV11-207.
To examine the effect of the drugs on the entry of DAF-using EV11-207 in RD cells directly, we used confocal microscopy of cells labeled with an anti-EV11 monoclonal antibody that binds to Vp3 in the capsid to detect virus early after infection. The nuclei were labeled with DAPI. Figure 7A shows that at 60 min postbinding, EV11-207 has been completely internalized and has localized to a juxtanuclear position. Pretreatment of cells with nystatin (Fig. 7B) and cytochalasin D (Fig. 7E) appeared to prevent internalization of EV11-207, and the virus can be seen at the periphery of the cell. The cell surface labeling was also confirmed with unpermeabilized cells (data not shown). Chlorpromazine treatment had little or no effect on the pattern of entry by EV11-207 since virus could be detected in the juxtanuclear position (Fig. 7C). Nocodazole treatment (Fig. 7D) allowed entry of EV11-207, but the virus appeared to be blocked in vesicular bodies scattered around the nucleus rather than localizing to the juxtanuclear position.
FIG. 7.
Localization of EV11-207 during entry after drug treatment. RD cells were pretreated with drugs for 30 min. EV11-207 at an MOI of 100 was added, followed by incubation at 4°C for 30 min. The cells were washed twice and transferred to 37°C to permit entry. Entry was halted after 60 min by fixation with 4% formaldehyde in PBS. Virus was detected by evaluating immunofluorescence with mouse anti-EV11 antisera and Alexa 594-conjugated rabbit anti-mouse secondary antisera. Immunofluorescence was visualized with a Leica confocal microscope. (A) Untreated cells; (B) nystatin; (C) chlorpromazine; (D) nocodazole; (E) cytochalasin D.
DAF-using EV11-207 copurifies with lipid raft-associated proteins.
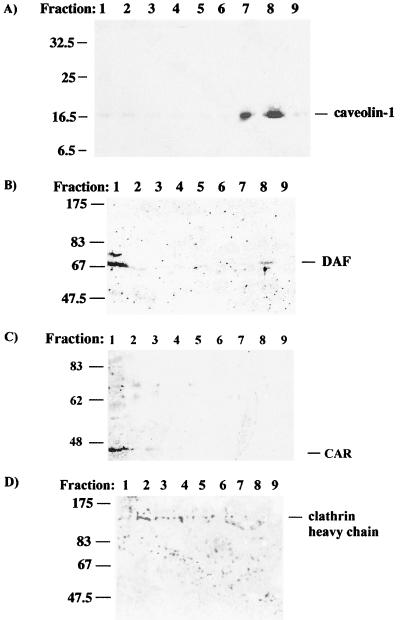
Lipid raft components can be isolated based on their insolubility in nonionic detergents such as Triton X-100 at 4°C (80). We used this fact to investigate the association between DAF-using and non-DAF-using EV11 with lipid rafts during entry. The raft components were isolated after [35S]methionine-labeled virus had been allowed to bind to cells at 4°C for 30 min, followed by incubation at 37°C for 60 min to allow entry. The Triton X-100-insoluble material was isolated by centrifugation through a discontinuous sucrose gradient; fractions were collected and analyzed by Western blot for the presence of marker proteins and by scintillation counting to detect labeled virus. Figure 8A shows the presence of caveolin-1 in the raft fractions prepared from RD cells. HT29 cells did not express detectable levels of caveolin-1 (data not shown). DAF could however be detected in raft fractions from HT29 cells as shown in Fig. 8B and in RD cells (data not shown). As a control for the raft preparations, fractions from both cell lines were probed with antibodies to nonraft proteins. Fractions from RD cells were probed with an antibody to clathrin heavy chain, and fractions from HT29 cells were probed with an antibody to CAR. CAR contains a sequence, YNQV, in its cytoplasmic tail. Such sequences have been shown to interact with clathrin adaptor proteins and direct proteins to clathrin-coated pits (55).
FIG. 8.
Western blot analysis of raft preparations. Fractions from Triton X-100-treated uninfected RD cells and HT29 cells were collected as described in Materials and Methods. The samples were analyzed for the presence of lipid raft and nonraft proteins. (A) Blot of RD cells probed for caveolin-1; (B) blot of HT29 cells probed for DAF; (C) blot of HT29 cells probed for CAR; (D) blot of RD cells probed for clathrin heavy chain. The fraction numbers shown start from the bottom of the sucrose gradient.
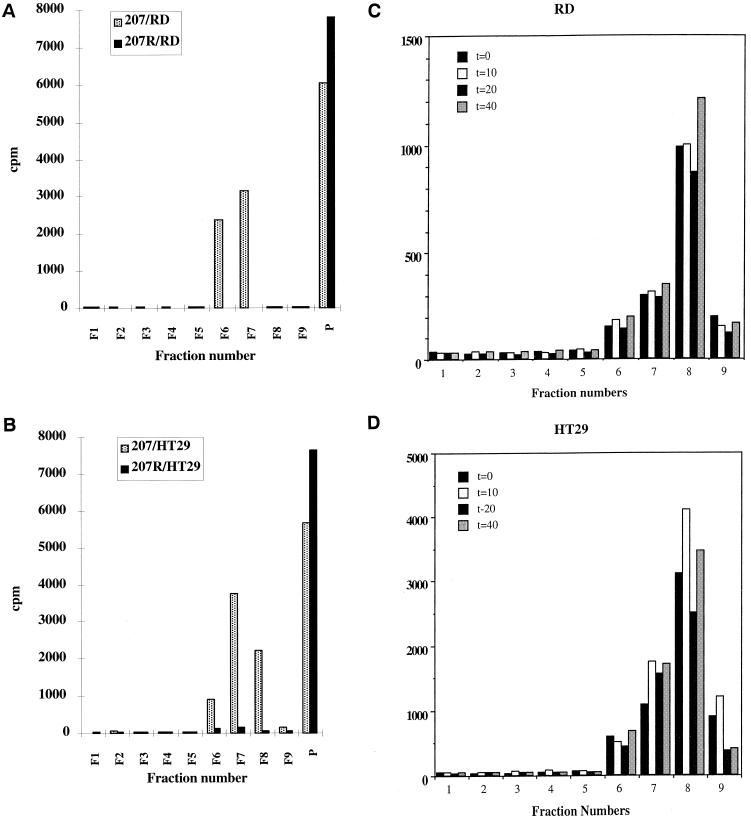
The presence of DAF and caveolin-1 in raft fractions 7 and 8 corresponds with the presence of radiolabeled EV11-207 in both cell lines (Fig. 9A and B), but not EV11-207R. The virus can be detected in raft fractions as early as 0 min after binding at 4°C (Fig. 9C). This shows that the virus binding to DAF is sufficient to lead to association with rafts. The virus can also be purified with raft components up to 90 min after entry (Fig. 9C), suggesting it remains associated with DAF and lipid rafts during intracellular trafficking.
FIG. 9.
Copurification of EV11-207 with raft fractions. RD (A) and HT29 (B) cells were infected with [35S]methionine-labeled EV11-207 or EV11-207R, and raft fractions were isolated as described in Materials and Methods. The fraction numbers shown start from the bottom of the sucrose gradient, and “P” represents the pellet resulting from the centrifugation. (C and D) Results of a time course of the association of [35S]methionine-labeled EV11-207 with lipid raft fractions in RD cells (C) and HT29 cells (D). Time points were taken at 0, 10, 20, and 40 min postbinding.
DISCUSSION
In this study we examined the consequence of virus-DAF interactions on the entry route(s) used by EV11-207 and its tissue culture-derived variant EV11-207R. These two viruses differ only in their capsid sequence, with six amino acids changes in EV11-207R compared to EV11-207 (Stuart et al., unpublished). The mutations are clustered in or around the so-called canyon region that surrounds the fivefold axis of symmetry (for a review, see reference 70). In a number of cases this region has been shown to be involved in receptor binding and specificity (5, 39, 90). EV11-207 has been shown previously to bind to DAF by using a hemagglutination assay with human RBC (Stuart et al., unpublished) and, more specifically, by using surface plasmon resonance techniques (42). This latter approach demonstrated that EV11-207 binds to DAF with a Kd of ca. 3 μM and that the reaction has fast on-off rates. EV11-207R was shown to be unable to hemagglutinate human RBC, suggesting that its ability to bind DAF is several orders of magnitude lower than that of the parent virus. These two viruses are identical in sequence apart from their capsid regions; thus, their receptor or coreceptor usage alone will determine differences in their cell tropism and entry mechanisms.
Determination of entry routes with drugs specific to particular endocytosis pathways.
In order to examine whether different entry route(s) are used by EV11-207 and EV11-207R, we studied infection by these viruses in two cell lines in the presence of drugs known to inhibit particular endocytic routes and internal trafficking pathways. These drugs are shown in Table 1 with their functions. The drugs were first tested at a range of concentrations to identify the optimum dose to use and also to look for any possible additional effects at higher doses. We have shown that the optimum concentrations to use were: nystatin at 25 μM, chlorpromazine at 14 μM, nocodazole at 20 μM, and cytochalasin D at 4 μM. We have also shown that at higher concentrations nonspecific effects of the drugs could be observed. Doses of nystatin at 50 μM and above interfere with other endocytosis pathways since inhibition of EV11-207R infection could be observed. It has been reported previously that excess depletion of cholesterol from the membrane leads to inhibition of clathrin-coated pit endocytosis as well as uptake through lipid rafts or caveolae (69, 82). At higher doses of chlorpromazine and cytochalasin D, an increase in cell death could be detected by DAPI staining, thus demonstrating that the reduction in the number of infected cells observed was due to decreased cell viability and not to the inhibition of specific entry pathways. Nocodazole proved to be remarkably specific for inhibiting EV11-207 infection and showed no side effects at higher doses.
From these data and the subsequent experiments to examine the effect of decreasing MOI, we have shown that nystatin and methyl-β-cyclodextrin (MCD [data not shown]) inhibit infection by the DAF-binding virus EV11-207 but not by EV11-207R in both HT29 and RD cells. EV11-207 therefore infects cells in a cholesterol-dependent manner. Moreover, the dependence upon cholesterol during infection by EV11-207 was shown to increase as the MOI of virus decreased. This suggests a specific role for lipid rafts and/or caveolae and may reflect a role for this infection route during the initial stages of enterovirus infection when low levels of incoming virus would be present. From the immunofluorescence images showing the localization of virus in nystatin-treated cells it would appear that this drug permits accumulation of EV11-207 at the cell surface but blocks virus entry. These data corresponds with the known ability of cholesterol-sequestering drugs to inhibit the endocytosis of GPI-linked proteins and also the entry through lipid rafts of pathogens such as SV40 and E. coli expressing Dr or FimH adhesins (19, 73, 79, 81).
The amount of virus used for the immune localization studies was necessarily high to enable us to visualize the virus without any replication. The data from the inhibition experiments carried out at various MOIs suggest that, at this high dose of virus, multiple entry pathways would be used. It may be that nystatin blocks the majority of virus at the cell surface by preventing entry through rafts but that at the higher MOIs some viruses can still enter through other pathways, resulting in full infection.
Two drugs that disrupt the cytoskeleton, nocodazole and cytochalasin D, which target microtubules and actin filaments, respectively, also inhibited infection by EV11-207 but not infection by EV11-207R. The inhibition of EV11-207 infection resulting from cytochalasin D treatment, as with nystatin and MCD, also increased with decreasing MOIs of virus, suggesting a role for actin in the more specific entry pathway used by EV11-207.
Cytochalasin D was less effective at inhibiting infection in RD cells compared to HT29 cells. This may simply reflect a phenotypic difference between these two cell lines. Thus, the HT29 cell line is a polarized gut epithelial cell line (32; unpublished data) expressing high levels of DAF (data not shown), whereas the RD cell line is a rhabdomyosarcoma cell line with the potential for limited myogenic differentiation (11) and expresses lower levels of DAF (data not shown). Deckert et al. (19) demonstrated that endocytosis of cross-linked GPI-anchored CD59 is inhibited by cytochalasin H in Jurkat cells but that CD59 internalization was unaffected by this drug in epithelial cell lines: A431 and CD59 transfected CHO cells. There have also been reports that endocytosis at the apical surface of polarized cells is sensitive to cytochalasin D (27); it may be that the polarized nature of HT29 cells renders them more susceptible to the effects of cytochalasin D in this assay.
Infection of RD cells with EV11-207 and EV11-207R is initiated much more quickly than in HT29 cells (unpublished data), possibly reflecting differences in entry pathways for these two cell lines. Anti-CD59 serum that inhibits the infection of RD cells by EV11-207 and EV11-207R has no effect in HT29 cells, also indicating that the entry routes available in these two cells lines may be subtly different (unpublished data). The immune localization studies demonstrated that in the presence of cytochalasin D the virus appeared blocked at the cell surface, in a fashion similar to that produced by nystatin treatment. This result suggests that cytochalasin D also blocks EV11-207 infection by inhibiting virus entry at the cell surface. The actin cytoskeleton has been shown to be important in the internalization of CD59 another GPI-anchored complement regulatory molecule similar to DAF (19). Disruption of the cytoskeleton by cytochalasin H prevents the internalization of GPI-linked CD59 through lipid rafts in T lymphocytes. Our data show that the DAF-dependent infection of EV11-207 through rafts is also sensitive to actin-disrupting drugs. Infection by EV11-207R is independent of actin filaments, which also suggests an alternative entry route to that used by EV11-207.
Inhibition of EV11-207 infection resulting from nocodazole treatment, however, was unaffected by the different MOIs of virus and remained constant at a 40-fold reduction. The action of nocodazole in disrupting microtubules affects events occurring after the virus has entered through the plasma membrane, when the endocytic vesicles containing virus are trafficking through the cytoplasm. The constant inhibition seen may result from the ability of nocodazole to block multiple trafficking pathways from both clathrin-coated pits and lipid rafts or caveolae. The appearance of the virus in nocodazole-treated cells in the vesicular structures scattered throughout the cytoplasm suggests the virus is being retained in a subcellular compartment. These vesicular structures may represent an intermediate compartment en route to subcellular compartments. Nocodazole has been shown to inhibit the trafficking of rhinovirus 2 in endosomal carrier vesicles (2). However, this drug does not prevent HRV2, parechovirus 1(34), or poliovirus (22) from replicating, suggesting that the transition to late endosomes is not required for efficient entry and uncoating of enterovirus RNA. EV11-207R was also able to replicate in the presence of nocodazole, suggesting that this virus can also efficiently uncoat prior to transition to late compartments. Nocodazole has also been shown to block the trafficking of caveolin-1 from the plasma membrane to the endoplasmic reticulum-Golgi and back again. In the presence of nocodazole the caveolin-1 is retained in the endoplasmic reticulum-Golgi intermediate compartment (16). The inhibition of EV11-207 infection by nocodazole suggests that, unlike EV11-207R, poliovirus, or rhinovirus, this virus requires transit to a different compartment for efficient infection.
Chlorpromazine, an inhibitor of clathrin-coated pit-mediated endocytosis, only weakly inhibited EV11-207 when a high MOI was used; at lower doses of virus this drug had no effect on EV11-207 infection. This suggests that any infection through clathrin-coated pits may result from the use of a less-specific pathway for this virus; such a pathway would be used when there is excess virus present. This concentration of chlorpromazine, however, was able to efficiently inhibit infection of both cell lines by the PR8 strain of influenza A virus (data not shown). Influenza is known to enter via receptor-mediated endocytosis (51). Chlorpromazine also partially inhibited infection by EV11-207R, reducing infection twofold regardless of the MOI used. This non-DAF-using virus may infect cells through clathrin-coated pits, but other, as yet unknown, infection routes may also play a role. The non-DAF-using variant EV11-207R was unaffected by the presence of nystatin, nocodazole, or cytochalasin D, demonstrating that this virus does not infect cells through lipid rafts or caveolae. Further work is under way to investigate possible infection routes for EV11-207R.
We have shown, by using binding assays and transfection with viral RNA, that the effect of all of the drugs tested takes place postbinding but prior to RNA uncoating, thus confirming that their primary effect in our assays is during entry and uncoating of the virus. This finding is of particular interest for nocodazole since the data suggest that, unlike poliovirus and rhinovirus, this virus requires transition to different intracellular compartments for uncoating of its RNA. Previous work has shown that poliovirus and coxsackievirus B3 convert to 135S particles at the cell surface. However, it has been suggested that during infection with hemagglutinating echoviruses the majority of the virions undergo particle conversion once they have been internalized (66). This would suggest a requirement for an intracellular compartment with internal factors necessary for promoting the uncoating process since soluble DAF has been shown to be insufficient to promote 135S particle formation.
Infection by both EV11-207 and EV11-207R was insensitive to the effects of chloroquine, demonstrating that acidification is not required for uncoating (data not shown); however, in control experiments infection by the PR8 strain of influenza virus was inhibited by the concentrations of chloroquine tested (data not shown). There is contradictory evidence for the requirement of acidification for poliovirus uncoating, although recent publications have indicated that it is not necessary. It is therefore likely that EV11-207 requires further factors to aid in its uncoating that are not present in the vesicles resulting from nocodazole treatment.
Colocalization of DAF-using EV11-207 with lipid rafts.
Lipid rafts can be separated from other cellular membrane components on the basis of their more-ordered structure. This leads to lipid raft domains being insoluble in certain nonionic detergents such as Triton X-100 at 4°C (72). This method also isolates cytoskeleton components that are then separated from the lipid rafts, on the basis of their density, by centrifugation through a discontinuous sucrose gradient. The majority of the cytoskeleton components penetrate the 40% sucrose layer, whereas the lipid rafts, having a lower density, are retained at the interface between 30% sucrose and 4% sucrose. By this method we have demonstrated that radiolabeled EV11-207, but not EV11-207R, copurifies with the raft components caveolin-1 and DAF during entry. This association of EV11-207 with lipid rafts can be observed after binding of labeled virus at 4°C for 30 min and up to 90 min postentry at 37°C in both cell lines. This shows that the binding of virus to DAF leads to their immediate association with lipid rafts after Triton X-100 extraction. SV40, a polyomavirus, binds to class I HLA and has been shown to enter through caveolae. This virus does not immediately associate with Triton X-100-insoluble fractions. It can be purified in the TX-100-insoluble fraction by 30 min postadsorption, reaching a maximum at 1 h, thus demonstrating that a transition phase is required for the virus to enter the caveolae (14). The detection of EV11-207 with DAF and lipid rafts postentry suggests that EV11-207 remains associated with lipid rafts domains during intracellular trafficking.
Based on the Western blot analysis of raft fractions from RD and HT29 cells, we observed that HT29 cells do not express detectable levels of caveolin-1. This would suggest that HT29 cells have very low levels of or no caveolae (43). The results from the drug inhibition studies would thus suggest that, in HT29 cells, EV11-207 enters through lipid rafts alone, whereas in RD cells it could enter through lipid rafts and/or caveolae; further work using electron microscopy is under way to confirm this.
Role of DAF and lipid rafts or caveolae in the cell entry of pathogens.
EV11-207 virions possess multiple DAF-binding sites with the potential for cross-linking DAF. Shafren et al. (76, 77) have reported that cross-linking of DAF by antibodies enhances the infection of RD cells by CAV21 and that this infection takes place through caveolae. The main receptor for CAV21 is ICAM-1, RD cells lack this protein and are nonpermissive to CAV21. These data suggest that for CAV21 virus alone is normally insufficient to cross-link DAF and induce internalization through lipid rafts or caveolae. CAV21 binds to the SCR1 domain of DAF, unlike EV11-207, which binds SCR3. Shafren et al. (78) have suggested that the close spacial arrangement between DAF and ICAM-1 allows interaction between ICAM-1 and the SCR3 domain of DAF and which in turn may promote cross-linking and internalization. The affinity of the binding between CAV21 and DAF is unknown, but it may be that the interaction of CAV21 with DAF at SCR1 does not allow the same ability for cross-linking in the absence of ICAM-1 or that interactions at SCR3 are required for efficient cross-linking.
E. coli expressing the Dr adhesin also infect cells in a DAF-dependent manner. These bacteria have been shown to enter through lipid rafts (73). The bacteria require a GPI-linked form of DAF for efficient invasion since cells expressing DAF molecules modified to include a transmembrane region from either HLA-B44 or membrane cofactor protein show reduced infection. The infection of epithelial cells by these DAF-binding bacteria is also inhibited very effectively by nocodazole and less so by cytochalasin D (25) in a pattern very similar to that observed for EV11-207, suggesting that there may be similar mechanisms involved during entry by these two pathogens.
Lipid rafts and caveolae have been shown to be the entry site for a growing number of pathogens. These pathogens include viruses such as SV40 and EV1, bacteria such E. coli expressing either Dr or FimH fimbriae, and Campylobacter and bacterial products such as cholera toxin and lipopolysaccharide. In the present study, we have demonstrated that the interaction between EV11-207 and DAF leads to the infection of cells through glycosphingolipid rafts. The identification of this infection route may aid in further studies to characterize additional proteins involved in the entry of this and other hemagglutinating enteroviruses.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72:9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bella, J., and M. G. Rossmann. 1999. Review: rhinoviruses and their ICAM receptors. J. Struct. Biol. 128:69-74. [DOI] [PubMed] [Google Scholar]
- 4.Belnap, D. M., D. J. Filman, B. L. Trus, N. Cheng, F. P. Booy, J. F. Conway, S. Curry, C. N. Hiremath, S. K. Tsang, A. C. Steven, and J. M. Hogle. 2000. Molecular tectonic model of virus structural transitions: the putative cell entry states of poliovirus. J. Virol. 74:1342-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belnap, D. M., B. M. McDermott, Jr., D. J. Filman, N. Cheng, B. L. Trus, H. J. Zuccola, V. R. Racaniello, J. M. Hogle, and A. C. Steven. 2000. Three-dimensional structure of poliovirus receptor bound to poliovirus. Proc. Natl. Acad. Sci. USA 97:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., M. P. Shepley, B. M. Chan, M. E. Hemler, and R. W. Finberg. 1992. Identification of the integrin VLA-2 as a receptor for echovirus 1. Science 255:1718-1720. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson, J. M., M. Chan, K. R. Solomon, N. F. St. John, H. Lin, and R. W. Finberg. 1994. Decay-accelerating factor (CD55), a glycosylphosphatidylinositol-anchored complement regulatory protein, is a receptor for several echoviruses. Proc. Natl. Acad. Sci. USA 91:6245-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., J. G. Mohanty, R. L. Crowell, N. F. St. John, D. M. Lublin, and R. W. Finberg. 1995. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J. Virol. 69:1903-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 10.Bergelson, J. M., J. F. Modlin, W. Wieland-Alter, J. A. Cunningham, R. L. Crowell, and R. W. Finberg. 1997. Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J. Infect. Dis. 175:697-700. [DOI] [PubMed] [Google Scholar]
- 11.Bouche, M., M. I. Senni, A. M. Grossi, F. Zappelli, M. Polimeni, H. H. Arnold, G. Cossu, and M. Molinaro. 1993. TPA-induced differentiation of human rhabdomyosarcoma cells: expression of the myogenic regulatory factors. Exp. Cell Res. 208:209-217. [DOI] [PubMed] [Google Scholar]
- 12.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell. Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 13.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. [DOI] [PubMed] [Google Scholar]
- 15.Clarkson, N. A., R. Kaufman, D. M. Lublin, T. Ward, P. A. Pipkin, P. D. Minor, D. J. Evans, and J. W. Almond. 1995. Characterization of the echovirus 7 receptor: domains of CD55 critical for virus binding. J. Virol. 69:5497-5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conrad, P. A., E. J. Smart, Y. S. Ying, R. G. Anderson, and G. S. Bloom. 1995. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J. Cell Biol. 131:1421-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crowell, R. L., and L. Philipson. 1971. Specific alterations of coxsackievirus B3 eluted from HeLa cells. J. Virol. 8:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowell, R. L., A. K. Field, W. A. Schleif, W. L. Long, R. J. Colonno, J. E. Mapoles, and E. A. Emini. 1986. Monoclonal antibody that inhibits infection of HeLa and rhabdomyosarcoma cells by selected enteroviruses through receptor blockade. J. Virol. 57:438-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deckert, M., M. Ticchioni, and A. Bernard. 1996. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J. Cell Biol. 133:791-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dinter, A., and E. G. Berger. 1998. Golgi-disturbing agents. Histochem. Cell. Biol. 109:571-590. [DOI] [PubMed] [Google Scholar]
- 22.Doedens, J., L. A. Maynell, M. W. Klymkowsky, and K. Kirkegaard. 1994. Secretory pathway function, but not cytoskeletal integrity, is required in poliovirus infection. Arch. Virol. Suppl. 9:159-172. [DOI] [PubMed] [Google Scholar]
- 23.Fan, D. P., and B. M. Sefton. 1978. The entry into host cells of Sindbis virus, vesicular stomatitis virus, and Sendai virus. Cell 15:985-992. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the He. La epithelial cell line. J. Infect. Dis. 176:158-167. [DOI] [PubMed] [Google Scholar]
- 26.Goodfellow, I. G., R. M. Powell, T. Ward, O. B. Spiller, J. W. Almond, and D. J. Evans. 2000. Echovirus infection of rhabdomyosarcoma cells is inhibited by antiserum to the complement control protein CD59. J. Gen. Virol. 81:1393-1401. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb, T. A., I. E. Ivanov, M. Adesnik, and D. D. Sabatini. 1993. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J. Cell Biol. 120:695-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grunert, H. P., K. U. Wolf, K. D. Langner, D. Sawitzky, K. O. Habermehl, and H. Zeichhardt. 1997. Internalization of human rhinovirus 14 into HeLa and ICAM-1-transfected BHK cells. Med. Microbiol. Immunol. 186:1-9. [DOI] [PubMed] [Google Scholar]
- 29.Harder, T., and K. Simons. 1997. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr. Opin. Cell Biol. 9:534-542. [DOI] [PubMed] [Google Scholar]
- 30.He, Y., V. D. Bowman, S. Mueller, C. M. Bator, J. Bella, X. Peng, T. S.Baker, E. Wimmer, R. J. Kuhn, and M. G. Rossmann. 2000. Interaction of the poliovirus receptor with poliovirus. Proc. Natl. Acad. Sci. USA 97:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hekmati, M., S. Polak-Charcon, and Y. Ben-Shaul. 1990. A morphological study of a human adenocarcinoma cell line (HT29) differentiating in culture: similarities to intestinal embryonic development. Cell. Differ. Dev. 31:207-218. [DOI] [PubMed] [Google Scholar]
- 33.Janes, P. W., S. C. Ley, A. I. Magee, and P. S. Kabouridis. 2000. The role of lipid rafts in T-cell antigen receptor (TCR) signalling. Semin. Immunol. 12:23-34. [DOI] [PubMed] [Google Scholar]
- 34.Joki-Korpela, P., V. Marjomaki, C. Krogerus, J. Heino, and T. Hyypia. 2001. Entry of human parechovirus 1. J. Virol. 75:1958-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joklik, W. K., and J. E. Darnell. 1961. The adsorbtion and early features of poliovirus in HeLa cells. Virology 13:439-447. [DOI] [PubMed] [Google Scholar]
- 36.Karnauchow, T. M., D. L. Tolson, B. A. Harrison, E. Altman, D. M. Lublin, and K. Dimock. 1996. The HeLa cell receptor for enterovirus 70 is decay-accelerating factor (CD55). J. Virol. 70:5143-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karnauchow, T. M., S. Dawe, D. M. Lublin, and K. Dimock. 1998. Short consensus repeat domain 1 of decay-accelerating factor is required for enterovirus 70 binding. J. Virol. 72:9380-9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitchens, R. L., P. Wang, and R. S. Munford. 1998. Bacterial lipopolysaccharide can enter monocytes via two CD14-dependent pathways. J. Immunol. 161:5534-5545. [PubMed] [Google Scholar]
- 39.Kolatkar, P. R., J. Bella, N. H. Olson, C. M. Bator, T. S. Baker, and M. G. Rossmann. 1999. Structural studies of two rhinovirus serotypes complexed with fragments of their cellular receptor. EMBO J. 18:6249-6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kronenberger, P., D. Schober, E. Prchla, O. Ofori-Anyinam, R. Vrijsen, B. Rombaut, D. Blaas, R. Fuchs, and A. Boeye. 1998. Uptake of poliovirus into the endosomal system of HeLa cells. Arch. Virol. 143:1417-1424. [DOI] [PubMed] [Google Scholar]
- 41.Kurzchalia, T. V., and R. G. Parton. 1999. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 11:424-431. [DOI] [PubMed] [Google Scholar]
- 42.Lea, S. M., R. M. Powell, T. McKee, D. J. Evans, D. Brown, D. I. Stuart, and P. A. van der Merwe. 1998. Determination of the affinity and kinetic constants for the interaction between the human virus echovirus 11 and its cellular receptor, CD55. J. Biol. Chem. 273:30443-30447. [DOI] [PubMed] [Google Scholar]
- 43.Lipardi, C., R. Mora, V. Colomer, S. Paladino, L. Nitsch, E. Rodriguez-Boulan, and C. Zurzolo. 1998. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J. Cell Biol. 140:617-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35.. [DOI] [PubMed] [Google Scholar]
- 45.Madshus, I. H., S. Olsnes, and K. Sandvig. 1984. Mechanism of entry into the cytosol of poliovirus type 1: requirement for low pH. J. Cell Biol. 98:1194-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36:107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marjomaki, V., V. Pietiainen, H. Matilainen, P. Upla, J. Ivaska, L. Nissinen, H. Reunanen, P. Huttunen, T. Hyypia, and J. Heino. 2002. Internalization of echovirus 1 in caveolae. J. Virol. 76:1856-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Belmonte, F., J. A. Lopez-Guerrero, L. Carrasco, and M. A. Alonso. 2000. The amino-terminal nine amino acid sequence of poliovirus capsid VP4 protein is sufficient to confer N-myristoylation and targeting to detergent-insoluble membranes. Biochemistry 39:1083-1090. [DOI] [PubMed] [Google Scholar]
- 50.Martino, T. A., M. Petric, M. Brown, K. Aitken, C. J. Gauntt, C. D. Richardson, L. H. Chow, and P. P. Liu. 1998. Cardiovirulent coxsackieviruses and the decay-accelerating factor (CD55) receptor. Virology 244:302-314. [DOI] [PubMed] [Google Scholar]
- 51.Matlin, K. S., H. Reggio, A. Helenius, and K. Simons. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maynell, L. A., K. Kirkegaard, and M. W. Klymkowsky. 1992. Inhibition of poliovirus RNA synthesis by brefeldin A. J. Virol. 66:1985-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muniz, M., and H. Riezman. 2000. Intracellular transport of GPI-anchored proteins. EMBO J. 19:10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ohno, H., M. Fournier, G. Poy, and J. S. Bonifacino. 1996. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 271:29009-29015. [DOI] [PubMed] [Google Scholar]
- 56.Orlandi, P. A., and P. H. Fishman. 1998. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 141:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parker, J. S., and C. R. Parrish. 2000. Cellular uptake and infection by canine parvovirus involves rapid dynamin-regulated clathrin-mediated endocytosis, followed by slower intracellular trafficking. J. Virol. 74:1919-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parton, R. G., B. Joggerst, and K. Simons. 1994. Regulated internalization of caveolae. J. Cell Biol. 127:1199-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parton, R. G. 1996. Caveolae and caveolins. Curr. Opin. Cell Biol. 8:542-548. [DOI] [PubMed] [Google Scholar]
- 60.Pasch, A., J. H. Kupper, A. Wolde, R. Kandolf, and H. C. Selinka. 1999. Comparative analysis of virus-host cell interactions of haemagglutinating and non-haemagglutinating strains of coxsackievirus B3. J. Gen. Virol. 80:3153-3158. [DOI] [PubMed] [Google Scholar]
- 61.Patel, J. R., J. Daniel, and V. I. Mathan. 1985. An epidemic of acute diarrhoea in rural southern India associated with echovirus type 11 infection. J. Hyg. 95:483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez, L., and L. Carrasco. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petrie, R. J., P. P. Schnetkamp, K. D. Patel, M. Awasthi-Kalia, and J. P. Deans. 2000. Transient translocation of the B cell receptor and Src homology 2 domain-containing inositol phosphatase to lipid rafts: evidence toward a role in calcium regulation. J. Immunol. 165:1220-1227. [DOI] [PubMed] [Google Scholar]
- 64.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74:2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pickl, W. F., F. X. Pimentel-Muinos, and B. Seed. 2001. Lipid rafts and pseudotyping. J. Virol. 75:7175-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powell, R. M., T. Ward, D. J. Evans, and J. W. Almond. 1997. Interaction between echovirus 7 and its receptor, decay-accelerating factor (CD55): evidence for a secondary cellular factor in A-particle formation. J. Virol. 71:9306-9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powell, R. M., V. Schmitt, T. Ward, I. Goodfellow, D. J. Evans, and J. W. Almond. 1998. Characterization of echoviruses that bind decay accelerating factor (CD55): evidence that some haemagglutinating strains use more than one cellular receptor. J. Gen. Virol. 79:1707-1713. [DOI] [PubMed] [Google Scholar]
- 68.Powell, R. M., T. Ward, I. Goodfellow, J. W. Almond, and D. J. Evans. 1999. Mapping the binding domains on decay accelerating factor (DAF) for haemagglutinating enteroviruses: implications for the evolution of a DAF-binding phenotype. J. Gen. Virol. 80:3145-3152. [DOI] [PubMed] [Google Scholar]
- 69.Rodal, S. K., G. Skretting, O. Garred, F. Vilhardt, B. van Deurs, and K. Sandvig. 1999. Extraction of cholesterol with methyl-β-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10:961-974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossmann, M. G. 1989. The canyon hypothesis. Viral Immunol. 2:143-161. [DOI] [PubMed] [Google Scholar]
- 71.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 72.Schroeder, R. J., S. N. Ahmed, Y. Zhu, E. London, and D. A. Brown. 1998. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Biol. Chem. 273:1150-1157. [DOI] [PubMed] [Google Scholar]
- 73.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shafren, D. R., D. J. Dorahy, R. A. Ingham, G. F. Burns, and R. D. Barry. 1997. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 71:4736-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shafren, D. R., D. T. Williams, and R. D. Barry. 1997. A decay-accelerating factor-binding strain of coxsackievirus B3 requires the coxsackievirus-adenovirus receptor protein to mediate lytic infection of rhabdomyosarcoma cells. J. Virol. 71:9844-9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shafren, D. R. 1998. Viral cell entry induced by cross-linked decay-accelerating factor. J. Virol. 72:9407-9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shafren, D. R., D. J. Dorahy, R. F. Thorne, T. Kinoshita, R. D. Barry, and G. F. Burns. 1998. Antibody binding to individual short consensus repeats of decay-accelerating factor enhances enterovirus cell attachment and infectivity. J. Immunol. 160:2318-2323. [PubMed] [Google Scholar]
- 78.Shafren, D. R., D. J. Dorahy, R. F. Thorne, and R. D. Barry. 2000. Cytoplasmic interactions between decay-accelerating factor and intercellular adhesion molecule-1 are not required for coxsackievirus A21 cell infection. J. Gen. Virol. 81:889-894. [DOI] [PubMed] [Google Scholar]
- 79.Shin, J. S., Z. Gao, and S. N. Abraham. 2000. Involvement of cellular caveolae in bacterial entry into mast cells. Science 289:785-788. [DOI] [PubMed] [Google Scholar]
- 80.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 81.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Subtil, A., I. Gaidarov, K. Kobylarz, M. A. Lampson, J. H.Keen, and T. E. McGraw. 1999. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc. Natl. Acad. Sci. USA 96:6775-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vihinen-Ranta, M., A. Kalela, P. Makinen, L. Kakkola, V. Marjomaki, and M. Vuento. 1998. Intracellular route of canine parvovirus entry. J. Virol. 72:802-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang, K., S. Huang, A. Kapoor-Munshi, and G. Nemerow. 1998. Adenovirus internalization and infection require dynamin. J. Virol. 72:3455-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang, L. H., K. G. Rothberg, and R. G. Anderson. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ward, T., P. A. Pipkin, N. A. Clarkson, D. M. Stone, P. D. Minor, and J. W. Almond. 1994. Decay-accelerating factor CD55 is identified as the receptor for echovirus 7 using CELICS, a rapid immuno-focal cloning method. EMBO J. 13:5070-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward, T., R. M. Powell, P. A. Pipkin, D. J. Evans, P. D. Minor, and J. W. Almond. 1998. Role for beta2-microglobulin in echovirus infection of rhabdomyosarcoma cells. J. Virol. 72:5360-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wien, M. W., M. Chow, and J. M. Hogle. 1996. Poliovirus: new insights from an old paradigm. Structure 4:763-767. [DOI] [PubMed] [Google Scholar]
- 89.Wooldridge, K. G., P. H. Williams, and J. M. Ketley. 1996. Host signal transduction and endocytosis of Campylobacter jejuni. Microb. Pathog. 21:299-305. [DOI] [PubMed] [Google Scholar]
- 90.Xing, L., K. Tjarnlund, B. Lindqvist, G. G. Kaplan, D. Feigelstock, R. H. Cheng, and J. M. Casasnovas. 2000. Distinct cellular receptor interactions in poliovirus and rhinoviruses. EMBO J. 19:1207-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zeichhardt, H., K. Wetz, P. Willingmann, and K. O. Habermehl. 1985. Entry of poliovirus type 1 and mouse Elberfeld (ME) virus into HEp-2 cells: receptor-mediated endocytosis and endosomal or lysosomal uncoating. J. Gen. Virol. 66:483-492. [DOI] [PubMed] [Google Scholar]