Abstract
Cytomegalovirus (CMV) retinitis is an important ocular complication in human immunodeficiency virus-infected individuals and the leading cause of blindness in those not undergoing highly active antiretroviral therapy. Murine CMV (MCMV) infection of mice has been shown to be a useful small-animal model for the study of CMV pathogenesis in the eye. The purpose of this study was to evaluate CMV persistence in ocular tissue and to determine the potential for reactivation. Following subretinal inoculation of immunocompetent BALB/c mice, tissues were tested for infectious virus by plaque assay and for the presence of viral DNA and RNA by PCR. The latent phase of the infection in mouse tissues was analyzed by plaque assay, PCR, and explantation cocultivation in both immunocompetent and cyclophosphamide-treated mice. The acute phase of the infection was resolved by 2 to 3 weeks postinfection, while viral DNA persisted beyond 12 months. Immediate-early 1 transcripts were detected in 100% of the ocular samples tested, and glycoprotein H transcripts were detected in 86% of the samples, but no difference in viral DNA or RNA levels between immunocompetent and immunosuppressed animals was measured. Irrespective of immune status, no in vivo reactivation was detected; however, reactivated virus was observed in 76 to 82% of the eyes following explantation onto a permissive cell layer. The transcriptional activity and relatively high frequency of explantation-induced reactivation in both immunocompetent and immunosuppressed mice suggest that control of MCMV latency in ocular tissue might involve other regulatory events that are not entirely dependent on intact specific immunity.
Cytomegalovirus (CMV) is a β-herpesvirus that is fairly ubiquitous in the human population and generally causes mild or subclinical disease in healthy individuals (47). Although the acute replication of CMV is eventually cleared by the host immune system, the virus persists by establishing a lifelong latent infection, defined as the presence of viral DNA without the detection of infectious virus. Human CMV (HCMV) has been shown to establish latency in cells of the monocyte/macrophage lineage including hematopoietic progenitor cells (16, 42, 56), and there is evidence of persistent HCMV infection in aortic endothelial cells (14, 15). The ability of CMV to reactivate from a latent state that is subsequently accompanied by asymptomatic viral shedding can periodically occur in healthy, seropositive individuals; however, the specific cell types from which recurrent virus comes are unknown. A significant amount of CMV morbidity can be attributed to reactivation events that are normally controlled by the immune system, based on the observation that immunosuppressed individuals often suffer from HCMV disease (54).
CMV has emerged as an important opportunistic pathogen in immunosuppressed persons, and reactivation of latent virus, which can cause significant morbidity in multiple organs including the eye, is frequently observed (20, 48). CMV retinitis is a focal, progressive, necrotic infection of the retina and is a sight-threatening result of ocular infection. It is the most common infectious ophthalmic complication in AIDS patients (17, 23, 25), affecting up to 40% of individuals not receiving highly active antiretroviral therapy (HAART), and is the leading cause of blindness in this population (24, 39).
The clinical features of CMV infections have been well characterized, but the pathogenesis of ocular infections, including the potential for establishment of a latent infection and subsequent reactivation, is not well understood. Murine models of CMV have been used successfully to study parameters of latency in visceral organs such as salivary gland, lung, and spleen (9, 32). Murine CMV (MCMV) is similar to HCMV with respect to pathogenesis and the ability to establish and reactivate from latent infections. It establishes latent infections in myeloid lineage cells of the blood and bone marrow (45), alveolar macrophages, endothelial cells of the kidney, liver, heart, and spleen (33), and the lung (5).
A murine model of ocular infection that uses subretinal inoculation of MCMV by a supraciliary injection has provided a system that histopathologically is similar to HCMV retinitis (2, 21, 31). It has previously been demonstrated that the use of immunosuppression increases both the incidence and the severity of CMV retinitis (12, 31); however, the molecular nature of CMV and immunological control following an acute infection remains largely unknown. Unlike other herpesviruses with characterized latent-associated transcriptional activity (28), viral gene expression in latent CMV infection is restricted. In CMV latency studies with the mouse, there is evidence at the molecular level of immediate-early 1 (ie-1) transcription activity in some instances (36, 65), and several studies have investigated the expression of viral RNA associated with productive infection in tissues of latently infected mice. Detectable levels of MCMV ie-1 transcripts in organs have been reported (6, 18, 37, 64, 65), but detection of ie-1 transcripts in tissues of latently infected mice is not universal (33). It is not clear whether this discrepancy is due to differences in sensitivity of detection, to methods used for infection, or to spontaneous reactivation of the virus.
Animal models of CMV have shown that reactivation can be induced by immunosuppressive therapies alone, such as total body irradiation, administration of cytotoxic drugs like cyclophosphamide (CY), or by immunodepletion of T cells or T-cell subsets (6, 7, 29, 40, 41, 49). Polic et al. (49) concluded that unlike other herpesviruses CMV latency in the lung is maintained primarily as a result of immune surveillance rather than by transcriptional control in latently infected cells. However, with less cytotoxic immunosuppressive regimens, such as the use of cyclosporine, the frequency of reactivation is much lower than what is observed with other therapies, both in animals and in clinical settings (7, 19, 52).
Since the establishment of combination therapy (HAART), AIDS patients responding to this treatment have shown an overall decrease in the incidence of opportunistic infection, including CMV retinitis (39). HAART responders have a suppression of human immunodeficiency virus (HIV) load, improvement in immune function, and a decline in the incidence of CMV retinitis. These results have led to discontinuing CMV maintenance therapy for some patients (26, 59, 60, 61), but the long-term benefit of HAART-induced immune reconstitution is not known and there are emerging reports of recurrent CMV retinitis in these patients (8, 27, 34).
Taken together, it is clear that control of CMV latency is likely organ dependent with respect to both gene expression and immune suppression. In this report, we have investigated MCMV latency in ocular tissue and evaluated both the ability of the virus to establish a latent infection and the ability of MCMV to reactivate to the level of infectious virus. We used a subretinal inoculation to infect immunocompetent BALB/c mice with MCMV, and both the acute and latent phases of the infection were analyzed by plaque assay, PCR, and explant cocultivation. There was no measurable viral reactivation in any organ tested in vivo, regardless of treatment with CY. However, we did observe an explantation-induced reactivation in a high percentage (>70%) of explanted eyes from both immunocompetent and immunosuppressed animals. Additionally, in the absence of detectable infectious virus, reverse transcriptase (RT)-PCR analysis showed ie-1 transcription activity in 100% of the samples tested and glycoprotein H (gH) transcription activity in 86% of the ocular samples. This pattern of persistence was independent of whether the mice were perfused prior to eye enucleation, and it continued beyond 12 months postinfection (p.i.). The relatively high level of reactivation from latently infected eyes, as well as the unique viral RNA expression pattern, suggests that latency in ocular tissue might be alternatively regulated compared to other organs.
MATERIALS AND METHODS
Virus and tissue culture.
The Smith strain of MCMV was prepared from murine salivary gland homogenates as described previously (29, 45). Mouse embryo fibroblast (MEF) cells used for viral titration and production of control DNA were prepared from 18- to 20-day gestation BALB/c mouse embryos (Harlan Sprague-Dawley, Indianapolis, Ind.) and maintained as previously described (58).
Infection of mice.
Adult (6- to 8-week-old) female BALB/c (H-2d) mice (Harlan Sprague-Dawley) were used for all experiments. For MCMV ocular inoculations, the right eye of each mouse was injected with 104 PFU per eye via the supraciliary route as described elsewhere (2, 31). A fresh aliquot of virus was thawed and diluted to the appropriate concentration in minimal essential medium (MEM) (Invitrogen, Gaithersburg, Md.) immediately before the inoculation for each experiment. The titers of the inocula were confirmed by plaque assay. Mock-infected mice received diluent only.
Reactivation and explant cocultivation.
Immunocompetent mice ocularly infected with MCMV or mock infected were rested for >3 months. In an effort to induce MCMV reactivation in vivo, mice were immunosuppressed with CY (Sigma, St. Louis, Mo.) or mock treated 0, 2, 5, 10, 16, and 23 days following the resting period (31, 46). One week after immunosuppressive treatment, the eyes (ipsilateral and contralateral), submandibular salivary glands, spleens, and lungs were removed, snap-frozen in liquid nitrogen, and stored at −70°C. Organs from each group were homogenized in MEM with 10% fetal calf serum (MEM-10) and analyzed for infectious virus by plaque assay. Organs from additional animals were homogenized in TRIzol (Invitrogen) for nucleic acid extraction and analyzed by PCR (45, 65). Some animals (as indicated in the text) were perfused with 30 ml of phosphate-buffered saline (PBS) prior to removal of ocular tissues for PCR analysis (3). For in vitro induction of MCMV reactivation, organs were harvested 1 week following CY treatment, minced in MEM-10, and explanted onto subconfluent MEF cell monolayers. The organs were cocultivated at 37°C and 5% CO2 and monitored daily for 60 days or until viral cytopathic effect (CPE) was observed.
Plaque assay.
Tissues from infected or control animals were harvested, snap-frozen in liquid nitrogen, and stored at −70°C. The eyes were thawed on ice and homogenized in 1 ml of MEM-10. For the other tissues, a 10% homogenate was made in 1 ml of MEM-10. Cell debris was removed by centrifugation at 3,000 × g. The titers of clarified homogenates were determined on monolayers of MEF cells in a 24-well plate as described previously (31, 45).
Reverse transcription and PCR analysis.
Total RNA and DNA were extracted separately from tissues by using TRIzol reagent (Gibco) and purified as described elsewhere (45, 65). RNA samples were treated with DNase (Invitrogen) and reverse transcribed by using pd(N)6 random primers in the presence or absence of Moloney murine leukemia virus reverse transcriptase (Invitrogen) as described previously (65). Ten percent of the reaction mixtures was used for PCR.
The primers and conditions for the PCR amplification of the MCMV ie-1 gene, the mouse adenine phosphoribosyltransferase (APRT) gene, and their transcripts were previously described (45). For amplification of gH DNA and RNA, the primers (sense, 5′CACCGATGTGGTGTTCCTGC; antisense, 5′CGCGTGCATCCCTGACGAGT) were designed from the published sequence (GenBank accession no. D10089) and amplified 406-bp products. Direct incorporation of [α-32P]dATP into the PCR products provided enhanced sensitivity of the assays (45, 65). All PCR conditions were optimized and tested to amplify their respective targets within the linear ranges of the reaction allowing for semiquantitation of the DNA or RNA present. Twenty percent of the completed PCR mixes were electrophoresed on nondenaturing 5% polyacrylamide gels, which were dried and exposed to X-ray film with intensifying screens.
Autoradiographs of PCRs were scanned with a Molecular Dynamics PDSI densitometer, analyzed with ImageQuaNT V4.2 software (Molecular Dynamics, Sunnyvale, Calif.), and compared to ie-1 standard curves of amplified plasmid DNA (31, 45, 65) containing the MCMV EcoRI E fragment (pRE1) (43) or reverse-transcribed RNA produced by in vitro transcription from a Bluescript plasmid (45, 65) containing the MCMV HindIII L fragment (43). Control DNA used for the amplification of gH was a pUC19 plasmid containing the MCMV HindIII C fragment (pHC1). Statistical analysis was performed with Sigma Stat v.2.1.
Confirmation of PCR products was done by Southern blot analysis with target-specific probes. Representative DNA and RNA samples from each group of animals were amplified as described above, except that no radionucleotide was included. Twenty percent of each product was run on a 5% polyacrylamide gel, electroblotted onto a nylon membrane, and hybridized under standard Southern blotting conditions (3). The membranes were probed with [γ-32P]-labeled probes for ie-1 (5′CCAACAAGATCCTCGAGTCTGG) or gH (5′CTGAGCGTTCTGGCGTCGGTA) specific for sequences internal to the predicted PCR products (30). Hybridization results were visualized by autoradiography.
RESULTS
MCMV replication kinetics and DNA persistence following subretinal inoculation.
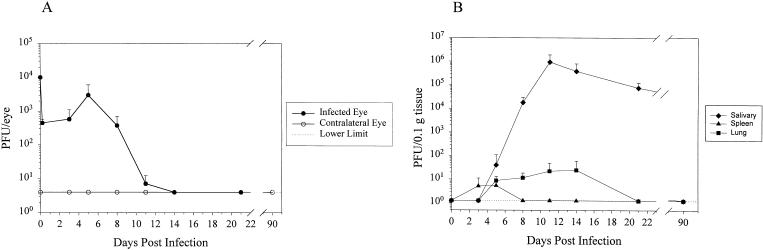
The acute replication kinetics of MCMV is dependent on both the route of inoculation and the particular organ in question. To determine the infection kinetics following an ocular inoculation, immunocompetent mice were infected with MCMV by subretinal inoculation. The infected eyes, contralateral eyes, submandibular salivary glands, spleens, and lungs were removed, and infectious MCMV titers were determined by plaque assay over a 3-week time period. In the inoculated eye, the virus reached a peak titer of 3 × 103 PFU on day 5 p.i. and decreased to below the level of detection between 2 and 3 weeks p.i. (Fig. 1A). The contralateral eye, however, had no detectable infectious virus over the 3-week time period, except in the case of one animal with one plaque detected on day 21 p.i. (Fig. 1A). Consistent with MCMV replication following other routes of inoculation, the salivary gland showed the greatest amount of viral replication, which peaked at around day 11 p.i., with titers remaining above 105 PFU/mg of tissue even at 3 weeks p.i. (Fig. 1B). By 3 months p.i., however, there was no detectable infectious virus in the salivary gland. Except for slight delays, typical replication kinetics were observed in both the spleen and the lung, and titers fell to near or below detectable levels by 3 weeks p.i. In addition to the delays, the peak titers were also lower than what is observed when MCMV is given by direct intravenous inoculation (65) (Fig. 1B).
FIG. 1.
Infectious MCMV titers in eyes and peripheral tissues of immunocompetent mice. Mice were infected by subretinal injection unilaterally with MCMV, and the virus titers in the eyes, salivary glands, spleens, and lungs were determined by kinetic plaque assays for 3 months. Each time point represents the mean (± standard deviation) PFU per eye of three animals. (A) MCMV titers in the infected (closed circles) and contralateral (open circles) eyes. (B) MCMV titers in salivary glands (diamonds), spleens (triangles), and lungs (squares).
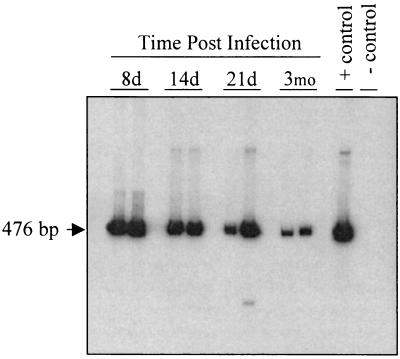
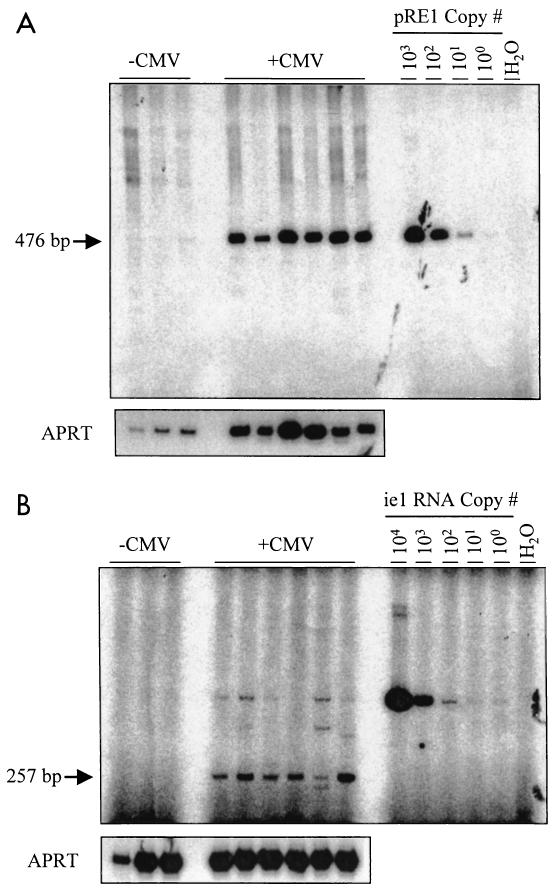
The persistence of MCMV DNA following the clearance of the acute infection in organs such as spleen, salivary gland, and lung is well described (9, 32, 65), but little is currently known about the possibility of ocular persistence. To determine if MCMV establishes a persistent infection following subretinal inoculation, the inoculated eyes were tested for the presence of viral DNA. MCMV DNA from individual eyes was PCR amplified with primers specific for the ie-1 region at 8, 14, and 21 days and 3 months p.i. (Fig. 2). Consistent with the establishment of a latent infection, MCMV DNA was present in the ocular tissue beyond the point of detectable infectious virus, even at 3 months p.i. To determine if the MCMV DNA detected in the eyes was due to latently infected blood cells within the vasculature of the ocular tissue, seven mice were subretinally inoculated. Three months p.i. the infected eyes were enucleated from three of the mice, while the remaining four animals were perfused with PBS prior to enucleation. Both APRT and ie-1 PCR analyses showed no significant differences in the amount of total DNA (P = 0.114) or MCMV DNA (P = 0.558) recovered between the perfused and nonperfused eyes.
FIG. 2.
Persisting MCMV DNA in ocular tissue of immunocompetent mice. Mice were infected by subretinal injection unilaterally with MCMV, and the eyes were analyzed kinetically for the presence of viral DNA. The DNA was extracted from infected eyes and amplified by PCR using primers specific for the MCMV ie-1 gene. The predicted PCR product for MCMV ie-1 with these primers is 476 bp. The plasmid pRE1, which contains the relevant region of the MCMV genome, was used as a positive control, and water was the negative control for the PCR. The panel shows a Southern blot analysis of two representative samples of each time point (n = 4/time point tested) using an ie-1-specific probe for the PCR product amplified from DNA at 8, 14, and 21 days and 3 months p.i.
In addition, the contralateral eye, salivary gland, spleen, and lung tissues were tested for the presence of viral DNA. Viral DNA was detected 3 months p.i. in 100% of these organs harvested from infected animals, with levels that correlated with the differences in replication kinetics following the ocular inoculation (data not shown). No viral DNA was detected in any tissue collected from mock-infected animals.
In vivo reactivation of MCMV in ocular tissue.
In an effort to determine if the persisting viral DNA following subretinal inoculation was biologically relevant, we used immunosuppression with CY in an attempt to induce reactivation of MCMV since immunosuppression is known to induce reactivation of herpesviruses (6, 7, 29, 40, 41, 49). Mice were infected subretinally with MCMV and rested for at least 3 months. To confirm the resolution of the lytic infection and the persistence of the viral DNA, one group of mice was tested for the presence of infectious virus by plaque assay and for viral DNA by PCR. As expected, no infectious virus was detected in any of the eyes, salivary glands, spleens, or lungs of the mice prior to CY treatment, but MCMV DNA was readily detected from all of the tissues tested (data not shown).
Following confirmation of the latent infection, the mice were mock treated or given a 4-week regimen of CY that induces reactivation of latent MCMV in a variety of organs following parenteral inoculation (B. M. Mitchell and J. G. Stevens, unpublished data). The dose of CY used induces a severe generalized immunosuppression in mice as measured by peripheral white blood cell counts and biological effect on acute viral replication in vivo (31, 46). The animals were tested for in vivo reactivation by plaque titer of the ocular and nonocular tissues, and the results are summarized in Table 1. In this set of experiments the CY-treated animals provided a measure of immunosuppressed-induced reactivation in vivo, while the mock-treated animals provided a measure for spontaneous in vivo reactivation. Regardless of whether or not the animals were immunosuppressed, infectious virus was not detected in the eyes or in the peripheral tissues, indicating that no measurable in vivo reactivation had occurred.
TABLE 1.
In vivo reactivation of MCMV following immunosuppressiona
| Organ | No. of organs positive for CMV/total no. tested (% positive)
|
|
|---|---|---|
| Mock-treated mice | CY-treated mice | |
| Infected eye | 0/10 (0) | 0/11 (0) |
| Contralateral eye | 0/3 (0) | 0/5 (0) |
| Salivary gland | 0/10 (0) | 0/11 (0) |
| Spleen | 0/7 (0) | 0/8 (0) |
| Lung | 0/3 (0) | 0/5 (0) |
Latently infected mice were given a 4-week regimen of cyclophosphamide or were mock treated. Animals from each of these groups were tested for infectious virus by plaque assay.
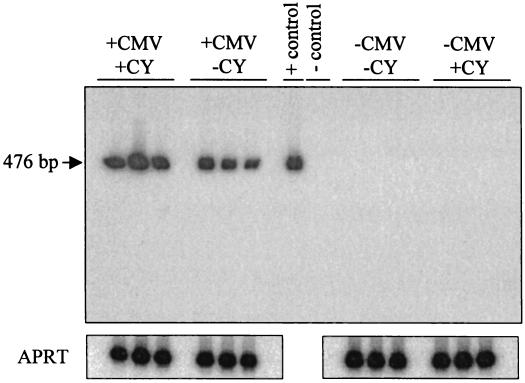
As an alternative approach to detecting any in vivo reactivation, the levels of viral DNA in ocular and nonocular tissues after immunosuppression were measured. MCMV DNA from individual eyes and peripheral tissues was PCR amplified with primers specific for the ie-1 region after CY treatment. Representative results are shown in Fig. 3. As expected, viral DNA was detected in the infected eyes in both groups, while no MCMV DNA was detected in the mock-infected eyes. Consistent with the results prior to CY-treatment, MCMV DNA was detected in 100% of the contralateral eyes, salivary glands, spleens, and lung tissues of infected mice following either CY treatment or mock immunosuppression (data not shown).
FIG. 3.
Latent MCMV DNA following immunosuppressive treatment. Latently infected (n = 10) or mock-infected (n = 3) mice were given a 4-week regimen of CY or mock treated in an effort to induce reactivation. The panel shows a Southern blot analysis of PCR-amplified ie-1 DNA from mouse eyes after CY treatment. Shown are three representative samples from each group of animals. Viral DNA was detected in all the eyes from mice infected with MCMV (+CMV), while mock-infected animals (−CMV) were negative. The plasmid pRE1, which contains the relevant region of the MCMV genome, was used as a positive control, and water was used as a negative control. PCR amplification of the mouse APRT gene was used as internal sample controls.
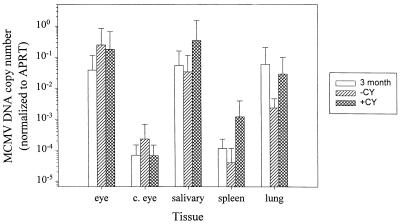
The levels of viral DNA in the various tissues before and after CY and mock treatments were quantitated by comparison to a standard curve of a known copy number, normalized to the APRT levels, and the relative genome equivalents were plotted for each group (Fig. 4). Three months p.i., the mean (± standard deviation) MCMV genome equivalents value in the ocular samples tested was 0.03 ± 0.08. Following CY treatment, the mean genome equivalents value in the CY-treated group was 0.18 ± 0.48, and in the mock-treated group it was 0.25 ± 0.58. There was no significant difference in the levels of DNA from the CY- or mock-treated animals in the eye (P = 0.36). The genome equivalents in the other organs tested ranged from a high in the salivary gland of 0.1 to 1 to a low of 10−3 to 10−4 in the contralateral eye. These data were consistent with the load of infectious virus detected during the course of the acute infection following the subretinal inoculation. Similarly, there was no significant difference in the levels of viral DNA among the three groups in the contralateral eye (P = 0.86), the salivary gland (P = 0.98), spleen (P = 0.23), or lung (P = 0.12).
FIG. 4.
Quantitative analysis of MCMV DNA in ocular and nonocular tissues. Latently infected mice were tested at 3 months p.i. (white bars) and following a 4-week regimen of CY (diagonally hatched bars) or mock treatment (cross-hatched bars). Semiquantitative PCR of MCMV DNA was performed and quantitated against a standard curve generated from pRE1 of known copy number. The amounts of ie-1 DNA were normalized against APRT levels for each sample and are expressed as MCMV genome equivalents. Shown is the mean genome equivalents ± standard deviation (n = 8 to 10/group) for infected eyes (eye), contralateral eyes (c. eye), salivary glands (salivary), spleens (spleen), and lung tissue (lung).
In vitro reactivation of MCMV from ocular tissue.
Since neither spontaneous nor CY-induced reactivation was observed in vivo, we used explantation of latently infected tissues with permissive cells, which is another established method to induce viral reactivation (44, 57). Organs from the remaining animals that had been mock- or CY-treated were explanted onto MEF cells and monitored up to 60 days for the development of virus-induced CPE.
Following explantation, reactivated virus from the infected eyes of immunosuppressed and immunocompetent animals was observed at the relatively high percentages of 76 and 82%, respectively (Table 2), while no specific viral CPE from uninfected control tissues was observed. Reactivation of other MCMV-positive organs was also observed at various percentages after cocultivation, but at frequencies lower than those of the infected eyes. The organ with the largest difference in reactivation frequency between the immunosuppressed and immunocompetent groups was the contralateral eye; however, this difference was not significant (P = 0.07) and might be explained by the fact that the contralateral eye had MCMV genome equivalents approximately 2 to 3 logs lower than the infected eyes, salivary gland, or lung (Fig. 4).
TABLE 2.
Explanation-induced reactivation of MCMV following immunosuppressiona
| Organ | No. of organs positive for CMV/total no. tested (% positive)
|
|
|---|---|---|
| Mock-treated mice | CY-treated mice | |
| Infected eye | 18/22 (82) | 16/21 (76) |
| Contralateral eye | 0/10 (0) | 4/9 (44) |
| Salivary gland | 7/11 (64) | 7/10 (70) |
| Spleen | 6/11 (55) | 4/10 (40) |
| Lung | 0/11 (0) | 1/10 (10) |
Latently infected mice were given a 4-week regimen of cyclophosphamide or were mock treated. Organs from animals from each of these groups were explanted onto MEF monolayers and cocultivated for 60 days or until viral CPE was observed.
The mean times of cocultivation until the observation of viral CPE were 11 days (range, 6 to 22 days) for mock-treated animals and 12 days (range, 3 to 54 days) for CY-treated animals. There was no significant difference in the mean times between the groups (P = 0.35). The observed kinetics of viral CPE following cocultivation was consistent with true reactivation events.
MCMV ie-1 RNA in latently infected ocular tissue.
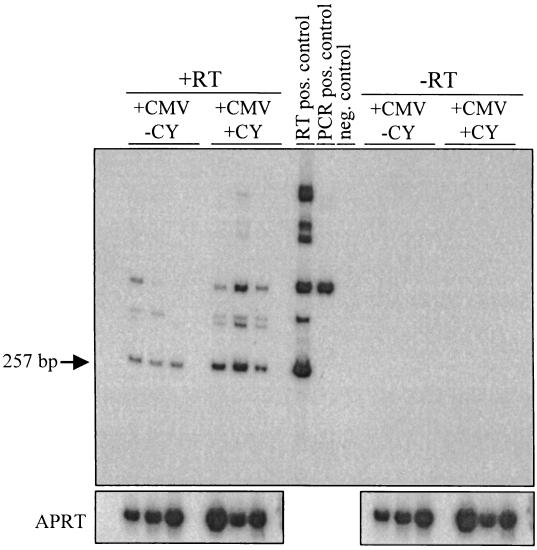
While the standard profile of MCMV latency is without detectable transcription, there are reports of rare, low-level transcriptional activity in various organs without the detection of infectious virus (11, 18, 37). To analyze the level of immediate-early transcription from the ocular samples, total RNA extracted from latently infected eyes, salivary gland, spleen, and lung tissue was RT-PCR amplified with ie-1 primers. MCMV ie-1 RNA was detected in 100% of the infected eyes tested in both the mock- and CY-treated groups (n = 10/group), while no RNA was detected in samples that did not receive reverse transcriptase (Fig. 5) or in uninfected controls (data not shown). As with the DNA analysis, RNAs extracted 3 months p.i. from the eyes of infected mice perfused with PBS prior to enucleation (n = 4) or nonperfused (n = 3) were tested in order to determine if the MCMV RNA detected in the eyes was due to reactivating virus from latently infected blood cells within the ocular tissue. Both APRT and ie-1 RT-PCR analysis showed no significant differences in the amount of total RNA (P = 0.937) or MCMV RNA (P = 0.719) between eyes from the perfused and nonperfused animals. Importantly, no ie-1 RNA was detected in the matched samples from the contralateral eye, salivary gland, spleen, or lung tissue by RT-PCR, even though the lower limit of sensitivity based upon amplification of in vitro-transcribed RNA was approximately 10 copies (data not shown).
FIG. 5.
MCMV ie-1 RNA following immunosuppressive treatment. Latently infected mice (+CMV) or mock-infected mice (−CMV) were given a 4-week regimen of CY (+CY) or mock treated (−CY). Total RNA was extracted from the eyes, DNase treated, and reverse transcribed in the presence (+RT) or absence (−RT) of reverse transcriptase. The panel shows a Southern blot analysis of PCR-amplified ie-1 cDNA. Shown are three representative samples from each group of animals (n = 10/group). CMV-infected MEF cDNA was used at the RT-positive control, which amplifies a spliced template to produce a 257-bp PCR product. The plasmid pRE1 was used as a positive control, and water was used as a negative control for the PCR.
MCMV gH RNA in latently infected ocular tissue.
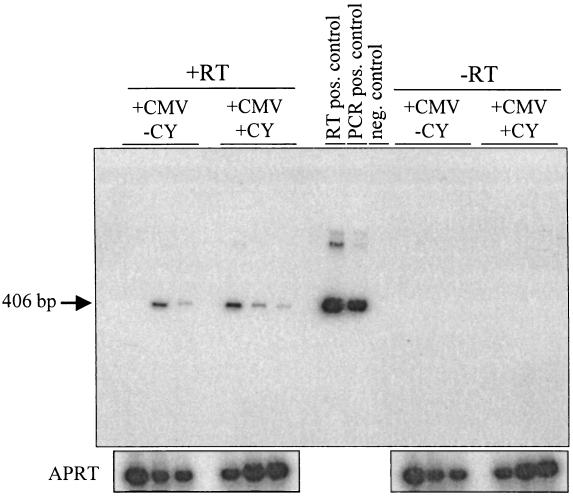
Because of the relatively high levels of ie-1 transcription observed in the infected eyes, we tested the ocular samples for gH RNA as a marker for late gene transcription (63). The gH RNA levels and prevalence were analyzed by RT-PCR amplification of the matched ie-1 cDNA samples with a level of sensitivity similar to that of ie-1 RT-PCR (data not shown). gH RNA was detected in 9 of 10 (90%) mock-treated animals and 8 of 10 (80%) CY-treated animals (Fig. 6). Like ie-1 RNA, gH RNA was not detected in salivary gland, spleen, or lung tissue of any CMV-infected or mock-infected animals (data not shown).
FIG. 6.
MCMV gH RNA following immunosuppressive treatment. Latently infected mice (+CMV) or mock-infected mice (−CMV) were given a 4-week regimen of CY (+CY) or mock-treated (−CY). Total RNA was extracted from the eyes, DNase treated, and reverse transcribed in the presence (+RT) or absence (−RT) of reverse transcriptase. The panel shows a Southern blot analysis of PCR-amplified gH cDNA. Shown are three representative samples from each group of animals (n = 10/group). CMV-infected MEF cDNA was used at the RT-positive control, which generates a 406-bp product. The plasmid pHC1 was used as a positive control, and water was used as a negative control for the PCR.
Quantitation of viral transcripts.
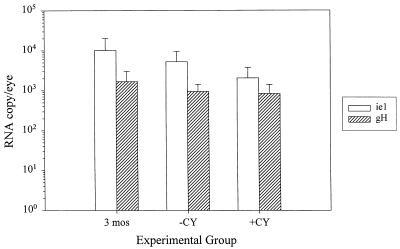
The ie-1 and gH RNA transcripts were quantitated and the RNA copy numbers were calculated for each experimental group prior to and following CY or mock treatments (Fig. 7). The amount of ie-1 RNA was well above the lower limit of sensitivity of the PCR and ranged from approximately 9 × 102 to 3 × 104 copies/eye. Similar to the DNA levels, the amounts of viral RNA were not significantly different among the three groups tested (P = 0.25). The levels of gH RNA were approximately 5- to 10-fold lower than the ie-1 levels and ranged from below the limit of sensitivity of the RT-PCR to 3.7 × 103 copies (Fig. 7), with no significant difference in the amount of gH RNA among the three groups tested (P = 0.49).
FIG. 7.
Quantitative analysis of MCMV RNA in ocular tissues. Latently infected mice were tested at 3 months p.i. (3 mos) or following a 4-week regimen of CY (+CY) or mock treatment (−CY). Semiquantitative PCR of MCMV cDNA for ie-1 and gH was performed and quantitated against a standard curve generated from in vitro-transcribed RNA. The levels are expressed as RNA copy number/eye. Shown is the mean RNA copy number/eye ± standard deviation of 8 to 10 eyes/group.
Long-term persistence of MCMV in ocular tissue.
To determine if the persistence of MCMV observed at 3 months p.i. was maintained for a longer period of time, eyes from mice were tested 1 year p.i. No virus was detected 12 months p.i. by plaque assay in ocular tissue harvested from either subretinally infected mice (n = 6) or mock-infected mice (n = 3). MCMV-infected mice (n = 6) and mock-infected mice (n = 3) were also analyzed for DNA and RNA at 12 months p.i. Consistent with the results from three months p.i., viral ie-1 DNA (Fig. 8A) and viral ie-1 RNA (Fig. 8B) were both detected in the eyes from infected animals, while eyes from mock-infected mice were negative.
FIG. 8.
Persistence of MCMV in ocular tissue 12 months p.i. DNA and RNA were extracted from the eyes of MCMV-infected (+CMV) mice (n = 6) or mock-infected (−CMV) mice (n = 3) 12 months p.i. The DNA was analyzed by PCR for ie-1 DNA (A), and the RNA was reverse transcribed and analyzed by RT-PCR for ie-1 RNA (B). Both PCR and RT-PCR analyses included direct incorporation of [α-32P]dATP into the PCR products and visualization by autoradiography. Serially diluted plasmid pRE1 of known copy number was used as a positive control for DNA analysis, and serially diluted in vitro-transcribed RNA of known copy number was reverse transcribed and used as a positive control for RNA analysis. Water was used as a PCR negative control, and APRT DNA or RNA was used as internal sample controls.
DISCUSSION
Latent CMV infection remains an important area of clinical investigation, since reactivation of CMV leads to significant morbidity and mortality in adult patients with AIDS and in individuals who undergo whole-organ and bone marrow transplantation. The molecular mechanisms of both the maintenance and recurrence of CMV are largely unknown, but it is becoming increasingly clear that both the genetic and immune controls of latent infections are dependent on the particular organ under study and the experimental model employed (11, 18, 22, 37, 45).
Relatively little is known about CMV latency in the eye compared to what is known regarding the lung, spleen, blood, and bone marrow (4, 11). In this report we have demonstrated that MCMV can both establish a latent infection in ocular tissues and reactivate. No measurable in vivo reactivation of latent MCMV was detected in any organ tested, not even following treatment with CY, supporting the idea that reactivation is an induced event and not simply an act of immune surveillance (38). We also report for the first time the induced reactivation of MCMV in a high percentage of explanted eyes from both immunocompetent (82%) and immunosuppressed (76%) animals. Additionally, in the absence of detectable infectious virus, RT-PCR analysis showed both ie-1 and gH transcriptional activities in the ocular samples tested.
With respect to the establishment of latency, our findings for ocular tissue are consistent with what is known about CMV latency in other organs and meet the criteria for the definition of latency. Following a subretinal inoculation, the acute infection in the eye was cleared within 2 to 3 weeks, but MCMV DNA was readily detected in the ocular tissue beyond the point of detection of infectious virus. This was the case even following perfusion, suggesting that latency was established directly in the ocular tissues and not simply in blood cells present within the vasculature of the eye. Atypically high transcriptional activity was observed in the eye after resolution of the acute infection and the establishment of latency. This was in contrast to what was measured in the nonocular tissues and to what has previously been observed in other organs (5, 6, 37, 38, 45, 65). The fact that we did not detect RNA in the other tissues further supports the conclusion that the signal was from the ocular tissue and not from MCMV-infected blood cells. In situ hybridization analysis would directly confirm this and might also identify the actual cell type(s) involved.
The molecular state of persisting CMV is still in question, and the virus might exist both in a true latent state and as a low-level chronic infection (50, 64, 65). There are several possibilities that could explain the transcriptional activity that we observed in the eye. Without the detection of infectious virus, transcription of ie-1 might represent a low-level persistent CMV infection, transient or incomplete reactivation of latent virus, or irrelevant transcripts due to alternative transcriptional control in ocular tissues. In studies of HCMV, ie-1 transcripts produced from a novel latency-specific promoter and antisense ie-1 transcripts have been identified in a small percentage of granulocyte-macrophage progenitor cells infected in vitro and in bone marrow cells of healthy, seropositive individuals (16, 35, 53), but their biological role is unclear (62). Initiation of ie-1 transcription is likely the first step in the reactivation process and is required to initiate the replicative cycle; however, our data and previous reports indicate that it is not necessarily a reliable indicator of productive CMV infection (18, 38). Although there is no definitive proof that there was complete absence of infectious virus when the ie-1 transcripts were detected, our data suggest in at least two ways, i.e., the minimal influence of immunosuppression and the level of transcriptional activity, that an active infection was not present.
Following treatment with CY, no differences in the amounts of DNA and RNA or in the levels of reactivation were measured. If the DNA and RNA levels in the ocular tissues were indicative of a low-level smoldering infection, treatment of the animals with CY would likely have allowed amplification of infectious virus to the level of detection by standard plaque assay. There was also no difference in the reactivation kinetics between the CY-treated and mock-treated groups. One argument could be that the CY regimen used did not have a biological effect in the eye. However, we have previously demonstrated that CY treatment induces a generalized immunosuppression (31, 46) and has a biological effect on acute replication of MCMV. Peak virus titers are increased within ocular tissue and clearance of the virus is delayed following CY treatment (31). Further evidence for true reactivation events and not just amplification of a smoldering productive infection is that during explantation, most of the cultures (>80%) showed no signs of CPE until after 1 week or more in culture.
A possible explanation for similar levels of transcriptional activity and reactivation frequencies in the immunocompetent and in the immunosuppressed animals might involve specific immunoregulatory factors not affected by the CY treatments either directly or indirectly. For instance, there could be a release of cytokines that in turn induce the expression of the ie-1 gene or other factors important in the reactivation pathway. It has been suggested for both MCMV and HCMV that the tumor necrosis factor (TNF) might play an important role in reactivation by inducing immediate-early gene expression through activation of NF-κB (10, 13, 55). TNF has recently been shown in an allogeneic transplantation model to induce MCMV ie-1 gene expression in the kidney and lung (22). Although we currently have no data to support that this occurs in ocular tissue, it is conceivable that the presence of cytokines in the relatively isolated environment of the eye could have a significant effect.
The second line of evidence for our data measuring true reactivation from latency is the level of transcription observed. The frequency and amount of transcriptionally active ocular tissue was high enough that infectious virus should have been detectable if ie-1 transcription was a consistent marker for productive infection. The levels of viral DNA in the latently infected ocular tissues were equivalent to at least 1 to 2 logs of infectious virus based on the estimated 500 MCMV genome copies/PFU previously calculated (36). If the transcripts detected were associated with permissive viral replication, then infectious virus would be expected to be detectable by plaque assay.
Transcriptional activity of ie-1 in the absence of detectable infectious virus is not necessarily in contradiction with other reports, but there are notable differences indicating that the molecular state of CMV persistence in the eye is unlike what is known for the spleen, lung, or salivary gland. The first difference is that significant ie-1 RNA levels were detected in 100% of the eye samples tested. This high frequency of ie-1 detection is intriguing in the context of recent work on MCMV latency in the lung. Kurz et al. have developed a mosaic model of ie-1 transcription in the lung following irradiation treatment, which indicates a focal pattern of activity, in which the molecular activity detected is randomly distributed throughout the organ, with large areas of no detectable activity (37, 38). The frequency of transcriptionally active tissue in the lung is relatively high (7 to 15 foci/lung) without the detection of infectious virus even by use of a previously described high-sensitivity focus expansion assay (36). Importantly, the pattern of transcriptional activity in the lung is not confined to the ie-1 gene and there is evidence of cotranscriptional regulation with the generation of the IE-3 transactivator, a protein known to be critical in the transition from latency to recurrence (1, 38).
Our data indicate that during latent infection of the eye, MCMV expresses a pattern of transcriptional activity that is not confined to the immediate early region. This further supports control of reactivation at checkpoints subsequent to ie-1 transcription (37) and provides evidence for control even after transcription of late viral genes. An important parameter will be to distinguish whether the multiple checkpoints required in the transition from latency to productive infection rely on the inherent differences in the tissues or if there are differences in the levels of molecular significance at each checkpoint. Additional testing for transcriptional activity of other viral genes in ocular tissue might help determine more-specific parameters of the viral persistence in the eye. Since recent work in cell culture and in the lungs of mice has indicated that the ie-3 gene is essential for viral growth and an important regulator in the transition from molecular latency to recurrent infection (1, 38), we are currently testing our ocular samples for the expression of this transcript. Although the precise molecular controls in the ocular tissue may be different from those in the lung, our findings are consistent with those for the lung and indicate the existence of several sequentially ordered control points in the transition from latency to recurrence. For the eye, these specific checkpoints appear to extend beyond the immediate-early and early genes to a late gene transcript, and this could have significance with respect to the frequency of reactivation in vitro and the overall level of regulation of CMV persistence in this tissue.
Transcriptional activity of ie-1 in the spleen has also been detected (18), but the frequency of detection is variable and dependent on the limit of sensitivity of the PCR. We extracted the RNA and DNA from entire eyes, so our results cannot confirm if the transcriptional activity that we observed was a focal event from one or more particular cell types in the eye or if the activity was randomly distributed throughout the tissue. Importantly, we did not detect any transcriptional activity of ie-1 or gH in any of the other latently infected tissues either before or after immunosuppressive treatment. These results are not necessarily in contrast to the previously reported transcriptional activity detected in the spleen and lung and might be explained by the initial level of virus that seeded these organs following the subretinal inoculation. Because the acute viral titers in the peripheral organs were 2 to 3 logs lower than that expected following an intraperitoneal or intravenous inoculation (65), our lack of ie-1 detection in the spleen and lung might be explained by the relatively low copy number of viral genomes in these organs (51). This could also account for the lack of measurable CY-induced reactivation in vivo.
The second difference between ocular transcription and that of other organs in our findings was our ability to detect late gene transcripts in a high frequency of both immunocompetent (90%) and immunosuppressed (80%) animals. In samples positive for gH RNA, the gH levels were 5- to 10-fold lower than the ie-1 RNA levels. The actual numbers of gH copies/eye might be slightly overestimated, since quantitation of gH was calculated from detection signals that were readily visible but on the border of the linear range of the RT-PCR. This could mean that the differences which we report are actually a minimal difference. Regardless of the actual ratios of gH and ie-1 RNA levels, our findings are similar to those of a previous study reporting that both ie-1 and gH transcriptional activities were detected in latently infected ocular tissue (11). However, it is noteworthy that the late gene transcripts in that study were detected only following immunosuppression (11). In the lung, the late transcript of glycoprotein B (gB) has been detected, but again, only after immunosuppressive treatment (38). To our knowledge, there have been no other reports of late gene transcriptional activity associated with CMV latency outside the context of immunosuppression or recurrent productive infection.
It is noteworthy that the levels of viral transcripts are several logs lower than that of DNA and that the gH transcript levels are lower than the levels of ie-1 transcripts. Clearly, most of the DNA persisting in the ocular tissue was not transcriptionally active at any given instant, suggesting the more typical profile of MCMV latency. However, at least some of this DNA was potentially biologically relevant as supported by the detection of immediate-early and late viral transcripts and the explantation-induced reactivation. The difference in levels of ie-1 and gH RNA is interesting and could provide important insight into the molecular regulation of MCMV latency and reactivation. Two obvious explanations for the differences are that transcriptionally active cells express both RNA populations but the ie-1 RNA is produced at higher levels or that only a portion of the ie-1-expressing cells are expressing gH RNA. The latter situation is likely and would suggest that different latently infected cells are at different points along the pathway of reactivation and that multiple checkpoints exist along that pathway to regulate full permissive reactivation from latency. In situ hybridization analysis with transcript-specific probes should provide additional information to delineate this issue.
The observations of MCMV in ocular tissue are interesting if reactivation in the eye is apparently not exclusively controlled by the intact immune system, and our data could provide insight into what is emerging in the clinical setting among patients with recurrent CMV retinitis. There might be other mechanisms for controlling CMV latency which are minimally influenced by immune reconstitution with HAART, and this will have implications in the long-term management of these patients. The fact that virus was able to spread from the inoculated eye to the contralateral eye might have significance in the development of bilateral eye disease seen in some AIDS patients. Future studies using this mouse model can further address this spread of virus. Finally, ocular infection of mice with MCMV will be a useful model for determining the molecular mechanism regulating CMV latency both within ocular tissue and in visceral organs.
Acknowledgments
This work was supported by the Retina Research Foundation, Houston, Tex.; the Baylor Center for AIDS Research (grant AI136211 from the National Institute for Allergy and Infectious Disease); the Abercrombie Foundation, Houston, Tex.; Research to Prevent Blindness, New York, N.Y.; and National Eye Institute (NEI) core grant 2P30EY02520-21. L.K. was supported by the National Institutes of Health predoctoral training grant EY07001-24 from the NEI.
REFERENCES
- 1.Angulo, A., P. Ghazal, and M. Messerle. 2000. The major immediate-early gene ie3 of mouse cytomegalovirus is essential for viral growth. J. Virol. 74:11129-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton, S. S., C. K. Newell, M. Y. Kanter, and S. W. Cousins. 1991. Retinitis in euthymic mice following inoculation of murine cytomegalovirus (MCMV) via the supraciliary route. Curr. Eye Res. 10:667-677. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, et al. (ed.). 2000. Current protocols in molecular biology. John Wiley and Sons, Inc., New York, N.Y.
- 4.Bale, J. F., Jr., M. E. O'Neil, B. Lyon, and S. Perlman. 1990. The pathogenesis of murine cytomegalovirus ocular infection. Anterior chamber inoculation. Investig. Ophthalmol. Vis. Sci. 31:1575-1581. [PubMed] [Google Scholar]
- 5.Balthesen, M., M. Messerle, and M. J. Reddehase. 1993. Lungs are a major organ site of cytomegalovirus latency and recurrence. J. Virol. 67:5360-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevan, I. S., C. C. Sammons, and C. Sweet. 1996. Investigation of murine cytomegalovirus latency and reactivation in mice using viral mutants and the polymerase chain reaction. J. Med. Virol. 48:308-320. [DOI] [PubMed] [Google Scholar]
- 7.Bruning, J. H., C. A. Bruggeman, and P. J. Breda Vriesman. 1988. The transfer of cytomegalovirus infection in rats by latently infected renal allografts, and the role of various immunosuppressive regimens in virus reactivation. Transplantation 46:623-624. [DOI] [PubMed] [Google Scholar]
- 8.Cassoux, N., B. Bodaghi, A. M. Fillet, G. Carcelain, C. Katlama, and P. Lehoang. 2000. Relapses of CMV retinitis after 2 years of highly active antiretroviral therapy. Br. J. Ophthalmol. 84:1203.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins, T., C. Pomeroy, and M. C. Jordan. 1993. Detection of latent cytomegalovirus DNA in diverse organs of mice. J. Infect. Dis. 168:725-729. [DOI] [PubMed] [Google Scholar]
- 10.Docke, W. D., S. Prosch, E. Fietze, V. Kimel, H. Zuckermann, C. Klug, U. Syrbe, D. H. Kruger, R. von Baehr, and H. D. Volk. 1994. Cytomegalovirus reactivation and tumour necrosis factor. Lancet 343:268-269. [DOI] [PubMed] [Google Scholar]
- 11.Duan, Y., and S. S. Atherton. 1996. Immunosuppression induces transcription of murine cytomegalovirus glycoprotein H in the eye and at non-ocular sites. Arch. Virol. 141:411-423. [DOI] [PubMed] [Google Scholar]
- 12.Duan, Y., Z. Ji, and S. S. Atherton. 1994. Dissemination and replication of MCMV after supraciliary inoculation in immunosuppressed BALB/c mice. Investig. Ophthalmol. Vis. Sci. 35:1124-1131. [PubMed] [Google Scholar]
- 13.Fietze, E., S. Prosch, P. Reinke, J. Stein, W. D. Docke, G. Staffa, S. Loning, S. Devaux, F. Emmrich, and R. von Baehr. 1994. Cytomegalovirus infection in transplant recipients. The role of tumor necrosis factor. Transplantation 58:675-680. [PubMed] [Google Scholar]
- 14.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fish, K. N., S. G. Stenglein, C. Ibanez, and J. A. Nelson. 1995. Cytomegalovirus persistence in macrophages and endothelial cells. Scand. J. Infect. Dis. Suppl. 99:34-40. [PubMed] [Google Scholar]
- 16.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderly, D. E., and L. M. Jampol. 1991. Diagnosis and treatment of cytomegalovirus retinitis. J. Acquir. Immune Defic. Syndr. 4(Suppl. 1):S6-S10. [PubMed] [Google Scholar]
- 18.Henry, S. C., and J. D. Hamilton. 1993. Detection of murine cytomegalovirus immediate early 1 transcripts in the spleens of latently infected mice. J. Infect. Dis. 167:950-954. [DOI] [PubMed] [Google Scholar]
- 19.Hibberd, P. L., N. E. Tolkoff-Rubin, A. B. Cosimi, R. T. Schooley, D. Isaacson, M. Doran, A. Delvecchio, F. L. Delmonico, H. Auchincloss, Jr., and R. H. Rubin. 1992. Symptomatic cytomegalovirus disease in the cytomegalovirus antibody seropositive renal transplant recipient treated with OKT3. Transplantation 53:68-72. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch, M. S. 1991. Cytomegalovirus and its role in the pathogenesis of acquired immunodeficiency syndrome. Transplant. Proc. 23:118-121. [PubMed] [Google Scholar]
- 21.Holland, G. N., E. N. Fang, B. J. Glasgow, A. M. Zaragoza, L. M. Siegel, M. C. Graves, E. H. Saxton, and R. Y. Foos. 1990. Necrotizing retinopathy after intraocular inoculation of murine cytomegalovirus in immunosuppressed adult mice. Investig. Ophthalmol. Vis. Sci. 31:2326-2334. [PubMed] [Google Scholar]
- 22.Hummel, M., Z. Zhang, S. Yan, I. DePlaen, P. Golia, T. Varghese, G. Thomas, and M. I. Abecassis. 2001. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J. Virol. 75:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabs, D. A. 1996. Acquired immunodeficiency syndrome and the eye-1996. Arch. Ophthalmol. 114:863-866. [DOI] [PubMed] [Google Scholar]
- 24.Jabs, D. A. 1998. Cytomegalovirus retinitis and the evolving AIDS epidemic. Retina 18:395-398. [DOI] [PubMed] [Google Scholar]
- 25.Jabs, D. A., and J. G. Bartlett. 1997. AIDS and ophthalmology: a period of transition. Am. J. Ophthalmol. 124:227-233. [DOI] [PubMed] [Google Scholar]
- 26.Jabs, D. A., S. G. Bolton, J. P. Dunn, and A. G. Palestine. 1998. Discontinuing anticytomegalovirus therapy in patients with immune reconstitution after combination antiretroviral therapy. Am. J. Ophthalmol. 126:817-822. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, S. C., C. A. Benson, D. W. Johnson, and A. Weinberg. 2001. Recurrences of cytomegalovirus retinitis in a human immunodeficiency virus-infected patient, despite potent antiretroviral therapy and apparent immune reconstitution. Clin. Infect. Dis. 32:815-819. [DOI] [PubMed] [Google Scholar]
- 28.Jones, C. 1998. Alphaherpesvirus latency: its role in disease and survival of the virus in nature. Adv. Virus Res. 51:81-133. [DOI] [PubMed] [Google Scholar]
- 29.Jordan, M. C., J. D. Shanley, and J. G. Stevens. 1977. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J. Gen. Virol. 37:419-423. [DOI] [PubMed] [Google Scholar]
- 30.Keil, G. M., A. Ebeling-Keil, and U. H. Koszinowski. 1987. Sequence and structural organization of murine cytomegalovirus immediate-early gene 1. J. Virol. 61:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kercher, L., and B. M. Mitchell. 2000. Immune transfer protects severely immunosuppressed mice from murine cytomegalovirus retinitis and reduces the viral load in ocular tissue. J. Infect. Dis. 182:652-661. [DOI] [PubMed] [Google Scholar]
- 32.Klotman, M. E., S. C. Henry, R. C. Greene, P. C. Brazy, P. E. Klotman, and J. D. Hamilton. 1990. Detection of mouse cytomegalovirus nucleic acid in latently infected mice by in vitro enzymatic amplification. J. Infect. Dis. 161:220-225. [DOI] [PubMed] [Google Scholar]
- 33.Koffron, A. J., M. Hummel, B. K. Patterson, S. Yan, D. B. Kaufman, J. P. Fryer, F. P. Stuart, and M. I. Abecassis. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komanduri, K. V., J. Feinberg, R. K. Hutchins, R. D. Frame, D. K. Schmidt, M. N. Viswanathan, J. P. Lalezari, and J. M. McCune. 2001. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J. Infect. Dis. 183:1285-1289. [DOI] [PubMed] [Google Scholar]
- 35.Kondo, K., J. Xu, and E. S. Mocarski. 1996. Human cytomegalovirus latent gene expression in granulocyte-macrophage progenitors in culture and in seropositive individuals. Proc. Natl. Acad. Sci. USA 93:11137-11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurz, S., H. P. Steffens, A. Mayer, J. R. Harris, and M. J. Reddehase. 1997. Latency versus persistence or intermittent recurrences: evidence for a latent state of murine cytomegalovirus in the lungs. J. Virol. 71:2980-2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurz, S. K., M. Rapp, H. P. Steffens, N. K. Grzimek, S. Schmalz, and M. J. Reddehase. 1999. Focal transcriptional activity of murine cytomegalovirus during latency in the lungs. J. Virol. 73:482-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurz, S. K., and M. J. Reddehase. 1999. Patchwork pattern of transcriptional reactivation in the lungs indicates sequential checkpoints in the transition from murine cytomegalovirus latency to recurrence. J. Virol. 73:8612-8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, V., I. Subak-Sharpe, S. Shah, C. Aitken, S. Limb, and A. Pinching. 1999. Changing trends in cytomegalovirus retinitis with triple therapy. Eye 13:59-64. [DOI] [PubMed] [Google Scholar]
- 40.Mayo, D., J. A. Armstrong, and M. Ho. 1978. Activation of latent murine cytomegalovirus infection: cocultivation, cell transfer, and the effect of immunosuppression. J. Infect. Dis. 138:890-896. [DOI] [PubMed] [Google Scholar]
- 41.Mayo, D. R., J. A. Armstrong, and M. Ho. 1977. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature 267:721-723. [DOI] [PubMed] [Google Scholar]
- 42.Mendelson, M., S. Monard, P. Sissons, and J. Sinclair. 1996. Detection of endogenous human cytomegalovirus in CD34+ bone marrow progenitors. J. Gen. Virol. 77:3099-3102. [DOI] [PubMed] [Google Scholar]
- 43.Mercer, J. A., J. R. Marks, and D. H. Spector. 1983. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith Strain). Virology 129:94-106. [DOI] [PubMed] [Google Scholar]
- 44.Mercer, J. A., C. A. Wiley, and D. H. Spector. 1988. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J. Virol. 62:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell, B. M., A. Leung, and J. G. Stevens. 1996. Murine cytomegalovirus DNA in peripheral blood of latently infected mice is detectable only in monocytes and polymorphonuclear leukocytes. Virology 223:198-207. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell, B. M., and J. G. Stevens. 1996. Neuroinvasive properties of herpes simplex virus type 1 glycoprotein variants are controlled by the immune response. J. Immunol. 156:246-255. [PubMed] [Google Scholar]
- 47.Mocarski, E. S. 1996. Cytomegaloviruses and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 48.Plachter, B., C. Sinzger, and G. Jahn. 1996. Cell types involved in replication and distribution of human cytomegalovirus. Adv. Virus Res. 46:195-261. [DOI] [PubMed] [Google Scholar]
- 49.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pollock, J. L., and H. W. Virgin. 1995. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J. Virol. 69:1762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin, R. H. 1990. Impact of cytomegalovirus infection on organ transplant recipients. Rev. Infect. Dis. 12 (Suppl. 7):S754-S766. [DOI] [PubMed] [Google Scholar]
- 53.Slobedman, B., and E. S. Mocarski. 1999. Quantitative analysis of latent human cytomegalovirus. J. Virol. 73:4806-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soderberg-Naucler, C., and J. Y. Nelson. 1999. Human cytomegalovirus latency and reactivation—a delicate balance between the virus and its host's immune system. Intervirology 42:314-321. [DOI] [PubMed] [Google Scholar]
- 55.Stein, J., H. D. Volk, C. Liebenthal, D. H. Kruger, and S. Prosch. 1993. Tumour necrosis factor alpha stimulates the activity of the human cytomegalovirus major immediate early enhancer/promoter in immature monocytic cells. J. Gen. Virol. 74:2333-2338. [DOI] [PubMed] [Google Scholar]
- 56.Taylor-Wiedeman, J., J. G. Sissons, L. K. Borysiewicz, and J. H. Sinclair. 1991. Monocytes are a major site of persistence of human cytomegalovirus in peripheral blood mononuclear cells. J. Gen. Virol. 72:2059-2064. [DOI] [PubMed] [Google Scholar]
- 57.Taylor-Wiedeman, J., P. Sissons, and J. Sinclair. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson, R. L., and J. G. Stevens. 1983. Biological characterization of a herpes simplex virus intertypic recombinant which is completely and specifically non-neurovirulent. Virology 131:171-179. [DOI] [PubMed] [Google Scholar]
- 59.Tural, C., J. Romeu, G. Sirera, D. Andreu, M. Conejero, S. Ruiz, A. Jou, A. Bonjoch, L. Ruiz, A. Arno, and B. Clotet. 1998. Long-lasting remission of cytomegalovirus retinitis without maintenance therapy in human immunodeficiency virus-infected patients. J. Infect. Dis. 177:1080-1083. [DOI] [PubMed] [Google Scholar]
- 60.van Roon, E. N., J. M. Verzijl, J. R. Juttmann, A. W. Lenderink, M. J. Blans, and A. C. Egberts. 1999. Incidence of discontinuation of highly active antiretroviral combination therapy (HAART) and its determinants. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:290-294. [DOI] [PubMed] [Google Scholar]
- 61.Vrabec, T. R., V. F. Baldassano, and S. M. Whitcup. 1998. Discontinuation of maintenance therapy in patients with quiescent cytomegalovirus retinitis and elevated CD4+ counts. Ophthalmology 105:1259-1264. [DOI] [PubMed] [Google Scholar]
- 62.White, K. L., B. Slobedman, and E. S. Mocarski. 2000. Human cytomegalovirus latency-associated protein pORF94 is dispensable for productive and latent infection. J. Virol. 74:9333-9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu, J., P. B. Dallas, P. A. Lyons, G. R. Shellam, and A. A. Scalzo. 1992. Identification of the glycoprotein H gene of murine cytomegalovirus. J. Gen. Virol. 73:1849-1854. [DOI] [PubMed] [Google Scholar]
- 64.Yu, Y., S. C. Henry, F. Xu, and J. D. Hamilton. 1995. Expression of a murine cytomegalovirus early-late protein in “latently” infected mice. J. Infect. Dis. 172:371-379. [DOI] [PubMed] [Google Scholar]
- 65.Yuhasz, S. A., V. B. Dissette, M. L. Cook, and J. G. Stevens. 1994. Murine cytomegalovirus is present in both chronic active and latent states in persistently infected mice. Virology 202:272-280. [DOI] [PubMed] [Google Scholar]