Abstract
Formation of small polykaryons by cell-cell fusion is characteristic of herpes simplex virus (HSV) lesions, but the great majority of viruses isolated from such lesions produce only limited cell fusion in tissue culture. Because of this, HSV laboratory strains that produce extensive cell fusion (syncytium formation) in culture are regarded as variants or mutants. Furthermore, the rarity of clinical isolates able to produce syncytia in culture suggests that extensive cell fusion is deleterious in vivo. Mutations that confer a syncytial phenotype can then be regarded as bypassing a mechanism that normally limits cell fusion. Determination of how these mutations, some of which are in the cytoplasmic tail of glycoprotein B (gB), lead to syncytium formation will likely reveal how fusion is controlled. Here we show the following. (i) Truncation of the cytoplasmic tail of HSV type 2 gB (gB-2) by a minimum of 25 residues or a maximum of 49 residues produces a syncytial phenotype. (ii) Truncation by 20 to 49 residues increases cell fusion when gB-2 is coexpressed with only gD-2, gH-2, and gL-2. (iii) Truncation by 25 or more residues removes a potential endocytosis motif and increases gB-2 cell surface expression. (iv) Mutation of this motif increases gB-2 cell surface expression but does not increase fusogenic activity, whereas mutation of another potential endocytosis motif does not increase surface expression but does increase fusogenic activity. Therefore, syncytial mutations in the cytoplasmic tail of gB-2 do not act by increasing cell surface levels of the protein.
Membrane fusion is a crucial facet in the life cycle of herpesviruses: in virus entry, in virus spread, probably in virus egress, and for some viruses, in cell-cell fusion. For herpes simplex viruses (HSV), entry into the cell occurs by fusion of the virus envelope with the cell plasma membrane (14, 24, 42) via a process that requires the concerted action of four viral glycoproteins, namely, glycoprotein B (gB), gD, gH, and gL (7, 13, 23, 32). HSV membrane proteins can also cause fusion of infected cells with neighboring cells to produce polykaryocytes or syncytia; this occurs to a limited extent in herpes lesions and in cell cultures infected with wild-type strains of the virus (2, 12, 34, 41), but variants that cause more extensive fusion in tissue culture can be isolated from laboratory stocks without mutagenesis and the resulting syncytia may contain thousands of nuclei (35).
Extensive cell-cell fusion in tissue culture requires the four glycoproteins involved in virus entry plus gE, gI, gM, and UL45 (10, 16); it also requires a syn mutation at any one of four loci (35), namely, gB, gK, UL20, or UL24. Because clinical isolates are rarely syncytial, whereas a wild-type virus can acquire a syncytial phenotype by a single conservative amino acid substitution in, for example, gB, it seems likely that a syncytial phenotype is actually deleterious to the virus in vivo. This possibility is supported by the finding that most of the syncytial variants tested are less virulent than nonsyncytial viruses after corneal infection of rabbits (41) or footpad (11) or vaginal (33) infection of mice. By extension, it is possible that syn mutations bypass a regulatory system for minimizing the extent of cell-cell fusion and that uncovering of the mechanism(s) by which they increase fusion could reveal this system. The following questions therefore need to be answered. (i) Why is a syncytial phenotype deleterious to the virus in vivo? (ii) How is cell-cell fusion controlled so as to minimize syncytium formation? (iii) How do syn mutations negate this control mechanism?
We have begun to study syn mutations in HSV type 2 (HSV-2) gB (gB-2) in order to address the third question. For HSV-1 gB (gB-1) and gB-2, all of the spontaneously occurring syn mutations and all of the engineered mutations resulting in a syncytial phenotype are in the cytoplasmic tail (1, 6, 7, 15, 16, 26, 39, 40). For example, truncation of the gB-1 cytoplasmic tail by 28 residues resulted in a syncytial phenotype (1). Similarly, truncation of equine herpesvirus 1 gB resulted in a syncytial phenotype (27) and truncation of pseudorabies virus (PRV) gB increased fusogenic activity, although whether syncytia were observed was not reported (28). In this study, we show that truncations of the gB-2 cytoplasmic tail produce a syncytial phenotype in infected cells and increase the extent of fusion when truncated gB-2 is coexpressed with gD, gH, and gL in a transfection-fusion system whereas they have no effect on virus-cell fusion. All of the truncations that increase cell-cell fusion remove a potential endocytosis motif, and absence of this motif results in higher levels of gB-2 at the cell surface. However, we show that this is not involved in controlling the extent of cell-cell fusion and that a different mechanism must therefore be invoked for the effects of syn mutations in gB-2. The relatively small amounts of wild-type gB-2 at the cell surface are not a limiting factor in cell-cell fusion.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
COS7 and CV1 cells were obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum. D6 cells, a gB-1-expressing cell line derived from Vero cells, were obtained from Stanley Person and grown in Dulbecco's modified Eagle's medium containing 5% fetal bovine serum and 0.2 mg of G418 per ml (9). HSV-2 strain 333 was obtained from Gary Cohen and Roselyn Eisenberg, and propagation and titer determination were done on CV1 cells. The gB-negative virus K082 was obtained from Stanley Person and propagated in D6 cells as described previously (9). Anti-gB polyclonal antibody R91 and monoclonal antibody (MAb) DL16 were provided by Gary Cohen and Roselyn Eisenberg, as was anti-gD polyclonal antibody R7. Anti-gB MAb SB3 was described previously (29). The anti-EEA1 MAb was obtained from BD Biosciences.
Plasmids and mutagenesis.
Plasmids pMM245, pMM346, pMM349, and pMM350, which express gB-2, gD-2, gH-2, and gL-2, respectively, were described previously (26). Mutagenesis of the gB-2 gene in pMM245 was performed by the QuikChange procedure (Stratagene) with Pfu DNA polymerase. All mutants were verified by sequencing.
Immunofluorescence assay.
Conditions for cell surface or intracellular staining of gB-2 in cells grown in Nunc Labtek chamber slides were as described previously (26). Antibodies used for detection of gB-2 were either rabbit polyclonal antibody R91 or mouse MAb SB3 or DL16, followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG), goat anti-mouse IgG-FITC, or goat anti-mouse IgG-Texas Red, as appropriate. For dual-label immunofluorescence assay to detect gB-2 and the early endosomal marker EEA1, a different fixation-and-permeabilization procedure was used (5). Briefly, cells were fixed with 2% formaldehyde and then permeabilized with 2% formaldehyde in methanol at −20°C. The primary antibodies were R91 and a MAb specific for EEA1, and the fluorescent conjugates were goat anti-rabbit IgG-Texas Red and goat anti-mouse IgG-FITC; the washing buffer was phosphate-buffered saline (PBS) containing saponin at 1 mg/ml. All fluorescent conjugates were purchased from Southern Biotechnologies.
Cell surface biotinylation and streptavidin-agarose precipitation.
COS7 cells in 35-mm-diameter dishes were transfected with a gB plasmid on day 1 and biotinylated on day 3. All of the steps in the biotinylation procedure were carried out at 4°C. Following three washes with PBS supplemented with 1 mM MgCl2 and 0.1 mM CaCl2 (complete PBS [C-PBS]), cells were incubated for 30 min with NHS-SS-biotin (0.5 mg/ml in C-PBS; Pierce) and for 10 min with C-PBS-10 mM glycine and then washed three times with C-PBS. Cytoplasmic extracts were prepared by lysing the cells with 100 μl of 10 mM Tris-HCl (pH 7.5)-10 mM NaCl-3 mM MgCl2-1% NP-40-0.5% sodium deoxycholate and then preadsorbed with 10% Pansorbin (Calbiochem) for 30 min before addition to 400 μl of binding buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 0.25% gelatin) together with 50 μl of 50% streptavidin-agarose (Pierce). After incubation for 1 h, the streptavidin-agarose beads were washed three times with 10 mM sodium phosphate (pH 7.2)-0.65 M NaCl-1 mM EDTA-1% Triton X-100, resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer, boiled for 3 min, and pelleted by centrifugation. The supernatants were used for denaturing gel electrophoresis.
Virus penetration (rate-of-entry) assay.
The virus penetration assay was performed essentially as described previously (18, 19). Briefly, COS7 cells were preincubated at 4°C for 30 min and then replicate dishes of cells were incubated with 250 PFU of virus for 1 h at 4°C to allow attachment but not penetration. The medium was replaced with medium prewarmed to 37°C to allow virus entry to begin, and at various time points, the cells were treated with acid-glycine (pH 3) to inactivate any virus that had not yet entered the cells. Plaques were counted after 24 h. The number of plaques produced on dishes that were not treated with acid-glycine was defined as 100%.
Complementation and syncytium assays.
The ability of gB mutants to function in virus entry was measured by complementation of K082, a gB-negative virus (8, 29). Briefly, COS7 cells in 60-mm-diameter dishes were transfected with a gB plasmid on day 1 and infected with 106 PFU of K082 virus on day 2, and the progeny virus was harvested (by freeze-thawing and sonication of cells) on day 3. Virus titers were subsequently determined on gB-expressing D6 cells. One hundred percent complementation is defined as the titer obtained after transfection with plasmid pMM245, which expresses wild-type gB-2. Complementation with a mutant is then defined by the following formula: % complementation = 100 × [(titer with mutant plasmid − titer with carrier DNA)/(titer with pMM245 − titer with carrier DNA)].
Syncytial activity was assayed by transfecting COS7 cells in 60-mm-diameter dishes with a gB plasmid on day 1, infecting them with 104 PFU of K082 virus on day 2, and observing them with a Nikon Diaphot microscope on day 3. For photography, cells were fixed with 3% paraformaldehyde-2% sucrose in PBS and stained with Giemsa stain.
Transfection-fusion assay.
The transfection-fusion assay was performed as described previously (26). Briefly, sparsely seeded COS7 cells were cotransfected with plasmids expressing gD-2, gH-2, gL-2, and either wild-type or mutant gB-2 on day 1. The cells were overlaid with an excess of freshly trypsinized untransfected cells on day 2 and subjected to Giemsa staining and photography on day 3.
RESULTS
Functional analysis of gB-2 truncation mutants.
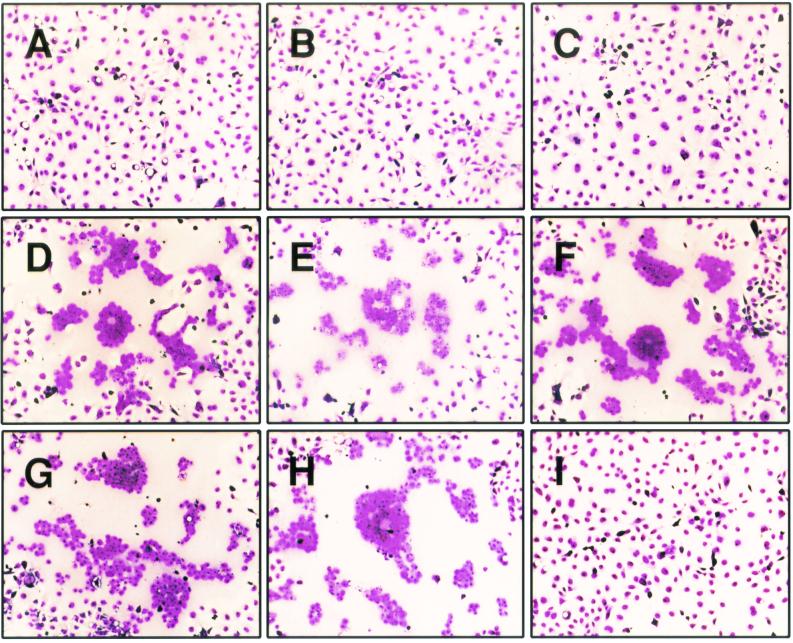
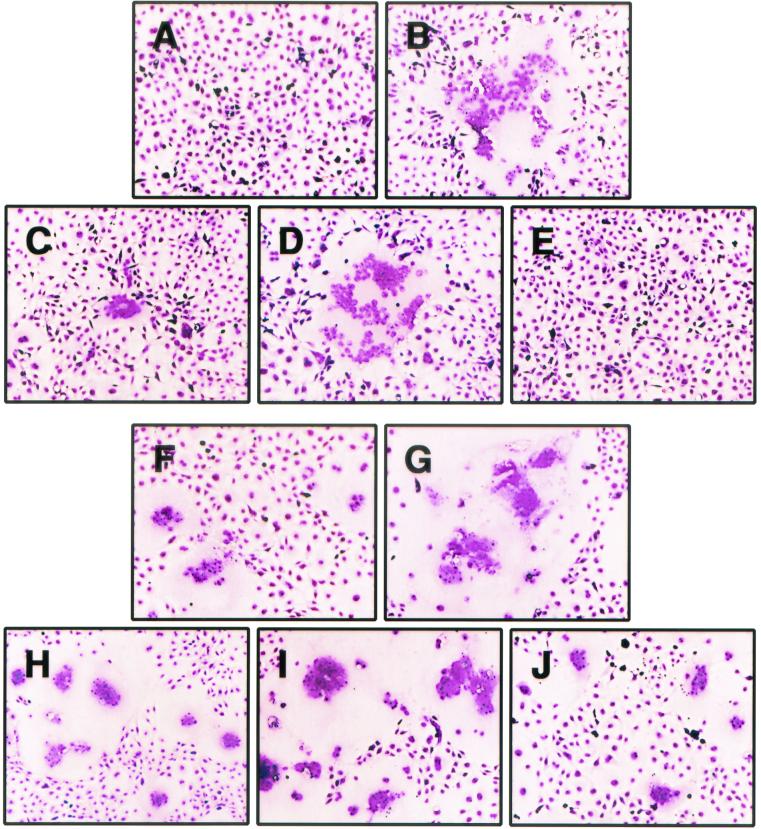
The cytoplasmic tail of gB-2 is 112 residues long, while that of gB-1 is 109 residues long. It was previously shown that truncation of the gB-1 cytoplasmic tail by 28 residues resulted in a syncytial phenotype (1). To determine if truncation of gB-2 has the same effect and, if it does, to determine how much of the cytoplasmic tail must be missing, a panel of eight truncation mutants was made by insertion of stop codons within the gB-2 gene in plasmid pMM245. The resulting proteins are referred to as 872T (which lacks the final 10 residues of the 882-residue protein), 862T, 857T, 852T, 844T, 836T, 833T, and 829T. COS7 cells were transfected with plasmids expressing either wild-type gB-2 or one of the truncation mutants and then infected with K082, an HSV-1 mutant that lacks a functional gB-encoding gene (9). Note that in this assay, only 104 PFU of virus was used per 106 cells so that most of the cells remained uninfected and therefore were potential targets for fusion with infected cells. The combination of wild-type gB-2 and K082 produced small nonsyncytial plaques at 48 h postinfection (hpi) due to limited spread of complemented virus (data not shown), but there was no visible cytopathic effect at 24 hpi (Fig. 1A). Similarly, expression of 872T, 862T, or 829T in infected cells produced no visible cytopathic effect at 24 hpi (Fig. 1B, C, and I, respectively). In contrast, combination of 857T, 852T, 844T, 836T, or 833T with K082 produced syncytial plaques at 24 hpi (Fig. 1D to H). Therefore, truncation of the cytoplasmic tail of gB-2 by 25 to 49 residues resulted in a syncytial phenotype.
FIG. 1.
Syncytial activity of gB-2 truncation mutants. COS7 cells were transfected with plasmids expressing either wild-type or truncated forms of gB-2 and infected on the next day with a gB-negative virus. At 24 hpi, the cells were stained with Giemsa stain. Magnification, ×100. Panels: A, wild-type gB-2; B, 872T; C, 862T; D, 857T; E, 852T; F, 844T; G, 836T; H, 833T; I, 829T.
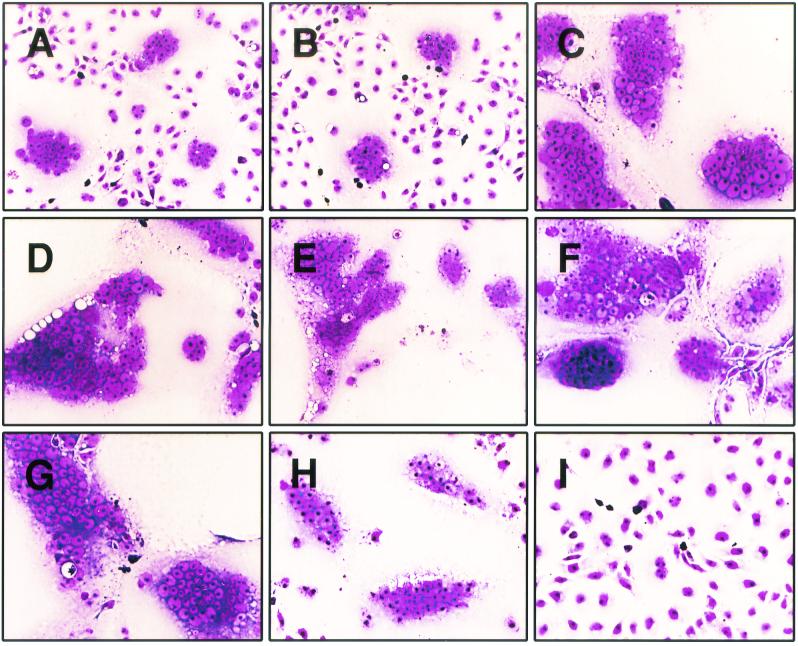
We have shown previously that coexpression of wild-type gB-2 with gD-2, gH-2, and gL-2 in COS7 cells results in cell-cell fusion (26). This had been demonstrated earlier for the equivalent HSV-1 proteins (38), and the assay was subsequently characterized further (4, 30). A similar system was reported for PRV, although in this case, the gD homolog was not required (21). Therefore, each of our gB-2 truncation mutants was next tested for the ability to cause cell-cell fusion when coexpressed in COS7 cells with gD-2, gH-2, and gL-2. A quantitative fusion assay previously developed in our laboratory proved to be unsuitable for mutants producing extensive cell fusion (i.e., greater than that associated with wild-type gB-2); however, the differences in the extent of fusion can readily be seen in Fig. 2. The 872T mutant (Fig. 2B) produced a degree of fusion similar to that produced by wild-type gB-2 (Fig. 2A), whereas 862T, 857T, 852T, 844T, and 836T all produced much larger foci of fusion (Fig. 2C to G). The 833T mutant (Fig. 2H) produced more fusion than wild-type gB-2 but less than the previous five mutants. In contrast, the 829T mutant produced no fusion at all (Fig. 2I). Thus, the two assays for cell-cell fusion give very similar, but not identical, results. The most notable difference is that truncation by 25 residues (857T) was necessary to produce a syncytial phenotype in infected cultures, whereas truncation by only 20 residues (862T) was necessary to greatly increase fusion in the transfection system. Truncation by 53 residues (829T) or more (data not shown) not only did not result in syncytial activity but also abolished activity in the transfection-fusion assay.
FIG. 2.
Effects of gB-2 truncations on cell-cell fusion in a transfection-fusion assay. COS7 cells were cotransfected with plasmids expressing gD-2, gH-2, gL-2, and either wild-type or mutated gB-2. They were overlaid with untransfected cells on the next day and stained with Giemsa stain after a further 36 h. Magnification, ×200. Panels: A, wild-type gB-2; B, 872T; C, 862T; D, 857T; E, 852T; F, 844T; G, 836T; H, 833T; I, 829T.
To determine if truncation of gB-2 alters virus-cell fusion activity, as well as cell-cell fusion activity, a complementation assay was used (8, 29). COS7 cells transfected with plasmids expressing either wild-type gB-2 or one of the truncation mutants were infected with K082 virus, and progeny virus was harvested at 24 h and its titer was determined on gB-expressing D6 cells. Note that in this assay, an inoculum of 106 PFU of virus per 106 cells was used so that a much larger percentage of cells was infected than in the assay used to measure syncytial activity. The complementation activity of most of the mutants was similar (within twofold) to that of wild-type gB-2 (Table 1). However, the 833T mutant produced only 15% activity in the complementation assay and 829T was nonfunctional. The finding that the increased cell-cell fusion activity of most of the truncation mutants is not matched by increased virus entry activity suggests that the proposed regulatory system for minimizing the extent of cell-cell fusion does not affect virus-cell fusion.
TABLE 1.
Complementation of gB-negative virus
| Truncation | No. of PFUa
|
Mean % complementation ± SD | |
|---|---|---|---|
| Mutant | Wild type | ||
| None (wild-type gB-2) | 463,000 | 463,000 | 100 |
| 872T | 360,000 | 400,000 | 90 ± 10 |
| 862T | 293,000 | 333,000 | 88 ± 33 |
| 857T | 1,093,000 | 613,000 | 194 ± 81 |
| 852T | 373,000 | 307,000 | 128 ± 30 |
| 844T | 1,280,000 | 800,000 | 156 ± 13 |
| 836T | 651,000 | 853,000 | 74 ± 26 |
| 833T | 27,000 | 183,000 | 15 ± 4 |
| 829T | 27 | 387,000 | 0 ± 0 |
Each value is the average of three independent experiments, together with the average obtained for wild-type gB-2 in the same three experiments. These are the values after subtraction of the background obtained with carrier salmon sperm DNA (generally, about 200 PFU).
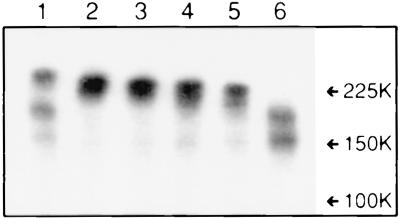
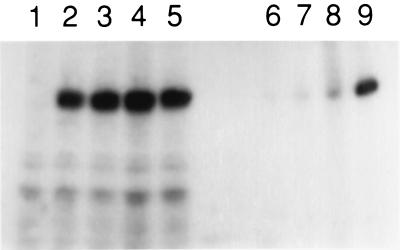
The inactivity of the 829T mutant in any of the functional assays might be explained by a critical role for residues 830 to 833 in either functional activity or maintenance of the correct conformation of gB. Two assays were therefore used to analyze folding of the truncation mutants. First, they were all found to interact with MAbs SB3 and DL16 in an immunofluorescence assay of permeabilized transfected cells (data not shown). Since both of these MAbs recognize conformational epitopes (29; G. H. Cohen and R. J. Eisenberg, personal communication), this result suggests that the ectodomain of each mutant folds correctly. The second assay consisted of electrophoresis of the proteins on a nondenaturing gel, followed by probing with an anti-gB polyclonal antibody (Fig. 3). A band corresponding to the gB dimer was detected for the wild-type protein and mutants 862T, 852T, 844T, and 833T (upper band in lanes 1 to 5) but not for mutant 829T (lane 6). The two additional bands seen with wild-type gB (lane 1) may result from partial proteolysis after endocytosis, since they were absent or barely detectable for mutants 862T, 852T, 844T, and 833T (see discussion of Fig. 5). The nature of the bands observed for 829T (lane 6) is unclear, since they migrated more slowly than expected for monomeric gB, and the truncation would be expected to prevent endocytosis (see Fig. 5). Nonetheless, the aberrant migration favors the interpretation that residues 830 to 833 are essential for formation or maintenance of the functional conformation of gB.
FIG. 3.
Nondenaturing gel electrophoresis of gB-2 truncation mutants. Cytoplasmic extracts of transfected COS7 cells were electrophoresed on a 7.5% nondenaturing polyacrylamide gel, transferred to nitrocellulose, and probed with polyclonal antibody R91, followed by 125I-protein A. Lanes: 1, wild-type gB-2; 2, 862T; 3, 852T; 4, 844T; 5, 833T; 6, 829T. The values on the right are molecular weights in thousands.
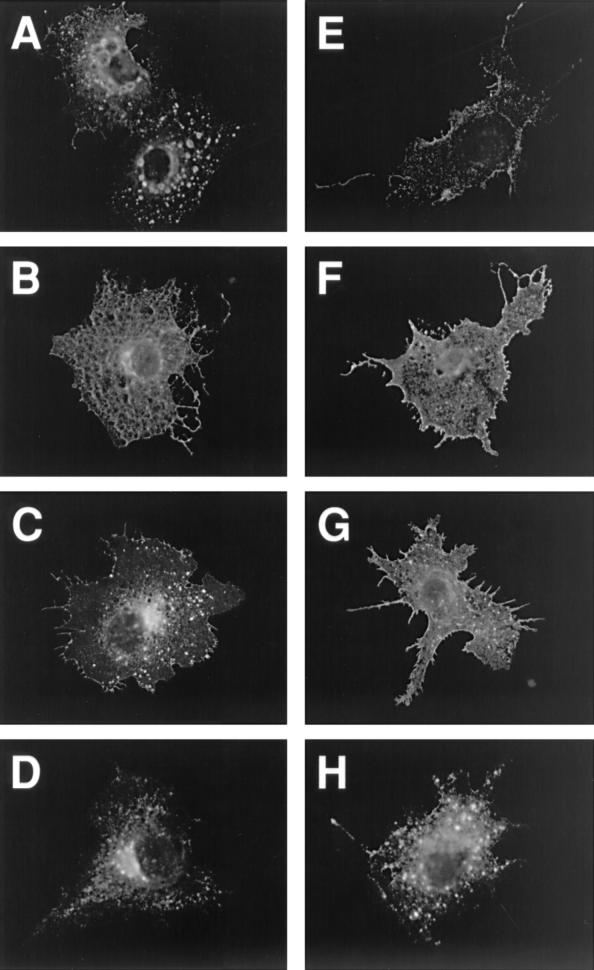
FIG. 5.
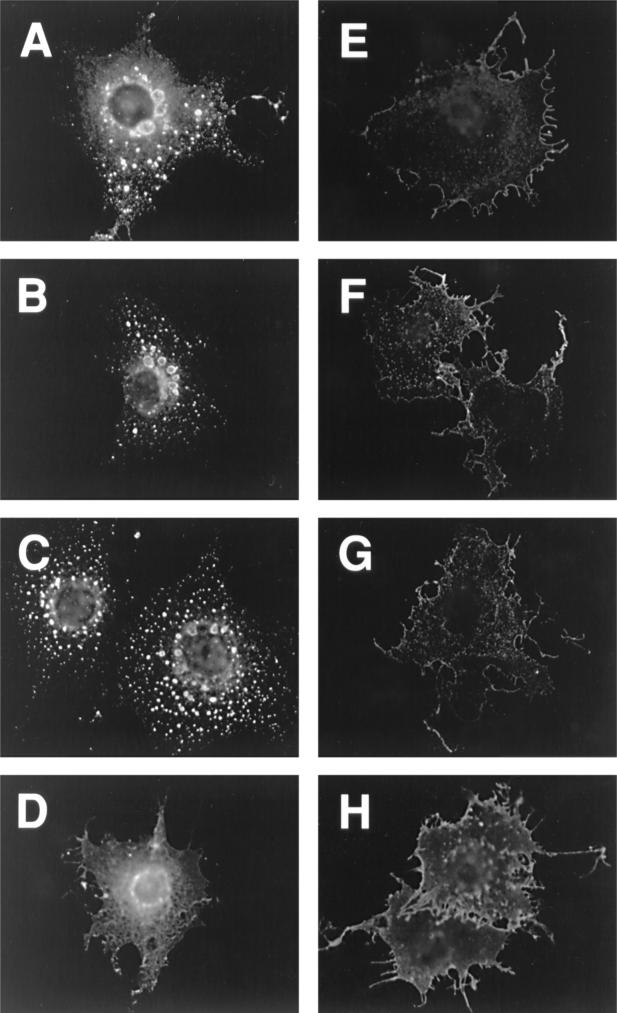
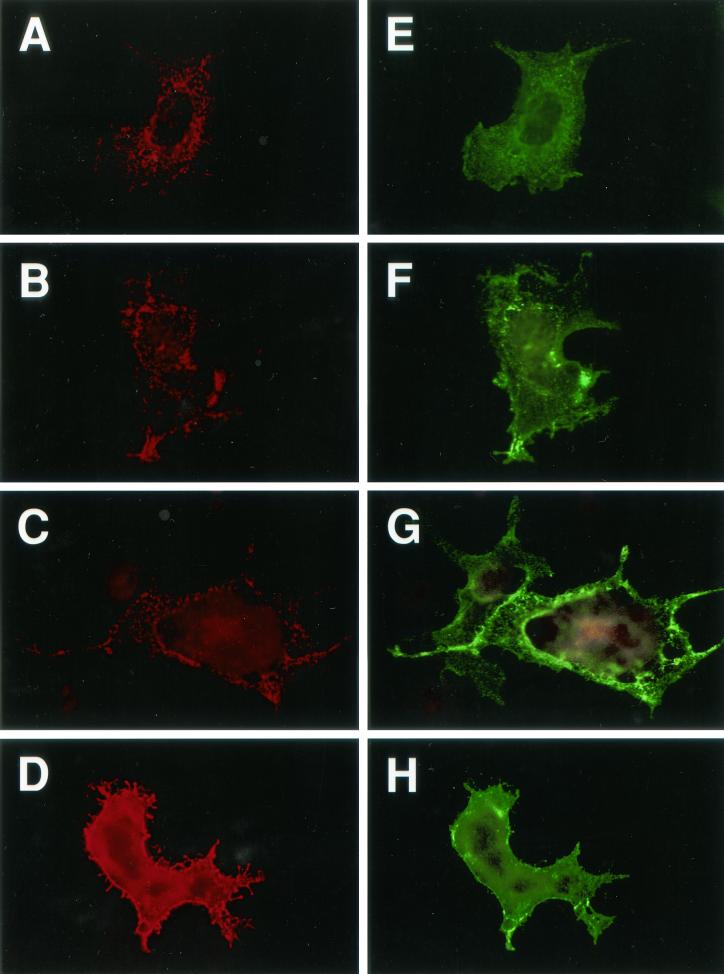
Immunofluorescence analysis of gB-2 truncation mutants in transiently transfected COS7 cells. For cell surface staining (E to H), cells were fixed with paraformaldehyde; for intracellular staining (A to D), they were fixed with paraformaldehyde and then permeabilized with Triton X-100. Cells were stained with polyclonal antibody R91, followed by a goat anti-rabbit IgG-FITC conjugate. Magnification, ×400. Panels: A and E, wild-type gB-2; B and F, 862T; C and G, 833T; D and H, 829T.
Cellular location of gB-2 truncation mutants.
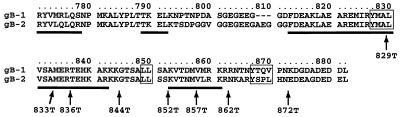
A potential YXXL endocytosis motif at residues 867 to 870 (Fig. 4) would be present in the 872T mutant, which behaved like wild-type gB-2 in both cell-cell fusion assays, but not in the mutants with increased activity in one or both assays. This motif is conserved (as YXXφ, where φ is a large hydrophobic residue) in the cytoplasmic tail of all sequenced gB homologs except that of Epstein-Barr virus and other lymphocryptoviruses, and for some viruses with this motif, endocytosis of gB has been shown to reduce cell surface expression (17, 28, 31, 36, 37). Even for EBV gB, truncation by 41 residues resulted in expression at the cell surface, where the wild-type protein is not detectable (22). However, the hypothesis that increasing the cell surface expression of gB might lead to increased cell-cell fusion has not previously been tested. Therefore, we examined the location of wild-type and truncated forms of gB-2 by immunofluorescence microscopy of transfected COS7 cells (Fig. 5). This analysis revealed four patterns of intracellular and cell surface expression. (i) Wild-type gB-2 showed a vesicular and punctate pattern of intracellular staining and a low level of cell surface staining (Fig. 5A and E, respectively). The 872T mutant closely resembled wild-type gB-2 (data not shown). (ii) In contrast, 862T, 857T, 852T, 844T, and 836T (862T is shown as an example) did not have a vesicular and punctate pattern of intracellular staining; instead, they appeared to be distributed between the endoplasmic reticulum and Golgi apparatus (Fig. 5B). Furthermore, cell surface staining (Fig. 5F) was much brighter than for 872T and the wild-type protein. (iii) The 833T mutant also showed bright cell surface staining (Fig. 5G), but intracellular staining was weaker than for the less truncated mutants, with some punctate staining also visible (Fig. 5C). (iv) Intracellular staining for the 829T mutant (Fig. 5D) was brighter near the center of the cell than at the edges, which is reminiscent of mutants misfolded in the ectodomain (29). In addition, cell surface staining for 829T, although brighter than for wild-type gB-2, was extremely punctate (Fig. 5H). In summary, the presence of the YXXL motif was associated with a relatively low level of gB-2 at the cell surface and a distinctive punctate and vesicular intracellular staining pattern, whereas the absence of this motif (which correlated with increased cell-cell fusion) was associated with a greater abundance of gB-2 at the cell surface and the absence of punctate/vesicular intracellular staining.
FIG. 4.
Alignment of the amino acid sequences of the cytoplasmic tails of gB-1 and gB-2. The amino acid numbers are for gB-2, and residue 1 is defined as the first one after removal of the signal sequence. Predicted α helices are indicated by bars underlining the sequence. Three potential endocytosis motifs are boxed, and the sites of truncations in gB-2 are indicated by arrows.
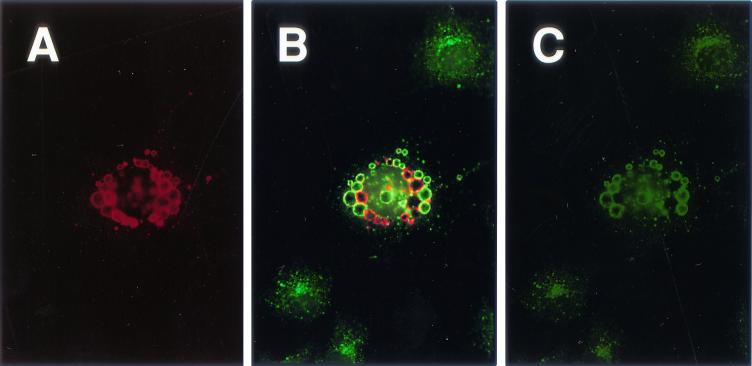
A preliminary analysis of the origin of the gB-2-containing vesicles was performed with a dual-label immunofluorescence assay. The objective was to determine if wild-type gB-2 colocalizes with EEA1, a peripheral membrane protein found on the outer surface of early endosomes (25). Comparison of Fig. 6A (gB-2 stained with Texas Red) and Fig. 6C (EEA1 stained with FITC) shows that many of the gB-2-containing vesicles do, indeed, contain EEA1. In contrast, EEA1 displayed a typical endosomal pattern of staining in cells not expressing gB-2 (peripheral cells in Fig. 6C). This result suggests that the vesicles are derived, at least in part, from early endosomes. Not all of the vesicles contained EEA1, as is evident in Fig. 6B, which shows the same cells when viewed through a set of filters that was optimized for FITC but allowed some bleedthrough of Texas Red fluorescence. Those vesicles that were not stained for EEA1 had likely followed the normal endosomal maturation pathway, which involves loss of EEA1, a tethering protein, from the external surface. This explanation is supported by the finding that a small number of gB-2-containing vesicles were stained for the lysosomal protein LAMP (data not shown).
FIG. 6.
Dual-label immunofluorescence assay to detect gB-2 and early endosomal protein EEA1. COS7 cells were transfected with a plasmid expressing wild-type gB-2 and processed 40 h later. They were fixed with formaldehyde and then permeabilized with methanol. Cells were stained with polyclonal antibody R91 and a MAb against EEA1, followed by goat anti-rabbit IgG-Texas Red and goat anti-mouse IgG-FITC. Magnification, ×400. Panels: A, gB-2 [Texas red channel]; B, EEA1 and gB-2 [FITC channel allowing bleedthrough of Texas Red]; C, EEA1 [FITC channel].
Mutation of potential endocytosis motifs.
The presence or absence of the residue 867-to-870 YXXL motif in the truncation mutants correlates with the presence of small or large amounts of gB-2 at the cell surface. Its absence also correlates with a syncytial phenotype. This suggested the possibilities that the degree of cell-cell fusion is dependent on the level of gB-2 at the cell surface and that increasing cell surface expression by preventing endocytosis will therefore increase fusion. Investigation of these possibilities is complicated by the fact that another YXXφ motif is found closer to the transmembrane anchor in most gB homologs, including gB-2, as well as a dileucine endocytosis motif between the two YXXφ motifs (Fig. 4).
Therefore, all three motifs were mutated independently in order to determine which of them affects cell surface levels of gB-2. For the two YXXφ motifs, the tyrosines were changed to alanines, producing mutants Y827A and Y867A; for the dileucine motif, both leucines were changed to alanine, producing mutant LL849/850AA.
The distribution of each mutant in transfected COS7 cells was examined first by immunofluorescence assay (Fig. 7). Y827A (Fig. 7B and F) and LL849/850AA (Fig. 7C and G) resembled wild-type gB-2 (Fig. 7A and E), with intracellular punctate and vesicular staining and relatively low levels of cell surface expression. In contrast, Y867A was more abundant at the cell surface (Fig. 7H) and did not show a punctate and vesicular intracellular pattern (Fig. 7D). Cell surface biotinylation, followed by precipitation with streptavidin-agarose and Western blotting with an anti-gB-2 polyclonal antibody (Fig. 8, lanes 6 to 9), confirmed the higher level of cell surface expression of Y867A (Fig. 8, lane 9). Note that the total expression levels of all three mutants (i.e., intracellular and cell surface combined) were similar to that of wild-type gB-2, as shown by parallel electrophoresis and Western blotting of cytoplasmic extracts that had not been through the biotinylation-and-precipitation procedure (Fig. 8, lanes 2 to 5). To determine if the Y867A mutation also affects cell surface expression levels in infected cells, COS7 cells were transfected with the appropriate plasmid and then infected with the gB-negative virus K082. An immunofluorescence assay was used to detect cell surface gB (Fig. 9A to D) and gD (Fig. 9E to H); the immunofluorescence of the latter was used to indicate infected cells. The results agree with those obtained in the absence of infection, i.e., that the Y867A mutation (Fig. 9D) increased the cell surface expression of gB relative to that of the wild type (Fig. 9A), whereas the Y827A and LL849/850AA mutations (Fig. 9B and C) did not. In summary, this series of experiments demonstrates that the YXXL motif closer to the C terminus of gB-2 is responsible for its intracellular distribution and a low level of cell surface expression.
FIG. 7.
Immunofluorescence analysis of gB-2 endocytosis mutants in transiently transfected COS7 cells. For cell surface staining (E to H), cells were fixed with paraformaldehyde; for intracellular staining (A to D), they were fixed with paraformaldehyde and then permeabilized with Triton X-100. Cells were stained with polyclonal antibody R91, followed by a goat anti-rabbit IgG-FITC conjugate. Magnification, ×400. Panels: A and E, wild-type gB-2; B and F, Y827A; C and G, LL849/850AA; D and H, Y867A.
FIG. 8.
Cell surface biotinylation analysis of gB-2 endocytosis mutants in transiently transfected COS7 cells. For lanes 1 to 5, cytoplasmic extracts were made from transfected cells by detergent lysis. For lanes 6 to 9, the surface proteins of transfected cells were biotinylated with NHS-SS-biotin and precipitated from cell extracts with streptavidin-agarose. The samples were electrophoresed on a 7.5% polyacrylamide gel under denaturing conditions, transferred to nitrocellulose, and probed with R91, a polyclonal antibody against gB-2. Lanes: 1, empty vector; 2 and 6, wild-type gB-2; 3 and 7, Y827A; 4 and 8, LL849/850AA; 5 and 9, Y867A.
FIG. 9.
Cell surface immunofluorescence analysis of gB-2 endocytosis mutants in infected COS7 cells. Cells were transfected with plasmids expressing either wild-type gB-2 or truncated forms of gB-2 and then infected with a gB-negative virus. At 10 hpi, the cells were fixed with paraformaldehyde and then incubated with mouse antibody SB3 (anti-gB) and rabbit antibody R7 (anti-gD), followed by goat anti-mouse IgG-Texas Red and goat anti-rabbit IgG-FITC conjugates. Magnification, ×400. Panels: A to D, gB; E to H, gD; A and E, cells expressing wild-type gB-2; B and F, cells expressing Y827A; C and G, cells expressing LL849/850AA; D and H, cells expressing Y867A.
The activity of each mutant in virus entry and cell-cell fusion was examined next. For the virus entry assay, COS7 cells expressing wild-type gB-2 or one of the mutants were infected with 106 PFU of the gB-negative virus K082 and progeny virus was harvested on the next day. For each mutant, the titer of progeny virus obtained and the rate of entry of this progeny virus into cells were comparable to the values obtained for progeny virus complemented with wild-type gB-2 (data not shown). Therefore, the mutations do not affect virus-cell fusion. To test for effects on cell-cell fusion, two assays were used (Fig. 10). First, transfected cells expressing each mutant were infected with 104 PFU of K082 virus and the cultures were examined at 24 hpi. Cells expressing either wild-type gB-2 or gB-2 carrying the known syn mutation E835D were used as controls. Syncytial plaques were observed in the cultures expressing gB-2 E835D (Fig. 10B) and LL849/850AA (Fig. 10D), and a low number of very small syncytial plaques was present in the culture expressing Y827A (Fig. 10C) but none were seen in the cultures expressing wild-type gB-2 (Fig. 10A) or Y867A (Fig. 10E). Second, each mutant was coexpressed with gD-2, gH-2, and gL-2, again with wild-type gB-2 and E835D as controls. The results were similar to those obtained in the previous assay, in that wild-type gB-2 (Fig. 10F) and Y867A (Fig. 10J) had the lowest activity, Y827A had slightly higher activity (Fig. 10H), and LL849/850AA (Fig. 10I) and E835D (Fig. 10G) had the greatest activity, comparable to that of the most fusogenic truncation mutants (Fig. 2C to G).
FIG. 10.
Effects of endocytosis mutations on cell-cell fusion. (A to E) COS7 cells were transfected with plasmids expressing either wild-type or mutated forms of gB-2 and infected on the next day with a gB-negative virus. At 24 hpi, the cells were stained with Giemsa stain. (F to J) COS7 cells were cotransfected with plasmids expressing gD-2, gH-2, gL-2, and either wild-type or mutated gB-2. They were overlaid with untransfected cells on the next day and stained with Giemsa stain after a further 36 h. Magnification, ×100. Panels: A and F, wild-type gB-2; B and G, E835D; C and H, Y827A; D and I, LL849/850AA; E and J, Y867A.
Several conclusions can be drawn from these results. (i) The double amino acid substitution in the LL849/850AA mutant represents a previously unidentified syn mutation. (ii) The Y827A mutation confers a borderline syncytial phenotype. (iii) The reason for the syncytial phenotype of the truncation mutants remains to be identified, since it apparently does not result from loss of the YXXL motif. (iv) The fact that the Y867A mutation increased cell surface expression of gB-2 but did not produce a syncytial phenotype, whereas the LL849/850AA mutation did not increase cell surface expression but did produce a syncytial phenotype, shows that syncytium formation is not the result of increased amounts of gB-2 on the cell surface.
DISCUSSION
HSV can enter uninfected cells by at least two, and possibly three, pathways. The first is by fusion of cell-free virions with the cell plasma membrane. The second is by fusion of infected cells with uninfected cells. A third pathway is suggested by the fact that plaque formation (and hence virus spread) can occur in the presence of antibodies that neutralize cell-free virus. One explanation would be that virions can transfer between cells without becoming extracellular at any point, possibly by passage through transiently formed intercellular fusion pores. Alternatively, the resistance to antibodies could be due to the inaccessibility of extracellular virions present between closely apposed cells, implying that the first and third pathways are similar. However, cell-to-cell spread (the third pathway) requires several virus-encoded membrane proteins that are not required for virus-cell fusion but are required for cell-cell fusion, suggesting that pathways two and three have some mechanistic feature in common.
Cell-cell fusion is probably not a major pathway of HSV spread; polykaryocytes are certainly seen in lesions but are not extensive, and most primary isolates and laboratory strains cause only a limited amount of cell-cell fusion in tissue culture. In contrast, variants that cause much more extensive cell-cell fusion can be readily isolated. Since extensive cell-cell fusion requires not only the proteins needed for cell-to-cell spread but also a syn mutation at any one of several loci in the viral genome, it could be hypothesized to result from a failure to control the limited amount of fusion involved in cell-to-cell spread.
Seen in this light, determining how syn mutations operate to increase cell-cell fusion is likely to provide important clues about the mechanism by which nonsyncytial viruses spread and possibly also about the mechanism of virus-cell fusion. The syn locus that we have chosen to focus on is gB.
All syn mutations in gB, whether present in spontaneously arising syncytial variants or introduced by targeted mutagenesis, are in the cytoplasmic tail, rather than the ectodomain or transmembrane region. In this study, we have shown, first, that truncation of the cytoplasmic tail of gB-2 by 25 residues (mutant 857T) confers a syncytial phenotype on the virus in tissue culture; this is in agreement with the earlier finding that truncation of gB-1 by 28 residues confers a syncytial phenotype (1). Second, truncation of gB-2 by 25 residues greatly increases the amount of cell-cell fusion when the protein is coexpressed with gD-2, gH-2, and gL-2 in transfected cells. In the latter assay, fusion is also increased by a truncation of 20 residues (862T), but for reasons that are not clear, this mutation does not produce a syncytial phenotype in infected cells. The two assays therefore produce similar but not identical results, suggesting that the presence of other viral proteins influences the outcome.
Truncation of gB-2 by 30, 38, 46, or 49 residues (852T, 844T, 836T, and 833T, respectively) produces a syncytial phenotype and also increases fusion in the transfection assay, although the effect of 833T in the latter assay is not as pronounced as for the other mutants. These results suggest a potential difference between gB-1 and gB-2 in that truncation of gB-1 by 41 residues was previously found not to produce a syncytial phenotype (20); however, the influence of cell type cannot be ruled out since Vero cells were used in that experiment and some syn mutations are known to be cell type dependent. Truncation of gB-2 by 53 or more residues (829T and others not shown) abolishes cell-cell fusion activity in both assays, possibly because of misfolding of these proteins. The likelihood that 829T is misfolded is increased by the finding that it has no activity in virus entry into cells.
In contrast to their enhanced cell-cell fusion activity in one or both assays, the activity of mutants 857T through 836T in virus entry is comparable to that of wild-type gB-2. Therefore, the predicted control step for cell-cell fusion (which the syn mutants overcome) does not operate during fusion of the viral envelope with the cell plasma membrane. Mutant 833T has reduced activity in virus entry; for technical reasons, we cannot distinguish between the possibility that it has inherently lower activity in virus-cell fusion and the possibility that the protein is inefficiently incorporated into virions. If the former explanation is correct, then the intermediate activity of this mutant in the transfection fusion assay might be the result of the following opposing factors: (i) increased activity due to the absence of residues downstream of 862 and (ii) decreased activity due to the absence of residues between 833 and 836.
The effects of the truncations on fusion activity presumably result either from the absence of certain residues or from changes in the structure of the remainder of the cytoplasmic tail. We looked first for a correlation between fusion activity and secondary structure. Since a three-dimensional structure has not been determined for any gB homolog, we previously made a secondary-structure prediction by using a neural network approach involving a multiple-sequence alignment of the gB homologs of 19 alphaherpesviruses (29). Regions in the cytoplasmic tail that are predicted to be α helical are shown in Fig. 4 as underlines. There is no convincing relationship between the positions of the predicted helices and the effects of the truncation mutants. Thus, loss of part or all of the helix between residues 853 and 861 correlates with syncytial activity, but activity in the transfection fusion assay is increased by loss of the final 20 residues (862T), which leaves that helix untouched. At least seven residues of the predicted long helix between residues 813 and 842 can be absent without reducing syncytial activity, but further shortening of the helix appears to result in misfolding of the protein.
We next looked at the amino acid sequence and realized that a potential endocytosis motif at residues 867 to 870 would be present in 872T, the one mutant that behaved like wild-type gB, but absent from all of the other mutants. Several gB homologs in other herpesviruses, including human cytomegalovirus (31, 37), PRV (28, 36), and varicella-zoster virus (17), have been shown to be endocytosed, and they all have this motif. Since endocytosis has been shown to reduce cell surface levels of some of these homologs, which might plausibly influence cell-cell fusion, we used an immunofluorescence assay to determine the distribution of wild-type and truncated forms of gB-2 in cells. Expression of wild-type gB-2 resulted in a prominent vesicular staining pattern, but this was not seen in any of the truncation mutants that lacked residues 867 to 870. Colocalization of gB-2 in these vesicles with the early endosome protein EEA1 is consistent with endocytosis of gB-2 from the cell surface. More importantly, the truncation mutant proteins lacking residues 867 to 870 were more abundant at the cell surface than was the wild-type protein.
Mutation of this motif (mutant Y867A) increased the amount of gB-2 at the transfected-cell surface, as shown by immunofluorescence and cell surface biotinylation assays, and produced the same effect when transfected cells were infected with a gB-negative virus. In contrast, mutation of additional potential endocytosis motifs at residues 827 to 830 (Y827A) and 849 to 850 (LL849/859AA) had no effect. Therefore, the sequence YSPL at residues 867 to 870 appears to be solely responsible for restricting the cell surface expression of gB-2. None of the mutations affected the activity of gB-2 in virus entry, as shown by complementation and by rate-of-entry assays, leaving the question of whether the increased cell surface expression of the Y867A mutant would result in increased cell-cell fusion. This was tested in both assays, and in neither one was there any evidence of increased fusion: the Y867A mutant did not confer a syncytial phenotype on the gB-negative virus K082, nor did it increase the extent of fusion when coexpressed with gD-2, gH-2, and gL-2. In contrast, the LL849/850AA mutant did confer a syncytial phenotype on K082 and did increase the extent of fusion when coexpressed with the other glycoproteins. Since a mutation that did not increase cell surface expression of gB-2 did increase cell-cell fusion activity but a mutation that did increase cell surface expression did not affect cell-cell fusion activity, it can be concluded that syn mutations in gB-2 do not operate by increasing its cell surface expression.
Therefore, the question of how syn mutations in gB-2 do exert their effect remains to be answered and is the subject of ongoing investigations. A second, and apparently unrelated, question is the role of endocytosis of herpesvirus glycoproteins, which appears to be a common feature of not only gB but also gE and gI homologs (3).
Acknowledgments
This investigation was supported by Public Health Service grant AI42146 from the National Institute of Allergy and Infectious Diseases. The support of the Louisiana State University Health Sciences Center Center for Excellence in Cancer Research is also acknowledged.
The excellent technical support of Annamarie Cowart and Tanja Minova was invaluable, as were the critical comments of John Sixbey during the preparation of the manuscript. We are grateful to Gary Cohen and Roz Eisenberg for providing antibodies R7, R91, and DL16 and to Stanley Person for providing the K082 virus and D6 cells.
REFERENCES
- 1.Baghian, A., L. Huang, S. Newman, S. Jayachandra, and K. G. Kousoulas. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 67:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blank, H., C. F. Burgoon, G. D. Baldridge, P. L. McCarthy, and F. Urbach. 1951. Cytologic smears in diagnosis of herpes simplex, herpes zoster, and varicella. JAMA 146:1410-1412. [DOI] [PubMed] [Google Scholar]
- 3.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 4.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 5.Buczynski, G., J. Bush, L. Zhang, J. Rodriguez-Paris, and J. Cardelli. 1997. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol. Biol. Cell 8:1343-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. [DOI] [PubMed] [Google Scholar]
- 7.Cai, W., B. Gu, and S. Person. 1988. Role of glycoprotein B of herpes simplex virus type 1 in viral entry and cell fusion. J. Virol. 62:2596-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, W., S. Person, C. DebRoy, and B. Gu. 1988. Functional regions and structural features of the gB glycoprotein of herpes simplex virus type 1. J. Mol. Biol. 201:575-588. [DOI] [PubMed] [Google Scholar]
- 9.Cai, W., S. Person, S. C. Warner, J. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis-Poynter, N., S. Bell, T. Minson, and H. Browne. 1994. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 68:7586-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dix, R. D., R. R. McKendall, and J. R. Baringer. 1983. Comparative neurovirulence of herpes simplex virus type 1 strains after peripheral or intracerebral inoculation of BALB/c mice. Infect. Immun. 40:103-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doane, F., A. J. Rhodes, and H. L. Ormsby. 1955. Tissue culture techniques in the study of herpetic infections of the eye. Am. J. Ophthalmol. 40:189-193. [PubMed] [Google Scholar]
- 13.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller, A. O., and P. G. Spear. 1987. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc. Natl. Acad. Sci. USA 84:5454-5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gage, P. J., M. Levine, and J. C. Glorioso. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 67:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haanes, E. J., C. M. Nelson, C. L. Soule, and J. L. Goodman. 1994. The UL45 gene product is required for herpes simplex virus type 1 glycoprotein B-induced fusion. J. Virol. 68:5825-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heineman, T. C., and S. L. Hall. 2001. VZV gB endocytosis and Golgi localization are mediated by YXXφ motifs in its cytoplasmic domain. Virology 285:42-49. [DOI] [PubMed] [Google Scholar]
- 18.Highlander, S. L., S. L. Sutherland, P. J. Gage, D. C. Johnson, M. Levine, and J. C. Glorioso. 1987. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J. Virol. 61:3356-3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, A., and R. Wagner. 1964. Penetration of herpes simplex virus into human epidermoid cells. Proc. Soc. Exp. Biol. Med. 116:863-869. [DOI] [PubMed] [Google Scholar]
- 20.Huff, V., W. Cai, J. C. Glorioso, and M. Levine. 1988. The carboxy-terminal 41 amino acids of herpes simplex virus type 1 glycoprotein B are not essential for production of infectious virus particles. J. Virol. 62:4403-4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, S. K., and R. Longnecker. 1997. The Epstein-Barr virus glycoprotein 110 carboxy-terminal tail domain is essential for lytic virus replication. J. Virol. 71:4092-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgan, C., H. M. Rose, and B. Mednis. 1968. Electron microscopy of herpes simplex virus. I. Entry. J. Virol. 2:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mu, F.-T., J. M. Callaghan, O. Steele-Mortimer, H. Stenmark, R. G. Parton, P. L. Campbell, J. McCluskey, J.-P. Yeo, E. P. C. Tock, and B.-H. Toh. 1995. EEA1, an early endosome-associated protein. J. Biol. Chem. 270:13503-13511. [DOI] [PubMed] [Google Scholar]
- 26.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 27.Neubauer, A., B. Braun, C. Brandmüller, O.-R. Kaaden, and N. Osterrieder. 1997. Analysis of the contributions of the equine herpesvirus 1 glycoprotein gB homolog to virus entry and direct cell-to-cell spread. Virology 227:281-294. [DOI] [PubMed] [Google Scholar]
- 28.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norton, D. D., D. S. Dwyer, and M. I. Muggeridge. 1998. Use of a neural network secondary structure prediction to define targets for mutagenesis of herpes simplex virus glycoprotein B. Virus Res. 55:37-48. [DOI] [PubMed] [Google Scholar]
- 30.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 31.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hübinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 32.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneweis, K. E., H. Forstbauer, M. Olbrich, and M. Tag. 1984. Pathogenesis of genital herpes simplex virus infection in mice. III. Comparison of the virulence of wild and mutant strains. Med. Microbiol. Immunol. 173:187-196. [DOI] [PubMed] [Google Scholar]
- 34.Scott, T. F. M., and D. L. McLeod. 1959. Cellular responses to infection with strains of herpes simplex virus. Ann. N. Y. Acad. Sci. 81:118-128. [DOI] [PubMed] [Google Scholar]
- 35.Spear, P. G. 1993. Membrane fusion induced by herpes simplex virus, p. 201-232. In J. Bentz (ed.), Viral fusion mechanisms. CRC Press, Inc., Boca Raton, Fla.
- 36.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walev, I., M. Lingen, M. Lazzaro, K. Weise, and D. Falke. 1994. Cyclosporin A resistance of herpes simplex virus-induced “fusion from within” as a phenotypical marker of mutations in the syn 3 locus of the glycoprotein B gene. Virus Genes 8:83-86. [DOI] [PubMed] [Google Scholar]
- 40.Weise, K., H. C. Kaerner, J. Glorioso, and C. H. Schröder. 1987. Replacement of glycoprotein B gene sequences in herpes simplex virus type 1 strain ANG by corresponding sequences of the strain KOS causes changes of plaque morphology and neuropathogenicity. J. Gen. Virol. 68:1909-1919. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler, C. E., Jr. 1964. Biologic comparison of a syncytial and a small giant cell-forming strain of herpes simplex. J. Immunol. 93:749-756. [PubMed] [Google Scholar]
- 42.Wittels, M., and P. G. Spear. 1990. Penetration of cells by herpes simplex virus does not require a low pH-dependent endocytic pathway. Virus Res. 18:271-290. [DOI] [PubMed] [Google Scholar]