Abstract
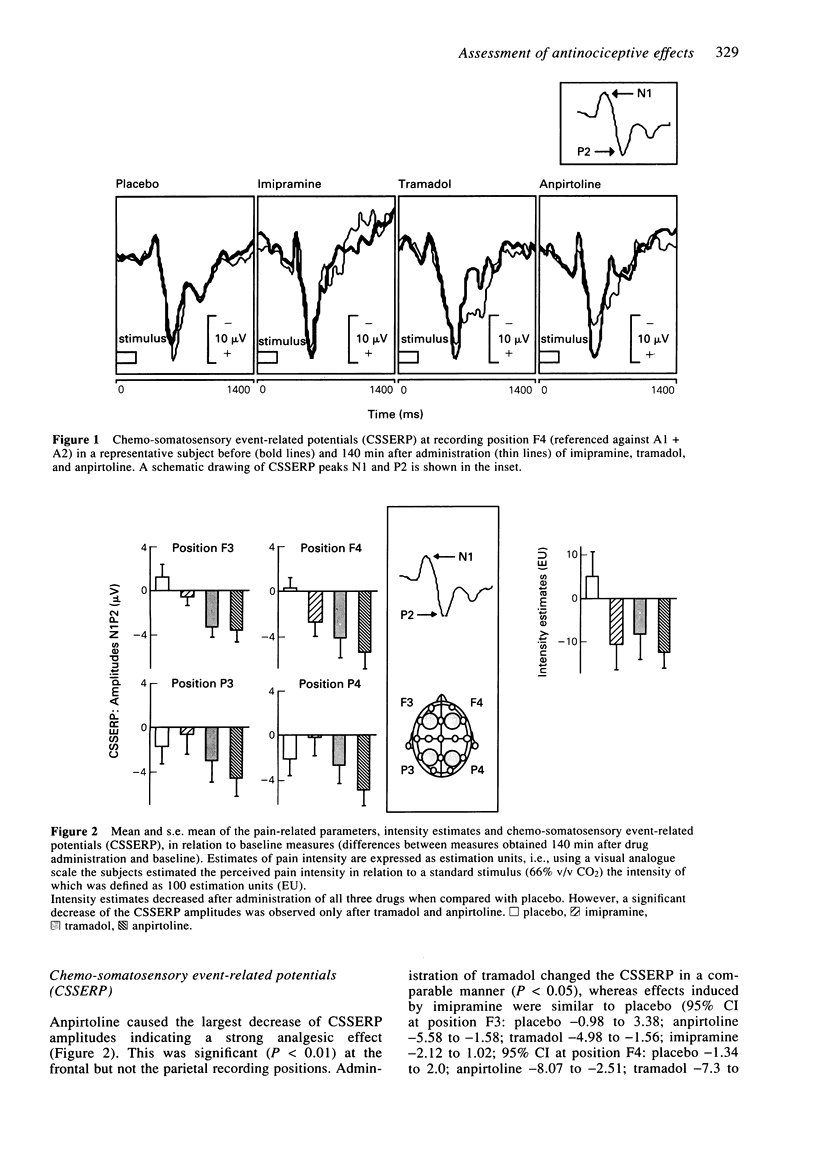
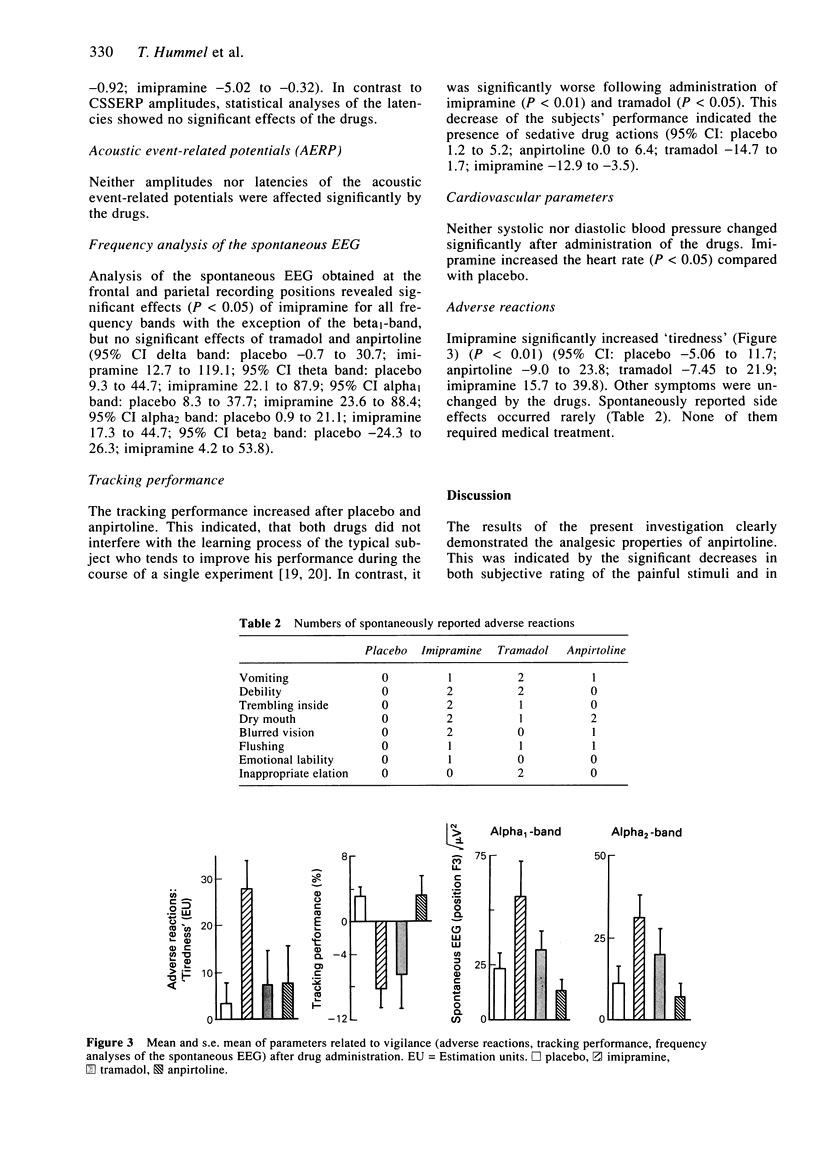
The pain relieving properties of imipramine (100 mg orally), tramadol (150 mg orally), and anpirtoline (60 mg orally) were compared in 16 healthy subjects in a cross-over, double-blind, randomized, and placebo-controlled study. Anpirtoline exhibits analgesia which is possibly mediated via serotoninergic pathways, whereas tramadol exerts its effects at opioid receptors. The pain-relieving effect of the tricyclic antidepressant imipramine may involve both serotoninergic and opioid mechanisms. Chemo-somatosensory event-related potentials (CSSERP) were recorded after painful stimulation of the nasal mucosa with carbon dioxide. Subjects rated the perceived intensity of the stimuli by means of a visual analogue scale. In addition, acoustically evoked responses were recorded, the spontaneous EEG was analyzed in the frequency domain, the subjects' vigilance was assessed in a tracking task, and side effects of the drugs were monitored. Anpirtoline and tramadol produced a decrease of both CSSERP amplitudes and subjective estimates of pain, the effects of the former compound being greater. In contrast, after administration of imipramine no change of CSSERP amplitudes could be detected, whereas the subjective estimate of pain intensity decreased significantly. This was accompanied by a significant decrease of arousal indicating that pain relief produced by acute administration of imipramine was primarily related to its sedation action. The analgesic properties of anpirtoline were demonstrated in man. Tramadol was characterized as a week opioid analgesic. In contrast, imipramine appeared to produce its pain-relieving effects predominantly by non-specific actions. It is hypothesized that different analgesics may change ERP sources in a drug-specific manner.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alon E., Schulthess G., Axhausen C., Hossli G. Doppelblindvergleichsstudie über die Wirkung von Tramadol und Buprenorphine auf die postoperativen Schmerzen. Anaesthesist. 1981 Dec;30(12):623–626. [PubMed] [Google Scholar]
- Anton F., Euchner I., Handwerker H. O. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain. 1992 Apr;49(1):53–60. doi: 10.1016/0304-3959(92)90187-G. [DOI] [PubMed] [Google Scholar]
- Ardid D., Eschalier A., Lavarenne J. Evidence for a central but not a peripheral analgesic effect of clomipramine in rats. Pain. 1991 Apr;45(1):95–100. doi: 10.1016/0304-3959(91)90169-X. [DOI] [PubMed] [Google Scholar]
- Becker D. E., Yingling C. D., Fein G. Identification of pain, intensity and P300 components in the pain evoked potential. Electroencephalogr Clin Neurophysiol. 1993 Jul-Aug;88(4):290–301. doi: 10.1016/0168-5597(93)90053-r. [DOI] [PubMed] [Google Scholar]
- Bernatzky G., Jurna I. Intrathecal injection of codeine, buprenorphine, tilidine, tramadol and nefopam depresses the tail-flick response in rats. Eur J Pharmacol. 1986 Jan 14;120(1):75–80. doi: 10.1016/0014-2999(86)90642-4. [DOI] [PubMed] [Google Scholar]
- Bromm B., Herrmann W. M., Scharein E. Zwei effektive Analgetika im Wirkungsvergleich. Experimentelle Studie: Tramadol versus Tilidin/Naloxon. Fortschr Med. 1989 Jun 10;107(17):385–389. [PubMed] [Google Scholar]
- Bromm B., Meier W., Scharein E. Imipramine reduces experimental pain. Pain. 1986 May;25(2):245–257. doi: 10.1016/0304-3959(86)90100-4. [DOI] [PubMed] [Google Scholar]
- Bromm B., Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol. 1984 Jul;6(7):405–410. [PubMed] [Google Scholar]
- Chudler E. H., Dong W. K., Kawakami Y. Tooth pulp-evoked potentials in the monkey: cortical surface and intracortical distribution. Pain. 1985 Jul;22(3):221–233. doi: 10.1016/0304-3959(85)90022-3. [DOI] [PubMed] [Google Scholar]
- Dong W. K., Chudler E. H., Martin R. F. Physiological properties of intradental mechanoreceptors. Brain Res. 1985 May 20;334(2):389–395. doi: 10.1016/0006-8993(85)90239-2. [DOI] [PubMed] [Google Scholar]
- Eide P. K., Hole K. Acute and chronic treatment with selective serotonin uptake inhibitors in mice: effects on nociceptive sensitivity and response to 5-methoxy-N,N-dimethyltryptamine. Pain. 1988 Mar;32(3):333–340. doi: 10.1016/0304-3959(88)90045-0. [DOI] [PubMed] [Google Scholar]
- Engel J., Bork A., Nubert I., Schönenberger H. Synthese von 14C-Anpirtolin. Arch Pharm (Weinheim) 1988 Nov;321(11):821–822. doi: 10.1002/ardp.19883211112. [DOI] [PubMed] [Google Scholar]
- Fialip J., Makambila M. C., Rigal F., Devoize J. L., Varoquaux O., Eschalier A. Chronic administration of clomipramine inhibits morphine hot plate analgesia. Life Sci. 1986 Mar 24;38(12):1097–1103. doi: 10.1016/0024-3205(86)90245-6. [DOI] [PubMed] [Google Scholar]
- Fields H. L., Heinricher M. M., Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Flohé L., Arend I., Cogal A., Richter W., Simon W. Klinische Prüfung der Abhängigkeitsentwicklung nach Langzeitapplikation von Tramadol. Arzneimittelforschung. 1978;28(1A):213–217. [PubMed] [Google Scholar]
- France R. D., Houpt J. L., Ellinwood E. H. Therapeutic effects of antidepressants in chronic pain. Gen Hosp Psychiatry. 1984 Jan;6(1):55–63. doi: 10.1016/0163-8343(84)90060-4. [DOI] [PubMed] [Google Scholar]
- Friedel B. Die Wirkung von Tramadol auf das Elektroenzephalogramm und Elektronystagmogramm. Arzneimittelforschung. 1978;28(1A):187–189. [PubMed] [Google Scholar]
- Hennies H. H., Friderichs E., Schneider J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung. 1988 Jul;38(7):877–880. [PubMed] [Google Scholar]
- Hummel T., Friedmann T., Pauli E., Niebch G., Borbe H. O., Kobal G. Dose-related analgesic effects of flupirtine. Br J Clin Pharmacol. 1991 Jul;32(1):69–76. doi: 10.1111/j.1365-2125.1991.tb05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J., Kobal G., Kaukoranta E., Hari R. Cortical responses to painful CO2 stimulation of nasal mucosa; a magnetoencephalographic study in man. Electroencephalogr Clin Neurophysiol. 1986 Oct;64(4):347–349. doi: 10.1016/0013-4694(86)90159-8. [DOI] [PubMed] [Google Scholar]
- Klose R., Ehrhart A., Jung R. Der Einfluss von Buprenorphin und Tramadol auf die CO2-Antwort in der unmittelbar postoperativen Phase nach Allgemeinanästhesie. Anasth Intensivther Notfallmed. 1982 Feb;17(1):29–34. [PubMed] [Google Scholar]
- Kobal G., Hummel C., Nuernberg B., Brune K. Effects of pentazocine and acetylsalicylic acid on pain-rating, pain-related evoked potentials and vigilance in relationship to pharmacokinetic parameters. Agents Actions. 1990 Mar;29(3-4):342–359. doi: 10.1007/BF01966467. [DOI] [PubMed] [Google Scholar]
- Kobal G., Hummel T. Effects of flupirtine on the pain-related evoked potential and the spontaneous EEG. Agents Actions. 1988 Feb;23(1-2):117–119. doi: 10.1007/BF01967210. [DOI] [PubMed] [Google Scholar]
- Kobal G. Pain-related electrical potentials of the human nasal mucosa elicited by chemical stimulation. Pain. 1985 Jun;22(2):151–163. doi: 10.1016/0304-3959(85)90175-7. [DOI] [PubMed] [Google Scholar]
- Lintz W., Barth H., Osterloh G., Schmidt-Böthelt E. Bioavailability of enteral tramadol formulations. 1st communication: capsules. Arzneimittelforschung. 1986 Aug;36(8):1278–1283. [PubMed] [Google Scholar]
- Magni G. The use of antidepressants in the treatment of chronic pain. A review of the current evidence. Drugs. 1991 Nov;42(5):730–748. doi: 10.2165/00003495-199142050-00002. [DOI] [PubMed] [Google Scholar]
- Richter W., Barth H., Flohé L., Giertz H. Clinical investigation on the development of dependence during oral therapy with tramadol. Arzneimittelforschung. 1985;35(11):1742–1744. [PubMed] [Google Scholar]
- Roberts M. H. Involvement of serotonin in nociceptive pathways. Drug Des Deliv. 1989 Mar;4(2):77–83. [PubMed] [Google Scholar]
- Rohdewald P., Granitzki H. W., Neddermann E. Comparison of the analgesic efficacy of metamizole and tramadol in experimental pain. Pharmacology. 1988;37(4):209–217. doi: 10.1159/000138468. [DOI] [PubMed] [Google Scholar]
- Sacerdote P., Brini A., Mantegazza P., Panerai A. E. A role for serotonin and beta-endorphin in the analgesia induced by some tricyclic antidepressant drugs. Pharmacol Biochem Behav. 1987 Jan;26(1):153–158. doi: 10.1016/0091-3057(87)90548-x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. The 1988 Merck Frosst Award. The role of ascending and descending noradrenergic and serotonergic pathways in opioid and non-opioid antinociception as revealed by lesion studies. Can J Physiol Pharmacol. 1989 Sep;67(9):975–988. doi: 10.1139/y89-154. [DOI] [PubMed] [Google Scholar]
- Schlicker E., Werner U., Hamon M., Gozlan H., Nickel B., Szelenyi I., Göthert M. Anpirtoline, a novel, highly potent 5-HT1B receptor agonist with antinociceptive/antidepressant-like actions in rodents. Br J Pharmacol. 1992 Mar;105(3):732–738. doi: 10.1111/j.1476-5381.1992.tb09047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen K. H., Reeh P. W., Anton F., Handwerker H. O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992 Jan;12(1):86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutfin T. A., DeVane C. L., Jusko W. J. The analysis and disposition of imipramine and its active metabolites in man. Psychopharmacology (Berl) 1984;82(4):310–317. doi: 10.1007/BF00427676. [DOI] [PubMed] [Google Scholar]
- Swedberg M. D., Shannon H. E., Nickel B., Goldberg S. R. D-16949 (anpirtoline): a novel serotonergic (5-HT1B) psychotherapeutic agent assessed by its discriminative effects in the rat. J Pharmacol Exp Ther. 1992 Dec;263(3):1015–1022. [PubMed] [Google Scholar]
- Thürauf N., Friedel I., Hummel C., Kobal G. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: is it a peripheral nociceptive event? Neurosci Lett. 1991 Jul 22;128(2):297–300. doi: 10.1016/0304-3940(91)90283-y. [DOI] [PubMed] [Google Scholar]
- Vogel W., Burchardi H., Sihler K., Valic L. Uber die Wirkung von Tramadol auf Atmung und Kreislauf. Arzneimittelforschung. 1978;28(1A):183–186. [PubMed] [Google Scholar]
- Walsh T. D. Antidepressants in chronic pain. Clin Neuropharmacol. 1983;6(4):271–295. [PubMed] [Google Scholar]
- Zemlan F. P., Behbehani M. M., Murphy R. M. Serotonin receptor subtypes and the modulation of pain transmission. Prog Brain Res. 1988;77:349–355. doi: 10.1016/s0079-6123(08)62801-0. [DOI] [PubMed] [Google Scholar]


