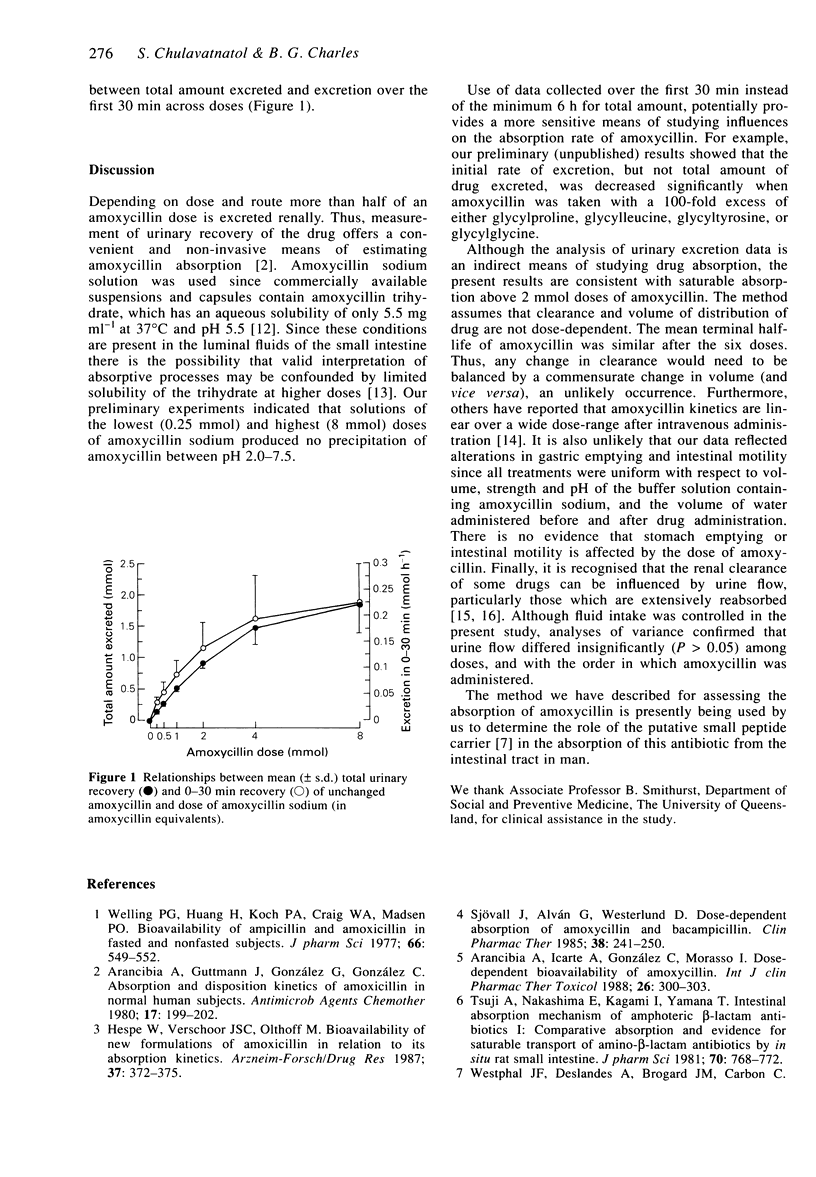
Abstract
Measurement of unchanged drug in urine was used to study the rate and extent of amoxycillin absorption after administration of amoxycillin sodium solution to six healthy subjects in a Latin-Square crossover design. The mean (95% CI) fraction of the dose excreted as unchanged amoxycillin decreased (P < 0.05) from 0.50 (0.44-0.56) after 97 mg amoxycillin sodium (= 0.25 mmol amoxycillin) to 0.23 (0.19-0.27) after 3103 mg (8 mmol), while the mean residence time determined from urinary excretion rate data increased (P < 0.05) from 1.54 (1.32-1.76) h to 2.16 (2.01-2.41) h. Plots of total urinary excretion and initial (0-30 min) excretion of unchanged drug vs dose indicated significant non-linearity above 776 mg doses. Michaelis-Menten parameters describing this relationship with respect to amount absorbed were 3.02 mmol for maximum amount absorbed and 1.93 mmol for amount absorbed at half maximum for 0-30 min. These results support a saturable absorption mechanism for amoxycillin which had clinical implications for high oral amoxycillin doses, and for competition with other drugs having capacity-limited absorption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arancibia A., Guttmann J., González G., González C. Absorption and disposition kinetics of amoxicillin in normal human subjects. Antimicrob Agents Chemother. 1980 Feb;17(2):199–202. doi: 10.1128/aac.17.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arancibia A., Icarte A., González C., Morasso I. Dose-dependent bioavailability of amoxycillin. Int J Clin Pharmacol Ther Toxicol. 1988 Jun;26(6):300–303. [PubMed] [Google Scholar]
- Birkett D. J., Miners J. O. Caffeine renal clearance and urine caffeine concentrations during steady state dosing. Implications for monitoring caffeine intake during sports events. Br J Clin Pharmacol. 1991 Apr;31(4):405–408. doi: 10.1111/j.1365-2125.1991.tb05553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chulavatnatol S., Charles B. G. High-performance liquid chromatographic determination of amoxicillin in urine using solid-phase, ion-pair extraction and ultraviolet detection. J Chromatogr. 1993 May 19;615(1):91–96. doi: 10.1016/0378-4347(93)80294-e. [DOI] [PubMed] [Google Scholar]
- Hespe W., Verschoor J. S., Olthoff M. Bioavailability of new formulations of amoxicillin in relation to its absorption kinetics. Arzneimittelforschung. 1987 Mar;37(3):372–375. [PubMed] [Google Scholar]
- Hill S. A., Jones K. H., Lees L. J. Pharmacokinetics of parenterally administered amoxycillin. J Infect. 1980 Dec;2(4):320–332. doi: 10.1016/s0163-4453(80)92720-6. [DOI] [PubMed] [Google Scholar]
- Paintaud G., Alván G., Dahl M. L., Grahnén A., Sjövall J., Svensson J. O. Nonlinearity of amoxicillin absorption kinetics in human. Eur J Clin Pharmacol. 1992;43(3):283–288. doi: 10.1007/BF02333024. [DOI] [PubMed] [Google Scholar]
- Sjövall J., Alván G., Westerlund D. Dose-dependent absorption of amoxycillin and bacampicillin. Clin Pharmacol Ther. 1985 Sep;38(3):241–250. doi: 10.1038/clpt.1985.166. [DOI] [PubMed] [Google Scholar]
- Torres-Molina F., Peris-Ribera J. E., García-Carbonell M. C., Aristorena J. C., Plá-Delfina J. M. Nonlinearities in amoxycillin pharmacokinetics. II. Absorption studies in the rat. Biopharm Drug Dispos. 1992 Jan;13(1):39–53. doi: 10.1002/bdd.2510130104. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Nakashima E., Hamano S., Yamana T. Physicochemical properties of amphoteric beta-lactam antibiotics I: Stability, solubility, and dissolution behavior of amino penicillins as a function of pH. J Pharm Sci. 1978 Aug;67(8):1059–1066. doi: 10.1002/jps.2600670810. [DOI] [PubMed] [Google Scholar]
- Tsuji A., Nakashima E., Kagami I., Yamana T. Intestinal absorption mechanism of amphoteric beta-lactam antibiotics I: Comparative absorption and evidence for saturable transport of amino-beta-lactam antibiotics by in situ rat small intestine. J Pharm Sci. 1981 Jul;70(7):768–772. doi: 10.1002/jps.2600700714. [DOI] [PubMed] [Google Scholar]
- Welling P. G., Huang H., Koch P. A., Craig W. A., Madsen P. O. Bioavailability of ampicillin and amoxicillin in fasted and nonfasted subjects. J Pharm Sci. 1977 Apr;66(4):549–552. doi: 10.1002/jps.2600660423. [DOI] [PubMed] [Google Scholar]



