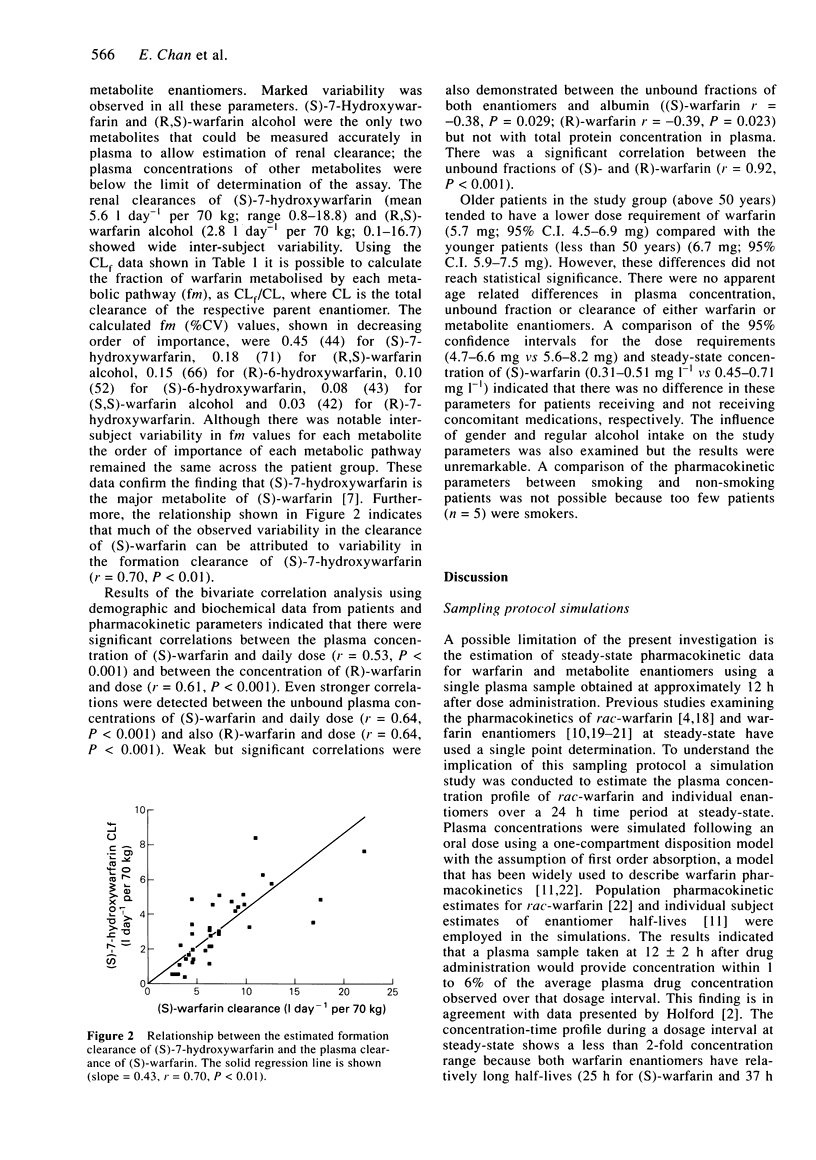
Abstract
1. The disposition of warfarin enantiomers and metabolites has been studied in 36 patients receiving chronic rac-warfarin therapy, titrated to approximately the same anticoagulant response. 2. A stereoselective h.p.l.c. assay was employed to determine the concentrations of (R)- and (S)-warfarin, (R,S)-warfarin alcohol and (S)-7-hydroxywarfarin in plasma and 24 h urine samples. The concentrations of (R)-7-hydroxywarfarin, (S,S)-warfarin alcohol and (R)-6- and (S)-6-hydroxywarfarin were also determined in urine samples. The fractions unbound of warfarin enantiomers were determined using equilibrium dialysis. 3. Wide variability was observed in daily dose requirements (mean 6.1 mg; range: 2.5-12 mg), in plasma concentrations of (S)-warfarin (0.48 mg l(-1); 0.11-1.02 mg l(-1)), (R)-warfarin (0.87 mg l(-1); 0.29-1.82 mg l(-1)), (R,S)-warfarin alcohol (0.31 mg l(-1); 0.02-0.72 mg l(-1)) and (S)-7-hydroxywarfarin (0.25 mg l(-1); 0.07-0.37 mg l(-1)) and the percentage unbound of (S)-warfarin (0.53%; 0.29%-0.82%) and (R)-warfarin (0.54%; 0.26%-0.96%). 4. The mean plasma clearances of warfarin enantiomers were 7.5 1 day-1 per 70 kg (2.5-22.1) for (S)-warfarin and 3.6 1 day-1 per 70 kg (1.6-8.8) for (R)-warfarin. There was a significant correlation between the estimated formation clearance of (S)-7-hydroxywarfarin and the clearance of (S)-warfarin, which accounted for much of the variability in the latter.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfield C., O'Reilly R., Chan E., Rowland M. Phenylbutazone-warfarin interaction in man: further stereochemical and metabolic considerations. Br J Clin Pharmacol. 1983 Dec;16(6):669–675. doi: 10.1111/j.1365-2125.1983.tb02239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfield C., Rowland M. Stereospecific fluorescence high-performance liquid chromatographic analysis of warfarin and its metabolites in plasma and urine. J Pharm Sci. 1984 Oct;73(10):1392–1396. doi: 10.1002/jps.2600731017. [DOI] [PubMed] [Google Scholar]
- Banfield C., Rowland M. Stereospecific high-performance liquid chromatographic analysis of warfarin in plasma. J Pharm Sci. 1983 Aug;72(8):921–924. doi: 10.1002/jps.2600720820. [DOI] [PubMed] [Google Scholar]
- Breckenridge A., Orme M. Kinetics of warfarin absorption in man. Clin Pharmacol Ther. 1973 Nov-Dec;14(6):955–961. doi: 10.1002/cpt1973146955. [DOI] [PubMed] [Google Scholar]
- Breckenridge A., Orme M., Wesseling H., Lewis R. J., Gibbons R. Pharmacokinetics and pharmacodynamics of the enantiomers of warfarin in man. Clin Pharmacol Ther. 1974 Apr;15(4):424–430. doi: 10.1002/cpt1974154424. [DOI] [PubMed] [Google Scholar]
- Choonara I. A., Haynes B. P., Cholerton S., Breckenridge A. M., Park B. K. Enantiomers of warfarin and vitamin K1 metabolism. Br J Clin Pharmacol. 1986 Dec;22(6):729–732. doi: 10.1111/j.1365-2125.1986.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. Q., Wainer I. W. The measurement of warfarin enantiomers in serum using coupled achiral/chiral, high-performance liquid chromatography (HPLC). Pharm Res. 1988 Oct;5(10):680–683. doi: 10.1023/a:1015991324316. [DOI] [PubMed] [Google Scholar]
- Holford N. H. Clinical pharmacokinetics and pharmacodynamics of warfarin. Understanding the dose-effect relationship. Clin Pharmacokinet. 1986 Nov-Dec;11(6):483–504. doi: 10.2165/00003088-198611060-00005. [DOI] [PubMed] [Google Scholar]
- James A. H., Britt R. P., Raskino C. L., Thompson S. G. Factors affecting the maintenance dose of warfarin. J Clin Pathol. 1992 Aug;45(8):704–706. doi: 10.1136/jcp.45.8.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch-Weser J. The serum level approach to individualization of drug dosage. Eur J Clin Pharmacol. 1975 Oct 10;9(1):1–8. doi: 10.1007/BF00613423. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis R. J., Trager W. F., Chan K. K., Breckenridge A., Orme M., Roland M., Schary W. Warfarin. Stereochemical aspects of its metabolism and the interaction with phenylbutazone. J Clin Invest. 1974 Jun;53(6):1607–1617. doi: 10.1172/JCI107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. J., Trager W. F. Warfarin metabolism in man: identification of metabolites in urine. J Clin Invest. 1970 May;49(5):907–913. doi: 10.1172/JCI106310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleer S. D., Chrystyn H., Foondun A. S. Measurement of the (R)- and (S)-isomers of warfarin in patients undergoing anticoagulant therapy. Chirality. 1992;4(8):488–493. doi: 10.1002/chir.530040806. [DOI] [PubMed] [Google Scholar]
- Mungall D. R., Ludden T. M., Marshall J., Hawkins D. W., Talbert R. L., Crawford M. H. Population pharmacokinetics of racemic warfarin in adult patients. J Pharmacokinet Biopharm. 1985 Jun;13(3):213–227. doi: 10.1007/BF01065653. [DOI] [PubMed] [Google Scholar]
- Murray B., Coleman R., McWaters D., Ludden T., Mungall D. Pharmacodynamics of warfarin at steady state. Ther Drug Monit. 1987;9(1):1–5. doi: 10.1097/00007691-198703000-00001. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Stevenson I. H., Ward C. A., Wood A. J., Crooks J. Determinants of anticoagulant control in patients receiving warfarin. Br J Clin Pharmacol. 1977 Jun;4(3):309–314. doi: 10.1111/j.1365-2125.1977.tb00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. A. Studies on the coumarin anticoagulant drugs: interaction of human plasma albumin and warfarin sodium. J Clin Invest. 1967 May;46(5):829–837. doi: 10.1172/JCI105582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly R. A. Studies on the optical enantiomorphs of warfarin in man. Clin Pharmacol Ther. 1974 Aug;16(2):348–354. doi: 10.1002/cpt1974162348. [DOI] [PubMed] [Google Scholar]
- Park B. K. Warfarin: metabolism and mode of action. Biochem Pharmacol. 1988 Jan 1;37(1):19–27. doi: 10.1016/0006-2952(88)90750-2. [DOI] [PubMed] [Google Scholar]
- Routledge P. A., Chapman P. H., Davies D. M., Rawlins M. D. Pharmacokinetics and pharmacodynamics of warfarin at steady state. Br J Clin Pharmacol. 1979 Sep;8(3):243–247. doi: 10.1111/j.1365-2125.1979.tb01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd A. M., Hewick D. S., Moreland T. A., Stevenson I. H. Age as a determinant of sensitivity to warfarin. Br J Clin Pharmacol. 1977 Jun;4(3):315–320. doi: 10.1111/j.1365-2125.1977.tb00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty H. G., Fennerty A. G., Routledge P. A. Clinical pharmacokinetic considerations in the control of oral anticoagulant therapy. Clin Pharmacokinet. 1989 Apr;16(4):238–253. doi: 10.2165/00003088-198916040-00003. [DOI] [PubMed] [Google Scholar]
- Toon S., Low L. K., Gibaldi M., Trager W. F., O'Reilly R. A., Motley C. H., Goulart D. A. The warfarin-sulfinpyrazone interaction: stereochemical considerations. Clin Pharmacol Ther. 1986 Jan;39(1):15–24. doi: 10.1038/clpt.1986.3. [DOI] [PubMed] [Google Scholar]
- Tozer T. N., Gambertoglio J. G., Furst D. E., Avery D. S., Holford N. H. Volume shifts and protein binding estimates using equilibrium dialysis: application to prednisolone binding in humans. J Pharm Sci. 1983 Dec;72(12):1442–1446. doi: 10.1002/jps.2600721218. [DOI] [PubMed] [Google Scholar]
- Wong Y. W., Davis P. J. Analysis of warfarin and its metabolites by reversed-phase ion-pair liquid chromatography with fluorescence detection. J Chromatogr. 1989 May 19;469:281–291. doi: 10.1016/s0021-9673(01)96463-5. [DOI] [PubMed] [Google Scholar]
- Yacobi A., Udall J. A., Levy G. Serum protein binding as a determinant of warfarin body clearance and anticoagulant effect. Clin Pharmacol Ther. 1976 May;19(5 Pt 1):552–558. doi: 10.1002/cpt1976195part1552. [DOI] [PubMed] [Google Scholar]


