Abstract
The Epstein-Barr virus immediate-early protein BZLF1 is a transcriptional activator that mediates the switch from latent to lytic infection. Here we demonstrate that BZLF1 induces both a G2 block and a mitotic block in HeLa cells and inhibits chromosome condensation. While the G2 block is associated with decreased cyclin B1 in host cells and can be rescued by overexpression of cyclin B1, the mechanism for the mitotic defect is as yet undetermined.
Epstein-Barr virus (EBV) is a gamma herpesvirus that infects approximately 85% of the population. EBV causes infectious mononucleosis and is also associated with nasopharyngeal carcinoma, Burkitt's lymphoma, Hodgkin's disease, and B-cell lymphomas in immunocompromised patients (25, 34). The EBV immediate-early protein BZLF1 is a transcriptional activator which binds to Ap1-like motifs in the promoters of early lytic viral genes and induces the switch from the latent to lytic life cycle in EBV (5, 7, 13, 36, 41).
Many viruses manipulate the host cell environment, in particular cell cycle progression, as a mechanism to enhance viral replication (39). Small DNA tumor viruses push cells into the S phase (38), while herpesviruses have been found to arrest cells in all phases of the cell cycle (8, 11, 22, 27, 37, 38). The cell cycle effects of herpesviruses are commonly mediated by the immediate-early proteins (20, 22, 26, 30, 46, 47). In the case of EBV, although BZLF1 expression in HeLa cells was reported to induce primarily a G1/G0 block, expression in U-2 OS cells induced a G2/M block (3, 4). In addition, when the Burkitt's lymphoma cell line Rael was induced into the lytic form of EBV infection using azacytidine, the BZLF1-positive subset of cells appeared to be blocked in the G2/M component of the cell cycle (35). However, the mechanism(s) by which BZLF1 induces a G2/M block in certain cell types have not been previously explored, nor is it known whether BZLF1 induces a G2 block, a mitotic block, or both.
The effect of BZLF1 expression on cell cycle progression in various cell types was examined using an adenovirus vector expressing BZLF1 (AdBZLF1) constructed as previously described (44). Cells were either mock infected, infected with AdLacZ (a control adenovirus vector expressing LacZ), or infected with AdBZLF1 at a titer equal to that of AdLacZ. Cells were harvested at various time points after infection and processed for cell cycle analysis as previously described (40). We have demonstrated that the cellular level of BZLF1 expression in HeLa cells infected with AdBZLF1 at a multiplicity of infection (MOI) of 50 is similar to that in the lytically infected population of EBV-positive gastric carcinoma AGS cells (29).
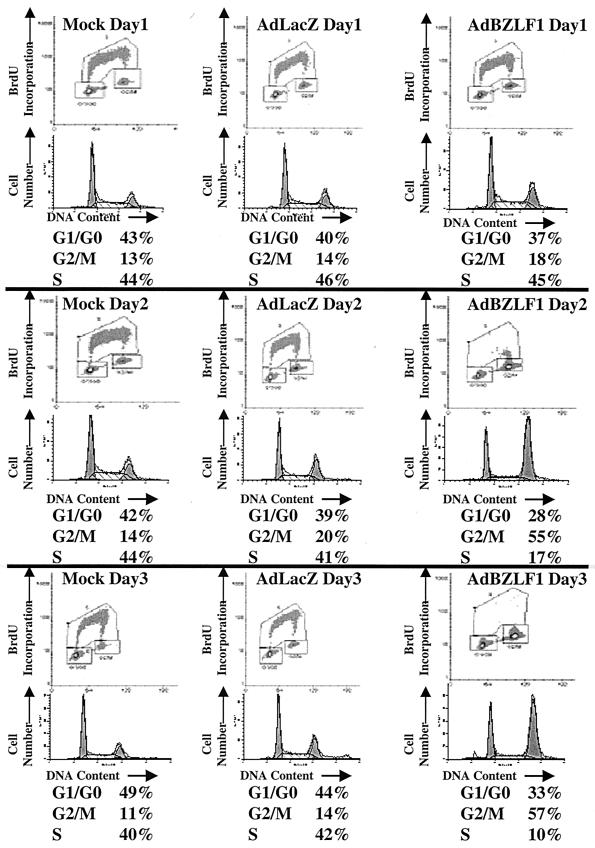
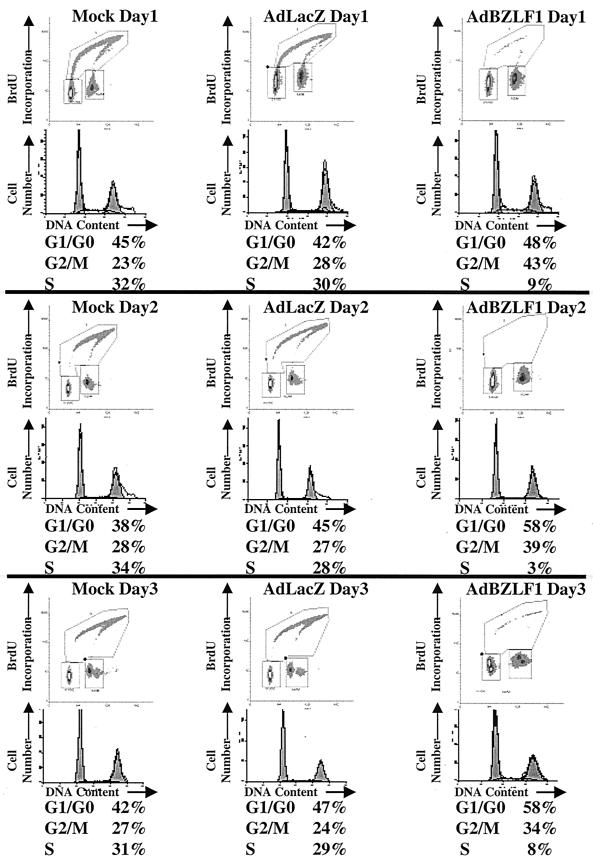
Although Cayrol and Flemington reported that BZLF1 expression using a plasmid vector in HeLa cells induced primarily a G1/S block (3, 4), we found that infection of HeLa cells with the AdBZLF1 vector resulted in primarily a G2/M block (Fig. 1). In contrast, infection of asynchronously growing normal human fibroblasts with the AdBZLF1 vector induced both a G1/S block and a G2/M block (Fig. 2). These results indicate that BZLF1 expression induces a G2/M block in at least some cell types.
FIG. 1.
BZLF1 causes a G2/M block in HeLa cells. Cells were mock infected or infected with the AdLacZ or AdBZLF1 vector (MOI of 50). Cell cycle was examined at various time points after infection by quantitating both bromodeoxyuridine (BrdU) incorporation and propidium iodine staining as previously described (40) using FACS analysis and a BrdU antibody.
FIG. 2.
BZLF1 causes both a G1/G0 block and a G2/M block in normal human fibroblasts. Normal human fibroblasts were infected with the AdLacZ or AdBZLF1 vector using an MOI of 250, which produces a level of cellular BZLF1 expression similar to that produced by an MOI of 50 in HeLa cells (data not shown). The cell cycle stage was determined at various time points after infection.
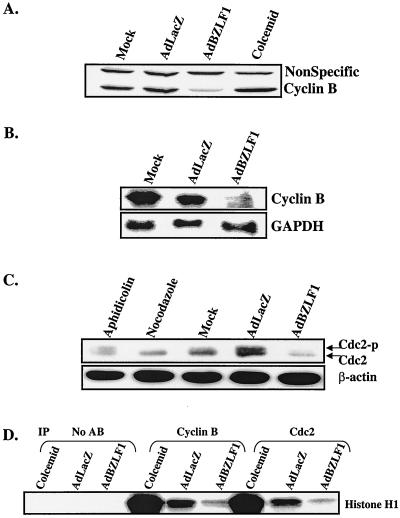
To further define the mechanism(s) by which BZLF1 expression results in a G2/M block in HeLa cells, we examined the levels of the cyclin B1 and cdc2 kinase proteins using immunoblot analysis as previously described (40) using antibodies specific to cyclin B1 and cdc2 (both a gift from Yue Xiong, University of North Carolina at Chapel Hill). The progression of cells from G2 to mitosis is orchestrated by the cyclin-dependent kinase cdc2 and cyclin B1 (32). BZLF1 expression in HeLa cells reduced the level of the cyclin B1 protein markedly (Fig. 3A) and, to a lesser extent, cdc2 (Fig. 3C). We also examined the level of cyclin B1 RNA in HeLa cells infected with the AdLacZ or AdBZLF1 vector (Fig. 3B). Northern blot analysis was performed as previously described (29) using a randomly primed 32P-labeled DNA probe containing the cyclin B1 sequence. AdBZLF1-infected HeLa cells had a greatly reduced cyclin B1 transcript level compared to AdLacZ-infected cells, while a control transcript, glyceraldehyde-3-phosphate dehydrogenase, was unaffected. These results suggest that BZLF1 reduces cyclin B1 at least partially at the RNA level, either by decreasing transcription or decreasing the stability of the cyclin B1 message.
FIG. 3.
BZLF1 decreases cyclin B1. HeLa cells were mock infected or infected with the AdLacZ vector or the AdBZLF1 vector. Two days postinfection, whole-cell extracts and RNA were prepared. (A) Immunoblot analysis was performed using an antibody against cyclin B1. (B) Northern blot analysis was performed using probes directed against cyclin B1 or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (C) Immunoblot analysis was performed to quantitate the expression and phosphorylation status of cdc2. Cells were also treated with aphidicolin and nocodazole, which block cells in S phase and M phase, respectively. Cdc2-p, phosphorylated cdc2. (D) Cyclin B1- and cdc2-associated kinase activity was quantitated as previously described (10) by immunoprecipitating (IP) extracts with cdc2 or cyclin B1 antibodies as indicated and then performing kinase assays using a histone H1 substrate. No AB, no antibodies.
cdc2 activity is inhibited when the protein is phosphorylated at two specific sites (threonine 14 and tyrosine 15) (9). Although BZLF1-expressing HeLa cells had less total cdc2 than AdLacZ-infected cells, most of the protein appeared to be in the active (unphosphorylated) form (Fig. 3C). Extracts from cells treated with nocodazole (which blocks cells in mitosis) served as a control for the dephosphorylated form of cdc2. The level of a control cellular protein, β-actin, was not affected by BZLF1. These results suggest that BZLF1 induces a G2 block in HeLa cells by decreasing the level of cyclin B1, as well as possibly cdc2.
To confirm that the cdc2- and cyclin B1-associated kinase activities are reduced in BZLF1-expressing HeLa cells, we performed immunoprecipitations of the AdLacZ- and AdBZLF1-infected HeLa cell extracts using a control antibody or cyclin B1 and cdc2 antibodies, and examined the ability of the immunoprecipitated proteins to phosphorylate the histone H1 substrate as previously described (10) (Fig. 3D). Extracts from HeLa cells treated with colcemid (which blocks cells in mitosis) served as a positive control in these experiments. Both the cyclin B1- and cdc2-associated kinase activities were greatly reduced in the AdBZLF1-infected HeLa cells compared to the AdLacZ-infected cells.
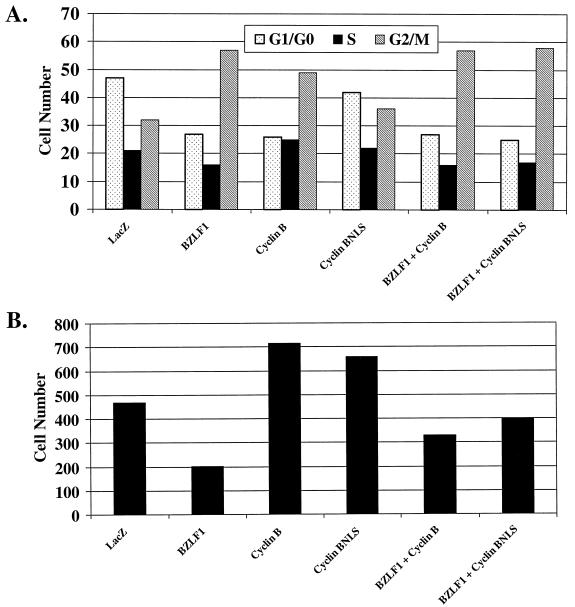
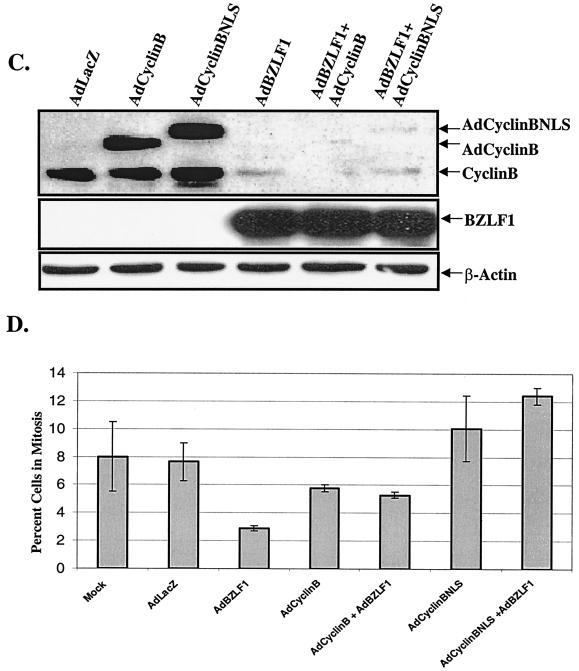
To determine whether the G2/M block in BZLF1-expressing HeLa cells is due to the lack of cyclin B1, HeLa cells were infected with the AdBZLF1 and AdLacZ vectors in the presence or absence of adenovirus vectors expressing cyclin B1 (a generous gift from David Morgan (23). One of these vectors expresses wild-type cyclin B1 (AdCyclinB), while the other expresses cyclin B1 fused to the simian virus 40 (SV40) nuclear localization signal (AdCyclinBNLS), allowing constitutive nuclear targeting of cyclin B1 independent of the cell cycle (23). Cells were first infected for 24 h with either the AdBZLF1 or AdLacZ vector and subsequently infected for an additional 24 h with either the AdLacZ or cyclin B vector. As shown in Fig. 4A, coinfection of HeLa cells with AdBZLF1 and either of the cyclin B1 vectors did not affect the number of cells in G2/M compared to infection with AdBZLF1 alone. However, since overexpression of cyclin B1 is known to induce a mitotic block (16, 21, 31), this effect may obscure its ability to rescue the BZLF1-induced G2 block unless the G2 and mitotic compartments are individually examined.
FIG. 4.
Cyclin B1 overexpression reverses the G2 block in AdBZLF1-infected HeLa cells. HeLa cells were infected with AdLacZ or AdBZLF1 or with an adenovirus vector expressing wild-type cyclin B1 (AdCyclinB) or cyclin B1 fused to the SV40 nuclear localization signal (AdCyclinBNLS) in the combinations indicated. (A) Cell cycle analysis was performed 2 days after infection as described in the legend to Fig. 1. (B) The number of cells in very early mitosis (in the same experiment shown in panel A) was quantitated by FACS analysis using an antibody directed against phosphorylated histone H3 (serine 10), combined with propidium iodine staining (to identify the G2/M compartment). A total of 28,000 cells were examined for each condition, and the total number of cells in very early mitosis for each condition is shown. (C) Immunoblot analysis was performed on cell extracts to quantitate the relative level of cyclin B in each condition. The adenovirus cyclin B proteins have lower mobility due to an epitope tag (23). (D) The percentages of cells specifically in mitosis in each condition from two separate experiments (average and range) were calculated as previously described (14).
Therefore, in the same experiment, we also examined the number of BZLF1-positive cells that had phosphorylation of histone H3 over serine 10 (a marker for early mitosis) (17) in the presence and absence of overexpressed cyclin B (Fig. 4B). The number of cells within the G2/M compartment of the cell cycle with phosphorylated histone H3 was quantitated by fluorescence-activated cell sorting (FACS) analysis as previously described (24) using an antibody that specifically recognizes histone H3 phosphorylated over serine 10 (catalog no. 06-570; Upstate Biotechnology), combined with propidium iodine staining. HeLa cells infected with AdBZLF1 alone had a lower number of G2/M cells with histone H3 phosphorylation than the AdLacZ-infected cells, and this effect was reversed by subsequent infection with the cyclin B adenovirus vectors. Immunoblot analysis of the same experiment (Fig. 4C) indicated that less cyclin B protein (both endogenous cyclin B and cyclin B derived from the adenovirus vectors) was present in cells infected with the BZLF1 vector versus cells infected with the AdLacZ vector. Since expression of cyclin B in the adenovirus vectors is driven by a heterologous promoter, BZLF1 may thus decrease cyclin B RNA stability (and possibly protein stability as well), rather than transcription.
To confirm that cyclin B reverses the BZLF1-induced G2 block, the percentage of total mitotic cells in two independent experiments was quantitated using propidium iodide staining in HeLa cells infected with the AdLacZ vector, the cyclin B1 vectors, the AdBZLF1 vector, or various combinations of these vectors (Fig. 4D). In comparison to cells infected with AdLacZ, HeLa cells expressing BZLF1 alone had fewer cells in mitosis (3 versus 8%), again suggesting that BZLF1 primarily induces a G2, rather than a mitotic, block in this cell type. When cells were coinfected with AdBZLF1 and either of the cyclin B1 vectors, the number of cells in mitosis was increased compared to cells infected with AdBZLF1 alone, particularly in the case of the AdCyclinBNLS construct. Thus, it appears that the G2 block caused by BZLF1 can be reversed by reconstituting cyclin B1 expression. However, the mitotic block caused by overexpression of cyclin B1 alone did not allow us to assess the effect of BZLF1 on mitosis in these experiments.
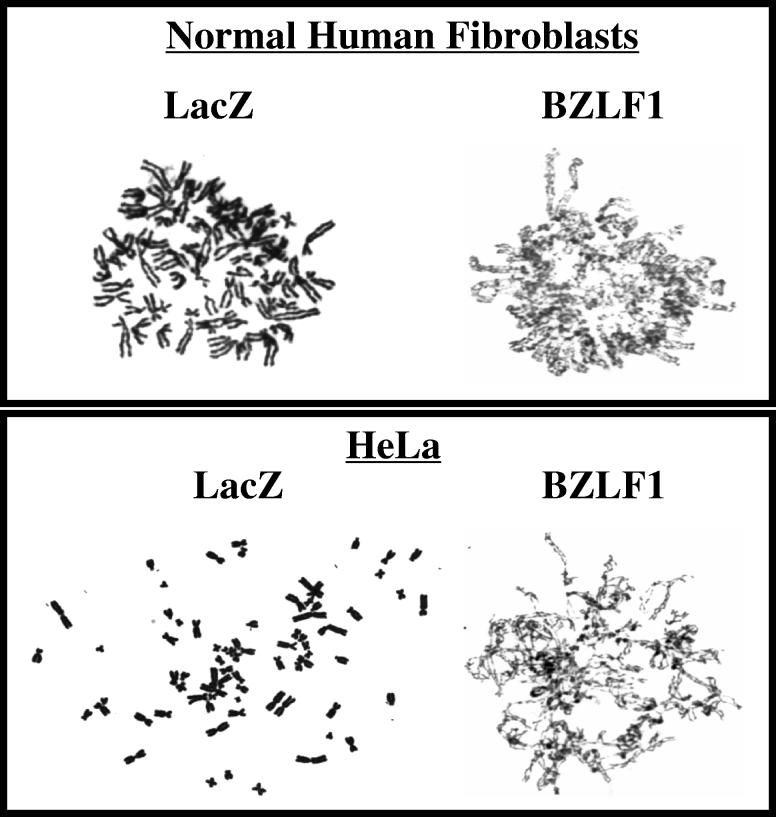
To further examine the effect of BZLF1 on mitosis in HeLa cells and normal human fibroblasts, the chromosomes in AdLacZ-infected and AdBZLF1-infected cells were examined as previously described using Giemsa staining (14). As shown in Fig. 5, in both of these cell types, the AdBZLF1-infected cells, but not the AdLacZ-infected cells, had a substantial number of cells in which mitosis appeared to be blocked due to a defect in chromosomal condensation and detangling. This defect was observed in 44% of the total metaphase population in BZLF1-infected normal human fibroblasts compared to only 4% of the total metaphase population in AdLacZ-infected normal human fibroblasts. Similar results were obtained in HeLa cells.
FIG. 5.
BZLF1 expression causes incomplete chromosomal condensation and detangling in HeLa cells and normal human fibroblasts. Cells were infected with AdLacZ or AdBZLF1 and mitotic figures were examined as previously described (14) 2 days postinfection.
In this report we have examined the mechanism(s) by which the EBV immediate-early protein BZLF1 induces a G2/M block in HeLa cells. We demonstrate that BZLF1 decreases the cyclin B1 transcript level, as well as the cyclin B1 protein level, in HeLa cells. Furthermore, we show that cyclin B1 overexpression reverses the BZLF1-mediated G2 block, suggesting that inhibition of cyclin B1 is the primary mechanism for this effect. Our results here also document for the first time that BZLF1 inhibits mitosis in both HeLa cells and normal human fibroblasts. This mitotic defect, which is characterized by incomplete chromosomal condensation and detangling, is reminiscent of the cell cycle block induced by inhibition of topoisomerase II (6). The herpes simplex virus ICP0 protein also induces a mitotic block, although in this case the defect is characterized by a “pseudoprometaphase” phenotype (12).
Rodriguez et al. previously reported that the lytically infected population of Rael Burkitt's lymphoma cells has a higher proportion of cells in G2/M than does the latently infected population (35). Therefore, the results obtained here using the BZLF1 adenovirus vector in EBV-negative cells may be relevant to the cell cycle effects occurring during authentic lytic EBV infection in some cell types. Although our results here clearly show that BZLF1 induces a G2/M block in certain cell types, the BZLF1 cell cycle effects appear to be cell type dependent (A. Mauser, E. Holley-Guthrie, A. Zanation, W. Yarborough, W. Kaufmann, A. Klingelhutz, and S. Kenney, unpublished data). Interestingly, we recently found that BZLF1 expression in telomerase-immortalized keratinocytes actually enhances expression of some S-phase-specific proteins (Mauser et al., unpublished), and the EBV-induced lesion, oral hairy leukoplakia, which contains the lytic form of EBV infection, appears to be a hyperproliferative lesion (43). Therefore, blocking the host cell cycle may be more advantageous for EBV lytic replication in some cell types.
Similar to the effect of lytic EBV infection, infection of some cell types with herpes simplex virus type 1 and human cytomegalovirus likewise induces a G2/M block which is at least partially due to the effects of the immediate-early proteins ICP0 and IE2-86, respectively (1, 2, 22, 26). Interestingly, a variety of other viral proteins have also been found to induce a G2/M block, including SV40 small-t antigen (15, 45), human parvovirus B19 (28), human immunodeficiency virus type 1 vpr (18, 19, 33), and a vaccinia virus-encoded protein (42). To our knowledge, BZLF1 is unique among these viral proteins in its ability to induce a G2 block by decreasing cyclin B1. The finding that so many different viruses block cells in G2/M suggests that either this stage of the cell cycle is particularly advantageous for viral replication and/or that inhibition of cellular DNA replication enhances the ability of some viruses to replicate.
Acknowledgments
This work was supported in part by Public Health Service grants RO1-CA58853 (to S.K.), RO1-CA81343, and RO1-CA42765 (both to W.K.) from the National Institutes of Health.
We thank Christine Campbell for valuable assistance in preparing the chromosomal spreads.
REFERENCES
- 1.Advani, S. J., R. Brandimarti, R. R. Weichselbaum, and B. Roizman. 2000. The disappearance of cyclins A and B and the increase in activity of the G2/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the α22/US1.5 and UL13 viral genes. J. Virol. 74:8-15. [PMC free article] [PubMed] [Google Scholar]
- 2.Castillo, J. P., A. D. Yurochko, and T. F. Kowalik. 2000. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 74:8028-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cayrol, C., and E. Flemington. 1996. G0/G1 growth arrest mediated by a region encompassing the basic leucine zipper (bZIP) domain of the Epstein-Barr virus transactivator Zta. J. Biol. Chem. 271:31799-31802. [DOI] [PubMed] [Google Scholar]
- 4.Cayrol, C., and E. K. Flemington. 1996. The Epstein-Barr virus bZIP transcription factor Zta causes G0/G1 cell cycle arrest through induction of cyclin-dependent kinase inhibitors. EMBO J. 15:2748-2759. [PMC free article] [PubMed] [Google Scholar]
- 5.Chevallier-Greco, A., E. Manet, P. Chavrier, C. Mosnier, J. Daillie, and A. Sergeant. 1986. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 5:3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke, D. J., R. T. Johnson, and C. S. Downes. 1993. Topoisomerase II inhibition prevents anaphase chromatid segregation in mammalian cells independently of the generation of DNA strand breaks. J. Cell Sci. 105:563-569. [DOI] [PubMed] [Google Scholar]
- 7.Countryman, J., and G. Miller. 1985. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc. Natl. Acad. Sci. USA 82:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittmer, D., and E. S. Mocarski. 1997. Human cytomegalovirus infection inhibits G1/S transition. J. Virol. 71:1629-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draetta, G., and J. Eckstein. 1997. Cdc25 protein phosphatases in cell proliferation. Biochim. Biophys. Acta 1332:M53-M63. [DOI] [PubMed] [Google Scholar]
- 10.Draetta, G., F. Luca, J. Westendorf, L. Brizuela, J. Ruderman, and D. Beach. 1989. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell 56:829-838. [DOI] [PubMed] [Google Scholar]
- 11.Ehmann, G. L., T. I. McLean, and S. L. Bachenheimer. 2000. Herpes simplex virus type 1 infection imposes a G1/S block in asynchronously growing cells and prevents G1 entry in quiescent cells. Virology 267:335-349. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R. D., W. C. Earnshaw, J. Findlay, and P. Lomonte. 1999. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 18:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell, P. J., D. T. Rowe, C. M. Rooney, and T. Kouzarides. 1989. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 8:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filatov, L., V. Golubovskaya, J. C. Hurt, L. L. Byrd, J. M. Phillips, and W. K. Kaufmann. 1998. Chromosomal instability is correlated with telomere erosion and inactivation of G2 checkpoint function in human fibroblasts expressing human papillomavirus type 16 E6 oncoprotein. Oncogene 16:1825-1838. [DOI] [PubMed] [Google Scholar]
- 15.Gaillard, S., K. M. Fahrbach, R. Parkati, and K. Rundell. 2001. Overexpression of simian virus 40 small-T antigen blocks centrosome function and mitotic progression in human fibroblasts. J. Virol. 75:9799-9807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallant, P., and E. A. Nigg. 1992. Cyclin B2 undergoes cell cycle-dependent nuclear translocation and, when expressed as a non-destructible mutant, causes mitotic arrest in HeLa cells. J. Cell Biol. 117:213-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hans, F., and S. Dimitrov. 2001. Histone H3 phosphorylation and cell division. Oncogene 20:3021-3027. [DOI] [PubMed] [Google Scholar]
- 18.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henklein, P., K. Bruns, M. P. Sherman, U. Tessmer, K. Licha, J. Kopp, C. M. de Noronha, W. C. Greene, V. Wray, and U. Schubert. 2000. Functional and structural characterization of synthetic HIV-1 Vpr that transduces cells, localizes to the nucleus, and induces G2 cell cycle arrest. J. Biol. Chem. 275:32016-32026. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs, W. E., and N. A. DeLuca. 1999. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J. Virol. 73:8245-8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holloway, S. L., M. Glotzer, R. W. King, and A. W. Murray. 1993. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73:1393-1402. [DOI] [PubMed] [Google Scholar]
- 22.Jault, F. M., J. M. Jault, F. Ruchti, E. A. Fortunato, C. Clark, J. Corbeil, D. D. Richman, and D. H. Spector. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J. Virol. 69:6697-6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin, P., S. Hardy, and D. O. Morgan. 1998. Nuclear localization of cyclin B1 controls mitotic entry after DNA damage. J. Cell Biol. 141:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juan, G., F. Traganos, W. M. James, J. M. Ray, M. Roberge, D. M. Sauve, H. Anderson, and Z. Darzynkiewicz. 1998. Histone H3 phosphorylation and expression of cyclins A and B1 measured in individual cells during their progression through G2 and mitosis. Cytometry 32:71-77. [DOI] [PubMed] [Google Scholar]
- 25.Kieff, E. 1996. Epstein-Barr virus and its replication, p. 2343-2395. In B. N. Fields, D. N. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 26.Lomonte, P., and R. D. Everett. 1999. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J. Virol. 73:9456-9467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu, M., and T. Shenk. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J. Virol. 70:8850-8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita, E., K. Tada, H. Chisaka, H. Asao, H. Sato, N. Yaegashi, and K. Sugamura. 2001. Human parvovirus B19 induces cell cycle arrest at G2 phase with accumulation of mitotic cyclins. J. Virol. 75:7555-7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison, T. E., A. Mauser, A. Wong, J. P. Ting, and S. C. Kenney. 2001. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 30.Murphy, E. A., D. N. Streblow, J. A. Nelson, and M. F. Stinski. 2000. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J. Virol. 74:7108-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray, A. W., M. J. Solomon, and M. W. Kirschner. 1989. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339:280-286. [DOI] [PubMed] [Google Scholar]
- 32.Parker, L. L., and H. Piwnica-Worms. 1992. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science 257:1955-1957. [DOI] [PubMed] [Google Scholar]
- 33.Re, F., D. Braaten, E. K. Franke, and J. Luban. 1995. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34cdc2-cyclin B. J. Virol. 69:6859-6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 35.Rodriguez, A., E. J. Jung, and E. K. Flemington. 2001. Cell cycle analysis of Epstein-Barr virus-infected cells following treatment with lytic cycle-inducing agents. J. Virol. 75:4482-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rooney, C. M., D. T. Rowe, T. Ragot, and P. J. Farrell. 1989. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J. Virol. 63:3109-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salvant, B. S., E. A. Fortunato, and D. H. Spector. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J. Virol. 72:3729-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song, B., J. J. Liu, K. C. Yeh, and D. M. Knipe. 2000. Herpes simplex virus infection blocks events in the G1 phase of the cell cycle. Virology 267:326-334. [DOI] [PubMed] [Google Scholar]
- 39.Swanton, C., and N. Jones. 2001. Strategies in subversion: de-regulation of the mammalian cell cycle by viral gene products. Int. J. Exp. Pathol. 82:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swenson, J. J., A. E. Mauser, W. K. Kaufmann, and S. C. Kenney. 1999. The Epstein-Barr virus protein BRLF1 activates S-phase entry through E2F1 induction. J. Virol. 73:6540-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takada, K., N. Shimizu, S. Sakuma, and Y. Ono. 1986. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J. Virol. 57:1016-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wali, A., and D. S. Strayer. 1999. Infection with vaccinia virus alters regulation of cell cycle progression. DNA Cell Biol. 18:837-843. [DOI] [PubMed] [Google Scholar]
- 43.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westphal, E. M., A. Mauser, J. Swenson, M. G. Davis, C. L. Talarico, and S. C. Kenney. 1999. Induction of lytic Epstein-Barr virus (EBV) infection in EBV-associated malignancies using adenovirus vectors in vitro and in vivo. Cancer Res. 59:1485-1491. [PubMed] [Google Scholar]
- 45.Whalen, B., J. Laffin, T. D. Friedrich, and J. M. Lehman. 1999. SV40 small T antigen enhances progression to G2 during lytic infection. Exp. Cell Res. 251:121-127. [DOI] [PubMed] [Google Scholar]
- 46.Wiebusch, L., and C. Hagemeier. 1999. Human cytomegalovirus 86-kilodalton IE2 protein blocks cell cycle progression in G1. J. Virol. 73:9274-9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiebusch, L., and C. Hagemeier. 2001. The human cytomegalovirus immediate early 2 protein dissociates cellular DNA synthesis from cyclin-dependent kinase activation. EMBO J. 20:1086-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]