Abstract
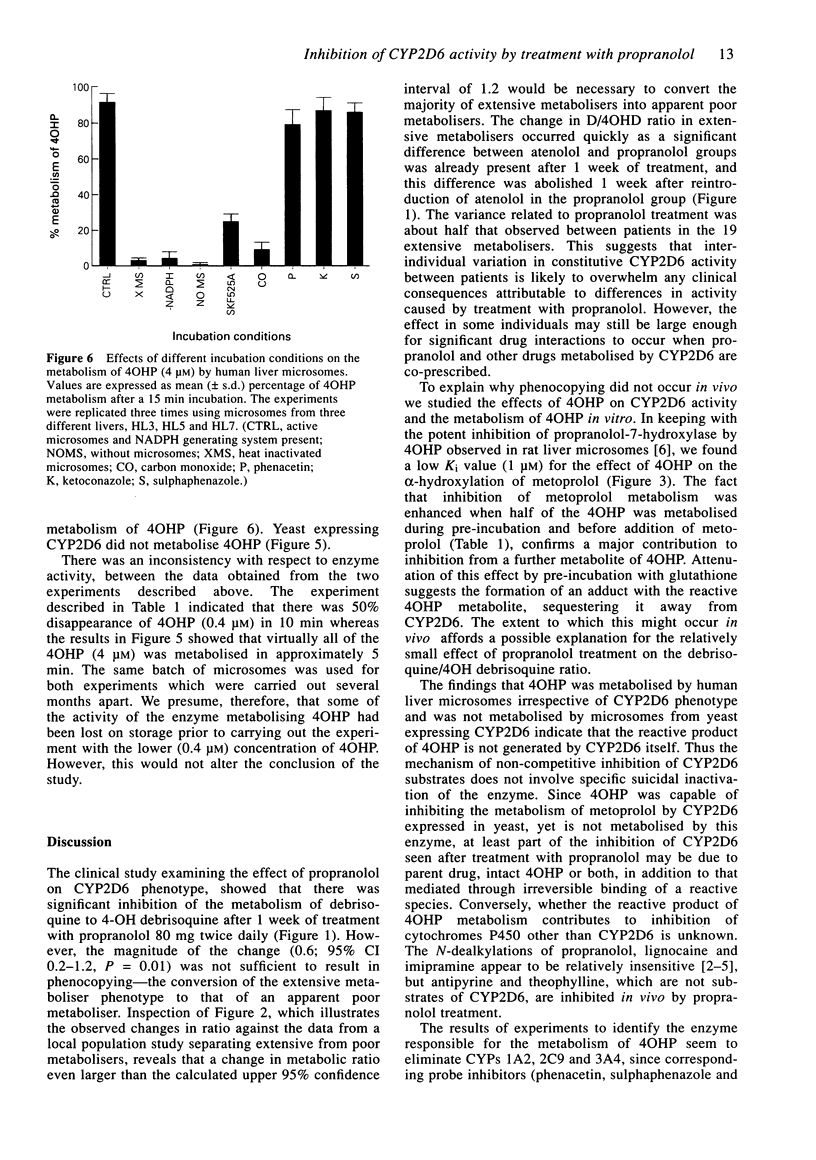
1. The 4-hydroxylation of propranolol by rat and human liver microsomes is associated with formation of a chemically reactive species which binds irreversibly to cytochrome P4502D6 (CYP2D6) destroying its catalytic function. Therefore, the effect of propranolol treatment (80 mg twice daily) on debrisoquine phenotype was examined, to see if it resulted in phenocopying in vivo. The role of 4-hydroxypropranolol (4OHP) in the inhibition of CYP2D6 activity was also studied using microsomes from yeast expressing CYP2D6 and from human livers; metoprolol was used as the CYP2D6 substrate. 2. Although a significant effect on apparent oxidation phenotype was demonstrated, the absolute change in the urinary debrisoquine/4-hydroxydebrisoquine ratio (D/4HD) was small, such that no extensive metaboliser who received propranolol treatment was reclassified as a poor metaboliser. The in vitro studies indicated that 4OHP is a potent inhibitor of metoprolol metabolism (Ki approximately 1 microM). This inhibitory effect was enhanced when 4OHP was pre-incubated in the presence of a NADPH generating system and human liver microsomes. The effect was decreased significantly when reduced glutathione was added to the pre-incubation mixture. Metabolism of 4OHP occurred when incubated with human liver microsomes in the presence of a NADPH generating system and irrespective of CYP2D6 phenotype; yeast expressing CYP2D6 did not metabolise 4OHP. 3. We conclude that, although treatment with propranolol 80 mg twice daily significantly decreases the catalytic function of CYP2D6, the inhibition is insufficient to result in phenocopying. The reactive intermediate produced by further metabolism of 4OHP is probably scavenged effectively in vivo by glutathione and other nucleophiles.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bax N. D., Lennard M. S., Tucker G. T. Inhibition of antipyrine metabolism by beta-adrenoceptor antagonists. Br J Clin Pharmacol. 1981 Dec;12(6):779–784. doi: 10.1111/j.1365-2125.1981.tb01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching M. S., Lennard M. S., Tucker G. T., Woods H. F., Kelly D. E., Kelly S. L. The expression of human cytochrome P450IA1 in the yeast Saccharomyces cerevisiae. Biochem Pharmacol. 1991 Jul 25;42(4):753–758. doi: 10.1016/0006-2952(91)90032-z. [DOI] [PubMed] [Google Scholar]
- Dybing E., Nelson S. D., Mitchell J. R., Sasame H. A., Gillette J. R. Oxidation of alpha-methyldopa and other catechols by cytochrome P-450-generated superoxide anion: possible mechanism of methyldopa hepatitis. Mol Pharmacol. 1976 Nov;12(6):911–920. [PubMed] [Google Scholar]
- Ellis S. W., Ching M. S., Watson P. F., Henderson C. J., Simula A. P., Lennard M. S., Tucker G. T., Woods H. F. Catalytic activities of human debrisoquine 4-hydroxylase cytochrome P450 (CYP2D6) expressed in yeast. Biochem Pharmacol. 1992 Aug 18;44(4):617–620. doi: 10.1016/0006-2952(92)90394-x. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Kumagai Y., Unger S. E., Cho A. K. Metabolism of methylenedioxymethamphetamine: formation of dihydroxymethamphetamine and a quinone identified as its glutathione adduct. J Pharmacol Exp Ther. 1990 Aug;254(2):521–527. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lennard M. S., Jackson P. R., Freestone S., Tucker G. T., Ramsay L. E., Woods H. F. The relationship between debrisoquine oxidation phenotype and the pharmacokinetics and pharmacodynamics of propranolol. Br J Clin Pharmacol. 1984 Jun;17(6):679–685. doi: 10.1111/j.1365-2125.1984.tb02403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard M. S., Silas J. H., Smith A. J., Tucker G. T. Determination of debrisoquine and its 4-hydroxy metabolite in biological fluids by gas chromatography with flame-ionization and nitrogen-selective detection. J Chromatogr. 1977 Mar 11;133(1):161–166. doi: 10.1016/s0021-9673(00)89216-x. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y., Fujita S., Chiba M., Kagimoto N., Umeda S., Suzuki T. Impairment of debrisoquine 4-hydroxylase and related monooxygenase activities in the rat following treatment with propranolol. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):861–865. doi: 10.1016/0006-2952(91)90189-c. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y., Suzuki K., Fujita S., Suzuki T. A possible mechanism of the impairment of hepatic microsomal monooxygenase activities after multiple administration of propranolol in rats. Biochem Pharmacol. 1992 Feb 18;43(4):757–762. doi: 10.1016/0006-2952(92)90240-j. [DOI] [PubMed] [Google Scholar]
- Otton S. V., Crewe H. K., Lennard M. S., Tucker G. T., Woods H. F. Use of quinidine inhibition to define the role of the sparteine/debrisoquine cytochrome P450 in metoprolol oxidation by human liver microsomes. J Pharmacol Exp Ther. 1988 Oct;247(1):242–247. [PubMed] [Google Scholar]
- Otton S. V., Gillam E. M., Lennard M. S., Tucker G. T., Woods H. F. Propranolol oxidation by human liver microsomes--the use of cumene hydroperoxide to probe isoenzyme specificity and regio- and stereoselectivity. Br J Clin Pharmacol. 1990 Nov;30(5):751–760. doi: 10.1111/j.1365-2125.1990.tb03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B. K. Metabolic basis of adverse drug reactions. J R Coll Physicians Lond. 1986 Jul;20(3):195–200. [PMC free article] [PubMed] [Google Scholar]
- Schneck D. W., Pritchard J. F. The inhibitory effect of propranolol pretreatment on its own metabolism in the rat. J Pharmacol Exp Ther. 1981 Sep;218(3):575–581. [PubMed] [Google Scholar]
- Shaw L., Lennard M. S., Tucker G. T., Bax N. D., Woods H. F. Irreversible binding and metabolism of propranolol by human liver microsomes--relationship to polymorphic oxidation. Biochem Pharmacol. 1987 Jul 15;36(14):2283–2288. doi: 10.1016/0006-2952(87)90592-2. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Ishida R., Matsui S., Masubuchi Y., Narimatzu S. Kinetic analysis of mutual metabolic inhibition of lidocaine and propranolol in rat liver microsomes. Biochem Pharmacol. 1993 Apr 6;45(7):1528–1530. doi: 10.1016/0006-2952(93)90055-2. [DOI] [PubMed] [Google Scholar]
- Walle T., Conradi E. C., Walle U. K., Gaffney T. E. O-methylated catechol-like metabolites of propranolol in man. Drug Metab Dispos. 1978 Jul-Aug;6(4):481–487. [PubMed] [Google Scholar]
- al-Asady S. A., Black G. L., Lennard M. S., Tucker G. T., Woods H. F. Inhibition of lignocaine metabolism by beta-adrenoceptor antagonists in rat and human liver microsomes. Xenobiotica. 1989 Sep;19(9):929–944. doi: 10.3109/00498258909043152. [DOI] [PubMed] [Google Scholar]


