Abstract
The α0 genes of herpes simplex virus 1 (HSV-1) contain three exons. Earlier studies have shown that the substitution of genomic sequences with a cDNA copy does not alter the capacity of the virus to replicate or establish latent infection. Other studies have demonstrated that HSV-1 may express alternatively spliced forms of α0 transcripts. The studies reported here centered on a mutant HSV-1(vCPc0) strain in which the genomic copies of the α0 gene were replaced with cDNA copies. From our research, we report the following observations. (i) In contrast to events transpiring in cells infected with wild-type virus, the expression of HSV-1(vCPc0) genes was delayed or restricted to α genes for many hours in rabbit skin cells and to a lesser extent in HEp-2 cells but not in Vero cells. This delay in the expression of HSV-1(vCPc0) β or γ genes was also multiplicity of infection dependent. (ii) Exposure to MG132, a proteasomal inhibitor, before infection with wild-type virus had no significant effect on the accumulation of viral proteins in Vero cells and caused an only slight delay in viral gene expression in rabbit skin cells in a multiplicity of infection-dependent fashion. The drug had no effect when it was added to the medium 3 h after infection. (iii) Rabbit skin or HEp-2 cells exposed to MG132 3 h after infection with the HSV-1(vCPc0) mutant accumulated only α proteins. This restriction was cell type dependent but not multiplicity of infection dependent. (iv) Both the delay in the expression of β and γ genes and the effect of MG132 added to the medium 3 h after infection were rescued by restoration of the intron 1 sequences in the HSV-1(vCPc0) mutant. However, cells transduced by baculoviruses expressing intron 1 RNA did not complement the HSV-1(vCPc0) mutant, suggesting that the function of intron 1 is in cis rather than in trans. We came to the following conclusions as a result. (i) Post-α gene expression requires the involvement of the proteasomal pathway in a cell type-dependent manner. Consistent with this requirement, the proapoptotic functions of MG132 are blocked in cells infected before exposure to the drug but not after exposure. (ii) A function encoded by the α0 gene that is absent from the cDNA copy is required for viral gene expression in a cell type- and multiplicity of infection-dependent fashion. The absence of this master function delays but does not ultimately block viral gene expression in the cell lines tested here.
The herpes simplex virus 1 (HSV-1) genome encodes very few genes whose mRNA is spliced. Most of these genes, i.e., α0, α22, and α47, are expressed immediately after infection and therefore before the splicing of mRNA is blocked by infected-cell protein no. 27 (ICP27) (43). A fourth gene, unique long sequence 15 (UL15), is expressed late in infection, after the splicing of mRNA is no longer suppressed or is at least suppressed at a reduced level (40). Of the four genes, two, α0 and UL15, contain three and two exons, respectively (8, 35). A question that has never been satisfactorily resolved is why HSV encodes multiexonal genes given that the virus disfavors the splicing of mRNA. Indeed, recombinant viruses carrying substitutions of genomic DNAs with cDNA copies of the genes appear to replicate in a manner similar to that of the wild-type virus (4). Recombinant viruses in which the wild-type α0 gene was replaced by cDNA copies have been studied extensively without evidence that the substitution is detrimental to the growth of the virus (11, 31, 34). One hypothesis for the evolutionary retention of spliced UL15 and α0 genes is that these genes may allow for the alternative splicing of mRNAs or for the expression of one exon (UL15) or both the first and second exon (α0) of the gene. Indeed, evidence that the transcription of α0 may generate alternatively spliced mRNAs and that expression of only one exon of UL15 or both the first and second exons of α0 has been reported (5, 7, 12, 42, 47, 48). Yet the existence and function of these alternative forms of ICP0 contravenes the nearly compelling evidence that the HSV-1 cDNA copies could readily substitute for the spliced forms of these genes. In this report, we reexamine the question as to whether the genomic sequence, at least in the case of the α0 gene, encodes functions missing from the cDNA.
This report presents the results of two converging studies. In the course of the analysis of the requirement for proteasome-dependent proteolysis during viral infection, we noted that viral gene expression is delayed in cells exposed to the proteasomal inhibitor MG132 before infection with wild-type virus but not in cells exposed to the drug 3 h after infection. This observation suggests that the initiation of viral gene expression is facilitated by a proteasome-dependent step in both a multiplicity of infection (MOI)- and cell type-dependent manner. This conclusion was reinforced by the observation that HSV-1 precluded apoptosis induced by the exposure to MG132 after infection but not before infection. In a parallel series of studies, we noted that, in rabbit skin cells and, to a lesser extent, in HEp-2 cells, there was a delay in the expression of the β and γ genes of a mutant HSV-1(vCPc0) strain in which the genomic copies of the α0 gene were replaced with cDNA copies. This defect was not apparent in Vero cells and was both cell type and MOI dependent. In addition, in contrast to the events transpiring in cells infected with the wild-type virus, rabbit skin cells infected with the HSV-1(vCPc0) mutant and treated with MG132 accumulated only α proteins. This effect was also cell type dependent. Furthermore, both the delay in the accumulation of β and γ gene expression in rabbit skin cells infected with the HSV-1(vCPc0) mutant and the drastic effect of MG132 added at 3 h after infection were rescued by restoration of the intron 1 sequences in the HSV-1(vCPc0) mutant. These results suggest that α0 plays a key intron 1- and proteasome-dependent role in the transition from α gene expression to later gene expression. The implication of these studies is that the genomic form of the α0 gene encodes a function that is expressed transiently early in infection, that it is sensitive to MG132, and that its expression in cis is essential for viral gene expression.
Relevant to this report are the known products and functions of the α0 gene. The well-characterized product is a 775-residue multifunctional protein designated ICP0. This protein appears to interact with many viral and cellular proteins and to perform a myriad of functions (15, 21-23, 27, 28). In recent studies, ICP0 has been shown to be dynamically associated with proteasomes, to be bound to proteasomes in the presence of MG132, and to function as an E3 ubiquitin ligase (6, 18, 46). ICP0 mediates the degradation of several cellular proteins (13-15, 25, 26). Another product of the α0 gene that has received scant attention is ICP0R, the product resulting from the transcription of the first and second exons of the α0 gene. This protein has been reported to accumulate at low but detectable levels during the late stages of infection (12) and to have properties antithetic to ICP0 (44, 45, 47-49).
MATERIALS AND METHODS
Cells and viruses.
Vero and HEp-2 cells were obtained from the American Type Culture Collection, the human 143 TK− cell line was obtained from Carlo Croce, and rabbit skin cells were originally obtained from J. McClaren. Cells were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum (HEp-2, 143TK−, and rabbit skin cells) or 5% newborn calf serum (Vero cells). HSV-1(F) is the prototype HSV-1 strain used in this laboratory (9). HSV-1(vCPc0) has been described previously (34). HSV-1(KOS) and HSV-1 (17) were obtained from P. A. Schaffer and J. Subak Sharpe, respectively.
Construction of recombinant HSV-1(vCPc0)R.
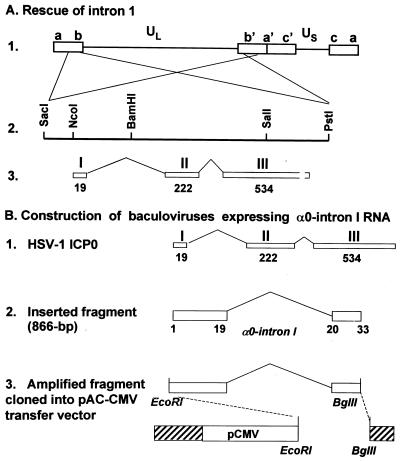
The 6.2-kb PstI-SacI fragment from plasmid pRB3710 (7) (Fig. 1A) containing the entire α0 gene of HSV-1(F) was used to repair HSV-1(vCPc0). Rabbit skin cell cultures in 25-cm2 flasks were cotransfected with the DNA of HSV-1(vCPc0) and purified plasmid DNA and were harvested at a 100% cytopathic effect. Dilutions of transfection cultures were plated on Vero cells to obtain isolated plaques. Single plaques were screened for the presence of a genomic α0 sequence by PCR with primers P6 (GCGACCCCCAGGGACCCTCC) and P14 (CACGGCGCACACGTCGCCCTCGT), which flank intron 1 of the α0 gene. Plaques that amplified the intron 1 sequence, giving rise to a 1,170-bp PCR product, were further purified and retested. One of these plaques, after four rounds of single-plaque purification and verification for the presence of the α0-intron 1 sequence from HSV-1(F), was amplified for further experiments. To confirm the identity of the recombinant virus, unique short sequence 11 (US11 sequence) was also amplified by PCR with primers P91 (CCGGAATTCATGAGCCAGACCCAACCC) and P92 (GGAAGATCTCTATACAGACCCGCGAG CCGTA) and the PCR product was compared with those amplified from HSV-1(F) and HSV-1(vCPc0) DNAs. The amplified products of the α0-intron 1 and US11 sequences from HSV-1(vCPc0)R had the same electrophoretic mobilities as those from HSV-1(F) and HSV-1(vCPc0), respectively (data not shown).
FIG. 1.
Schematic diagrams of the construction of recombinant virus in which the intron 1 sequences were restored and baculoviruses expressing α0-intron 1 RNA. (A) Recombinant HSV-1(vCPc0)R. Line 1, linear representation of the HSV-1 genome. The unique sequences are represented as the unique long (UL) and unique short (US) sequences. The terminal repeats flanking the unique sequences are shown as open rectangles with their designation letters above. Lines 2 and 3, enlargement of the domain of the α0 gene present in each of the b sequences and contained in the plasmid pRB3710. A purified SacI-PstI DNA fragment was used to rescue the α0-intron 1 missing from HSV-1(vCPc0). (B) Baculoviruses expressing α0-intron 1 sequences. Line 1, domains of HSV-1 ICP0. The three exons of the α0 gene are shown as open rectangles with their designation letters above and the number of amino acids below. Line 2, fragment of α0 used for the isolation of recombinant baculoviruses. The DNA fragment contains intron 1 and the first 33 codons of the α0 gene with the initiation ATG codon mutated to the GCA codon. Line 3, plasmids containing HSV-1 α0-intron 1 sequences used for generation of baculoviruses. The fragments, generated by PCR from viral DNAs of strain HSV-1(F), HSV-1(KOS), or HSV-1 (17), were digested with EcoRI and BglII restriction endonucleases, purified, and cloned into EcoRI- and BglII-digested pAc-CMV transfer vector.
Extraction of viral DNA and Southern blotting.
Viral DNAs used for the generation of recombinant viruses or amplification of HSV sequences for cloning purposes were purified by equilibrium centrifugation in NaI density gradients as previously described (36). For analysis of DNA synthesis, cultures in 25-cm2 flasks of Vero cells or rabbit skin cells were infected with 5 PFU of virus per cell and harvested at either 18 or 24 h after infection. Total viral DNA was isolated as previously described (5, 37), digested with BamHI restriction endonuclease, electrophoresed on 0.7% agarose gels, transferred to zeta-probe membranes, and hybridized with the radiolabeled plasmid pRB143, which contains the terminal BamHI S fragment (29).
Construction of baculoviruses expressing α0-intron 1 sequences.
DNA fragments containing the first 33 codons of the α0 (first ATG codon mutated to GCA codon) and intron 1 sequences of HSV-1(F), HSV-1(KOS), and HSV-1 (17) were amplified from purified viral DNAs by PCR with primers P1A (GGGGAATTCGCAGAGCCCCGCCCCGGAGCGAGTACC) and P1B (GAAGATCTCTACAGGTCTCGGTCGCAGGGAAA), which included restriction sites EcoRI (underlined) at the 5′ end and BglII (underlined) at the 3′ end in the final products (Fig. 1B). The PCR products were digested with EcoRI and BglII. Purified DNA fragments were cloned into the EcoRI-BglII site of baculovirus expression vector pAc-CMV (50) immediately after the cytomegalovirus promoter. Sf9 insect cells were cotransfected with baculoGold linearized viral DNA (Pharmingen) and plasmid DNA. Transfected cultures were harvested, and viral stocks were prepared according to the manufacturer's instructions. The presence of α0 sequences in the baculoviruses was verified by PCR with primers P1A and P1B.
Preparation of cell lysates, electrophoretic separation of proteins, and immunoblotting.
Replicate cultures of Vero, HEp-2, 143TK−, or rabbit skin cells in 25-cm2 flasks were either mock infected or infected with 5 PFU of virus per cell and maintained at 37°C in 199V medium consisting of a mixture of 199 medium supplemented with 1% calf serum. Cells were exposed to 10 μM MG132 (Biomol) at 3 h after infection if required and were harvested at 18 or 24 h after infection. At that time, cells were harvested, washed three times with phosphate-buffered saline, and then solubilized in 200 μl of disruption buffer (50 mM Tris-HCl [pH 7], 2% sodium dodecyl sulfate, 710 mM β-mercaptoethanol, 3% sucrose). Fifty-microliter aliquots of lysates were boiled for 5 min, and the solubilized proteins were subjected to electrophoresis in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, blocked with 5% nonfat milk, reacted with primary antibody followed by the appropriate secondary antibody conjugated to alkaline phosphatase (Bio-Rad), and visualized according to the manufacturer's instructions.
Extraction of total RNA and Northern blotting.
Cultures in 25-cm2 flasks were either mock infected or infected with 5 PFU of HSV-1(F) virus per cell or exposed to recombinant baculoviruses (10 to 40 PFU per cell). Cultures were harvested at 18 h after infection. Total RNA was extracted by using Trizol reagent (Gibco BRL) according to the manufacturer's instructions. Aliquots of 7.5 μg of total RNA were separated on 1% formaldehyde agarose gels, transferred to membranes, and hybridized with a 32P-labeled probe. The bound probe was visualized by autoradiography.
DNA fragmentation assay.
Replicate sets of cultures in 25-cm2 flasks were either mock infected or infected with HSV-1 viruses at an MOI of 10 in the presence or absence of 10 μM MG132. At 24 h after infection, cells were harvested, washed with phosphate-buffered saline, and lysed in a solution containing 10 mM Tris-HCl (pH 8), 10 mM EDTA, and 0.5% Triton X-100. The lysates were digested with 0.1 mg of RNase/ml at 37°C for 1 h and centrifuged at 12,000 rpm in an Eppendorf microcentrifuge (model 5415C) for 25 min to pellet chromosomal DNA. Supernatants were digested with 1 mg of proteinase K/ml at 50°C for 2 h in 1% sodium dodecyl sulfate, extracted with phenol and chloroform, precipitated in cold ethanol, and subjected to electrophoresis on 1.5% agarose gels containing 0.5 μg of ethidium bromide per ml. Oligonucleosomal DNA fragments were visualized by UV light transillumination. Photographs were taken with the aid of Eagle Eye II (Stratagene).
Antibodies.
Mouse monoclonal antibody LP1 against α-trans-inducing factor (αTIF) was a kind gift from A. Minson. Monoclonal antibodies to ICP0, ICP4, ICP8, and ICP27 were purchased from the Goodwin Cancer Research Institute (Plantation, Fla.). Mouse monoclonal antibody against US11 and rabbit polyclonal antibodies R77 (against the amino-terminal region of ICP22) and W2 (against the carboxyl-terminal region of ICP22) have been described previously (1, 24, 41).
RESULTS
Characteristics of proteins encoded by the HSV-1(vCPc0) mutant.
In mutant HSV-1(vCPc0), both copies of the α0 genes of strain HSV-1 (17) were replaced with cDNA copies of HSV-1(KOS). To verify the genomic composition of HSV-1(vCPc0) and also clarify the electrophoretic mobility of proteins made by this mutant in studies described here, replicate cultures of rabbit skin cells were either mock infected or infected with 5 PFU of HSV-1(F), HSV-1(vCPc0), HSV-1(KOS), or HSV-1 (17) per cell. The cells were harvested at 18 h after infection, lysed, subjected to electrophoresis on denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP0 and US11. As shown in Fig. 2B, ICP0 of HSV-1(vCPc0), like that of HSV-1(KOS), migrated slower than that of HSV-1(F) or HSV-1 (17). The US11 protein of HSV-1(vCPc0), like that of HSV-1 (17), also had a mobility slower than that of HSV-1(F) or HSV-1(KOS). These results are consistent with the genomic structure of HSV-1(vCPc0).
FIG. 2.
DNA and protein analyses of wild-type and recombinant viruses. (A) Autoradiographic images of 32P-labeled BamHI S fragment (pRB143) hybridized to electrophoretically separated BamHI digests of total DNA extracted from rabbit skin cells infected with HSV-1(F), HSV-1(vCPc0), and HSV-1(vCPc0)R. Replicate cultures in 25-cm2 flasks of rabbit skin cells were infected with 5 PFU of HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R per cell. The cells were harvested at 24 h after infection. Total DNA was extracted, digested with BamHI restriction endonuclease, electrophoretically separated in a 0.7% agarose gel, transferred to a zeta-probe membrane, and hybridized with 32P-labeled BamHI S fragment (pRB143). The bound probe was visualized by autoradiography. The probe hybridized with both the junction fragment (BamHI SP) and the terminal fragment (BamHI S). The loss of a restriction site resulting from the deletion of the intron 1 led to the formation of a chimeric BamHI fragment in the digest of HSV-1(vCPc0) (lane 2). The restriction site was restored in HSV-1(vCPc0)R along with intron 1 (lane 3). (B) Photographs of immunoblots of electrophoretically separated proteins from rabbit skin cells infected with HSV-1(F), HSV-1(vCPc0), HSV-1(KOS), or HSV-1 (17). Replicate cultures in 25-cm2 flasks were either mock infected or exposed to 5 PFU of virus per cell. The cells were harvested at 18 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP0 and US11.
US11 proteins of different strains contain variable numbers of PRX repeats in their carboxyl-terminal regions. Our sequencing analysis showed that the US11 sequences contained 24 PRX repeats in the HSV-1 (17) and HSV-1(vCPc0) sequences and 21 and 20 repeats in the HSV-1(F) and HSV-1(KOS) sequences, respectively. Thus, the larger number of PRX repeats accounted for the slower mobility of the US11 protein of HSV-1 (17). It is not clear what causes the slower mobility of HSV-1(KOS) ICP0. Since ICP0 of the partially repaired virus HSV-1(vCPc0)R, which retained to a large extent exon 3 of HSV-1(vCPc0), had the same slow mobility as that of HSV-1(vCPc0) (see Fig. 8A and B, lanes 5 through 8), the determinant controlling the electrophoretic mobility resided in exon 3 of the HSV-1(KOS) ICP0 sequence.
FIG. 8.
Photographs of immunoblots of electrophoretically separated proteins from lysates of rabbit skin cells infected with HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R in the presence or absence of MG132. Replicate cultures in 25-cm2 flasks of rabbit skin cells were either mock infected or exposed to 5 (A) or 10 to 100 (B) PFU of corresponding virus per cell. At 3 h after infection, one set of cultures was replenished with fresh untreated medium (lanes 1, 3, 5, and 7) whereas a second set was replenished with medium containing 10 μM MG132 (lanes 2, 4, 6, and 8). The cells were harvested at 24 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP0, ICP4, ICP8, ICP27, αTIF, or US11 or polyclonal antibody to ICP22.
Partial rescue of HSV-1(vCPc0).
The objective of these experiments was to rescue HSV-1(vCPc0) with viral DNA sequences containing the domains of the α0 gene. To define precisely the rescued domain, the DNA fragment used in the rescue was derived from HSV-1(F) DNA. The recombinant virus designated HSV-1(vCPc0)R, obtained as described in Materials and Methods, was first analyzed by restriction endonuclease analysis. Subsequently, relevant portions of the gene were sequenced. The restoration of intron 1 is readily apparent from the restriction endonuclease digest shown in Fig. 2A. Thus, the loss of a restriction site resulting from the deletion of intron 1 led to the formation of a chimeric BamHI fragment in the digest of HSV-1(vCPc0) (Fig. 2A, lane 2). The restriction site was restored in HSV-1(vCPc0)R along with intron 1 (Fig. 2A, lane 3).
Analysis of the DNA sequences of the HSV-1(vCPc0)R recombinant showed that intron 1 was restored (Fig. 3A) and that the sequences of the intron were those of HSV-1(F) rather than HSV-1(KOS) or HSV-1 (17). The sequencing data also showed that recombination occurred in exon 2 and led to the failure in the insertion of intron 2 (Fig. 3B). Finally, as detailed in the legend to Fig. 3, the sequence of a stretch of viral DNA in exon 3 near the exon 2-exon 3 junction indicates that the 3′ sequence of HSV-1(vCPc0)R is exactly the same as that of the mutant HSV-1(vCPc0).
FIG. 3.
Alignment of ICP0 sequences from HSV-1(vCPc0)R and HSV-1 (17). The PstI-SacI fragment containing the genomic α0 gene (Fig. 1A) from HSV-1(vCPc0)R was cloned into pGEM3Z. The plasmid was digested with restriction endonucleases BamHI and SalI. The 2,556-bp BamHI-SalI fragment containing both intron 1-exon2 and exon 2-intron 2-exon 3 junctions and most of the sequence of the α0 gene (Fig. 1A) was purified and sequenced by using primers designed to read through both junctions and the 5′ region of exon 3. (A) Sequence alignment at the junction between intron 1 and exon 2. (B) Sequence alignment at the exon 2-intron 2-exon 3 junction. Intron-exon junctions are shown and flanked by arrows. Dots denote missing nucleotides. Bars above nucleotide sequences denote differences between HSV-1(vCPc0)R and HSV-1 (17). The numbers 1, 2, and 3 identify 3′ regions of intron 1 that differed in nucleotide sequence. We had previously sequenced and confirmed the nucleotide sequences of this region of α0 genes from HSV-1(F), HSV-1 (17), and HSV-1(KOS). The corresponding sequences are x GTG x 9C x 6C 6T 4C TTAG for strain HSV-1 (17), x GCG x 6C x 6C 7T 4C TTAG for HSV-1(F), and x GCG x 6C x 7C 6T 4C TCAG for HSV-1(KOS), where “x” denotes intervening sequences. The corresponding sequence from HSV-1(vCPc0)R is that of HSV-1(F). In addition, codon 228 for Pro is CCG in HSV-1 (17) and CCT in HSV-1(F) and HSV-1(KOS). (C) Alignment of the sequence between codons 293 and 340 in exon 3 of the α0 gene. The HSV-1 sequences differ at sites indicated by numbers 5, 6, and 7. First, there are four GGC codons for Gly (codons 305 through 308) in HSV-1 (17) and HSV-1(KOS) and five GGC codons in HSV-1(F). Codon 316 is GCG for Ala in HSV-1 (17) and HSV-1(F) and GTG for Val in HSV-1(KOS). At residues 326 through 333, there are four copies of GGGGTT codons for Gly-Val in HSV-1 (17) and HSV-1(F) but only two copies in HSV-1(KOS). The 3′ sequences (panels B and C) of HSV-1(vCPc0)R are, to a large extent, characteristic of HSV-1(KOS) from which the mutant HSV-1(vCPc0) obtained the α0-cDNA copies. Subsequently, we sequenced the corresponding fragment from HSV-1(vCPc0) mutant DNA and found that it was the same as that of HSV-1(vCPc0)R shown above.
Delay of HSV-1(vCPc0) mutant gene expression is cell type dependent.
In preliminary experiments, we noted that the gene expression of the HSV-1(vCPc0) mutant was delayed by several hours in some cell lines but not in others. The experiments described in this section illustrate the results in some detail. Briefly, replicate cultures of rabbit skin, HEp-2, or Vero cells were exposed to 5 PFU of HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R per cell. The cells were harvested at the times shown in Fig. 4 and 5. The lysates were electrophoretically separated on denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with the antibodies shown in the figures.
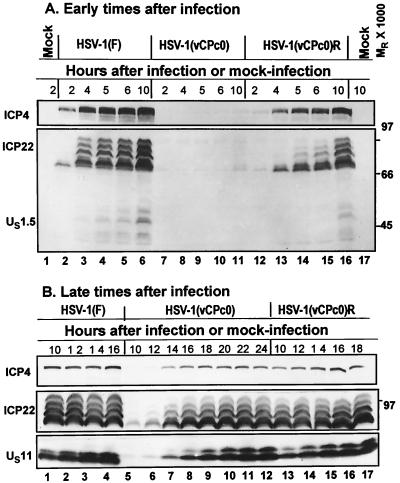
FIG. 4.
Photographs of immunoblots of electrophoretically separated proteins from lysates of rabbit skin cells infected with HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R in the early stages (A) and late stages (B) of infection. Replicate cultures in 25-cm2 flasks of rabbit skin cells were either mock infected or exposed to 5 PFU of virus per cell. At the indicated times after infection, cells were harvested as described in Materials and Methods. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP4 and US11 or polyclonal antibody to ICP22 as described in Materials and Methods.
FIG. 5.
Photographs of immunoblots of electrophoretically separated proteins from lysates of HEp-2 or Vero cells infected with HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R. The experimental design was similar to that described in the legend to Fig. 4. (A) Profiles of ICP4, ICP22, and US11 in infected HEp-2 cells. (B) Similar data for infected Vero cells. Note that a HEp-2 cell protein reacted with the anti-ICP22 antibody and had an electrophoretic mobility slightly slower than that of the slowest migrating form of ICP22 (Fig. 5A, lanes 1 and 15).
The results of this series of experiments were as follows. (i) The appearance of HSV-1(vCPc0) proteins, primarily ICP4 and ICP22, was barely detected by 6 h after infection in rabbit skin cells (Fig. 4A, lanes 7 through 11). Late in infection (Fig. 4B), the amounts of accumulated ICP4, ICP22, and US11 proteins were similar to those in HSV-1(F) or the partially restored HSV-1(vCPc0)R-infected cells. Both wild-type and HSV-1(vCPc0)R proteins were detected at 2 h after infection (Fig. 4A, lanes 2 through 6 and 12 through 16).
(ii) A smaller delay in the appearance of HSV-1(vCPc0) proteins was noted in infected HEp-2 cells (Fig. 5A, lanes 6 through 9). In this instance, ICP4 and small amounts of unprocessed ICP22 accumulated for at least 10 h. In contrast, processed forms of ICP22 from HSV-1(F) and HSV-1(vCPc0)R were present at 6 h after infection (Fig. 5A, lanes 3 and 12).
(iii) In contrast, the accumulation of HSV-1(vCPc0) proteins did not differ significantly from those of HSV-1(F) or HSV-1(vCPc0)R in Vero cells (Fig. 5B).
We conclude that the HSV-1(vCPc0) mutant exhibits a delay in the appearance of viral proteins in a cell type-dependent manner. The mutation maps in ICP0 inasmuch as the partially rescued mutant is similar in terms of the temporal pattern of gene expression to that of HSV-1(F).
Delay in the expression of HSV-1(vCPc0) mutant genes is dependent on MOI.
The purpose of this series of experiments was to determine whether the delay in mutant HSV-1(vCPc0) gene expression in rabbit skin cells was MOI dependent. In this series of experiments, replicate cultures of rabbit skin cells were exposed to 0.5 or 5 PFU of the HSV-1(vCPc0) mutant per cell. The cultures were harvested at the times shown in Fig. 6 and processed as described above. The results were as follows. Trace amounts of ICP4 and ICP22 were detected in lysates of cells exposed to 0.5 PFU of HSV-1(vCPc0) per cell. But even as late as 11 h after infection, there was no accumulation of processed forms of ICP22 and no appreciable amounts of US11 protein (Fig. 6, lanes 2 through 5). ICP4 and ICP22 were readily detected in lysates of cells infected with 5 PFU of HSV-1(vCPc0) per cell, and both processed forms of ICP22 and US11 proteins were present in lysates of cells harvested at 9 h after infection (Fig. 6, lanes 6 through 9).
FIG. 6.
Effect of MOI on the gene expression of HSV-1(vCPc0) in rabbit skin cells. Replicate cultures in 25-cm2 flasks of rabbit skin cells were either mock infected or exposed to 0.5 (lanes 2 through 5) or 5 (lanes 6 through 9) PFU of virus per cell. At the indicated times after infection, cells were harvested as described in Materials and Methods. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP4 and US11 or polyclonal antibody to ICP22 as described in Materials and Methods.
We conclude from these experiments that the delay in HSV-1(vCPc0) gene expression is both cell type and MOI dependent.
Proteasomal inhibitor MG132 arrests the expression of viral genes in cells exposed to the drug before, but not after, infection with wild-type virus.
In this series of experiments, replicate cultures of Vero or rabbit skin cells were incubated in medium containing 10 μM MG132 either 2 h before (Fig. 7, lanes 5 through 7) or 3 h after (Fig. 7, lanes 8 through 10) exposure to 1, 10, or 100 PFU of HSV-1(F) per cell. The cells were harvested at 22 h after infection and processed as described above.
FIG. 7.
Effect of the time of MG132 exposure on viral gene expression. Replicate cultures in 25-cm2 flasks of Vero (A) or rabbit skin (B) cells were either mock infected or exposed to 1, 10, or 100 PFU of HSV-1(F) per cell. Cells were either untreated (lanes 1 through 4) or treated with 10 μM MG132 at 2 h before (lanes 5 through 7) or 3 h after (lanes 8 through 10) virus infection. At 22 h after infection, cells were harvested, solubilized in disruption buffer, electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibody to ICP0 or US11 or polyclonal antibody to ICP22 as described in Materials and Methods.
The results shown in Fig. 7 were as follows. (i) In rabbit skin cells treated with the drug before infection (panel B, lanes 5 through 7), there was a reduction in the accumulation of ICP0 and processed forms of ICP22. The effect was most prominent when cells were exposed to 10 PFU or less per cell (panel B, lanes 5 and 6). In those cells, only small amounts of ICP0 and unprocessed ICP22 accumulated. A small amount of US11 protein was detected only when cells were exposed to 100 PFU per cell (panel B, lane 7). Cells treated with the drug 3 h after infection contained ICP0, processed forms of ICP22, and US11 (panel B, lanes 8 through 10). This reduction in viral gene expression at an early stage in viral replication was virus independent (data not shown).
(ii) In Vero cells (panel A), the effect of MG132 was less significant. Again, MG132 added 3 h after infection had no effect on the accumulation of viral proteins (panel A, lanes 8 through 10). In cells treated with the drug before viral infection, accumulation of ICP0, processed forms of ICP22, and US11 proteins were not affected when cells were exposed to 10 PFU or more per cell (panel A, lanes 6 and 7), although smaller amounts of US11 accumulated in treated cells rather than in untreated cells (panel A, lanes 3, 4, 6, and 7). Only at 1 PFU per cell did MG132 added before infection result in a significant decrease in the accumulation of viral proteins, notably US11 (panel A, lane 5).
These results indicate that the reduction in viral gene expression by MG132 in the early stages of virus replication is dependent on both cell type and MOI.
Proteasomal inhibitor MG132 arrests the expression of viral genes in rabbit skin cells exposed to the drug after infection with the HSV-1(vCPc0) mutant.
In this series of experiments, replicate cultures of rabbit skin cells were exposed to 5 PFU of HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R per cell. The cells were mock treated or exposed to 10 μM MG132 at 3 h after infection. The cells were harvested at 24 h after infection and processed as described above. The results (Fig. 8A) were as follows. Except for a decrease in the accumulation of US11 protein, the accumulation of all other HSV-1(F) or HSV-1(vCPc0)R proteins tested in this study was not significantly affected by the exposure of rabbit skin cells to MG132 at 3 h after infection. In contrast, only unprocessed forms of ICP22 were readily detected in rabbit skin cells exposed to MG132 at 3 h after infection with the HSV-1(vCPc0) mutant (Fig. 8A, lane 6). Neither HSV-1(KOS) nor HSV-1 (17) was affected by MG132 when tested under the same conditions (data not shown).
In a second series of experiments, we examined the effect of MOI on the expression of viral genes in rabbit skin cells exposed to 10 μM MG132 at 3 h after infection. In these experiments, cells were infected with 10 or 100 PFU of virus per cell, as shown in Fig. 8B. Cells were harvested at 24 h after infection and processed as described above. The significant observation shown in Fig. 8B was that cells exposed to 100 PFU of HSV-1(vCPc0) per cell accumulated small amounts of ICP0, ICP27, ICP4, and ICP22. We did not detect αTIF or US11 proteins in these cells (Fig. 8B, lane 6).
We therefore conclude the following. (i) Unlike that of wild-type virus, expression of the HSV-1(vCPc0) mutant is arrested by the proteasomal inhibitor MG132 at a very early stage in viral gene expression. The defect in the accumulation of viral proteins in the presence of MG132 maps to the α0 gene inasmuch as the partially rescued virus HSV-1(vCPc0)R cannot be differentiated from the wild-type virus.
(ii) The stage of arrest of the HSV-1(vCPc0) mutant in cells exposed to MG132 at 3 h after infection is similar to that of wild-type HSV-1(F) in cells exposed to MG132 before infection.
(iii) Even a very high MOI did not overcome the inhibitory effect of MG132 in cells exposed to the drug at 3 h after infection with the HSV-1(vCPc0) mutant.
One potentially interesting observation was of the patterns of accumulation of the posttranslationally processed forms of ICP22. Earlier studies have shown an interdependence in the electrophoretic mobility between the isoforms of ICP22 and ICP0 (7, 39). In addition, earlier studies have shown that accumulation of the US11 protein is in part dependent on the presence of functional ICP22 (32, 38, 39). In light of the fluctuation in the levels of accumulated US11 protein, it was important to determine whether these observations reflected mutations in ICP22 or the differential effects of MG132 on the accumulation of viral proteins. To meet this objective, we sequenced the ICP22 open reading frame encoded by HSV-1(F), HSV-1(vCPc0), HSV-1(KOS), and HSV-1 (17). The results showed that the sequence of ICP22 encoded by HSV-1(vCPc0) was identical to that of HSV-1 (17) and HSV-1(F) but different with respect to several codons from those of HSV-1(KOS). These differences include codons for Ala-10, Ser-34, Asp-46, Asp-48, Tyr-116, and Thr-281 that were replaced by codons for Val, Ala, Glu, Glu, Cys, and Ala, respectively, in the HSV-1(KOS) sequence. The similarity of the ICP22 sequences of HSV-1(vCPc0) with those of HSV-1 (17) and HSV-1(F) suggests that the differences in the electrophoretic mobility of the isoforms of ICP22 shown in Fig. 8 and 9 do not reflect the primary sequence of ICP22.
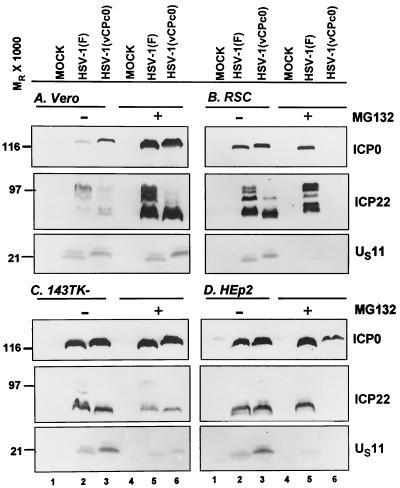
FIG. 9.
Photographs of immunoblots of electrophoretically separated proteins from Vero, rabbit skin (RSC), 143TK−, and HEp-2 cells infected with HSV-1(F) or HSV-1(vCPc0) in the presence or absence of MG132. Replicate cultures in 25-cm2 flasks of Vero cells (A), rabbit skin cells (RSC) (B), 143TK− cells (C), or HEp-2 cells (D) were either mock infected or infected with 5 PFU of virus per cell. At 3 h after infection, the medium of one set of cultures was replaced with fresh medium (lanes 1 through 3) whereas a replicate set was replenished with medium supplemented with 10 μM MG132 (lanes 4 through 6). The cells were harvested at 24 h after infection. Proteins were solubilized in disruption buffer and electrophoretically separated in 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP0 and US11 or polyclonal rabbit antibody to ICP22 as described in Materials and Methods.
It should be noted that ICP0 of the partially repaired virus HSV-1(vCPc0)R had the same slower mobility as the mutant HSV-1(vCPc0) ICP0 (Fig. 8A and B, lanes 5 through 8). The reason is that HSV-1(vCPc0)R had retained to a large extent the exon 3 sequence of the HSV-1(vCPc0) ICP0 gene (Fig. 3C), and this indicates that ICP0 exon 3 of the HSV-1(KOS) sequence contains an element which causes the slow mobility of the ICP0 protein.
Inhibitory effect of MG132 on the HSV-1(vCPc0) mutant in cells exposed to the drug at 3 h after infection is cell type dependent.
Earlier in this paper (Fig. 4 and 5), we showed that the delay in the expression of HSV-1(vCPc0) mutant genes was cell type dependent. In the experiments described above (Fig. 8), we showed that the expression of HSV-1(vCPc0) mutant genes was arrested at a very early stage in rabbit skin cells exposed to MG132 at 3 h after infection. We also showed that both defects map to the α0 gene inasmuch as the partially repaired HSV-1(vCPc0)R cannot be differentiated from wild-type HSV-1(F) with respect to these properties. The question arose as to whether the arrest in HSV-1(vCPc0) gene expression by the exposure of cells to MG132 at 3 h after infection was also cell type dependent. In this series of experiments, replicate cultures of Vero, HEp-2, rabbit skin, and 143TK− cells were infected with 5 PFU of HSV-1(F) or HSV-1(vCPc0) per cell. The cultures were mock treated or exposed to 10 μM MG132 at 3 h after infection, harvested at 24 h after infection, and processed as described above.
The results shown in Fig. 9 were as follows. (i) ICP0, ICP22, and US11 were readily detected in all cultures of cells infected with HSV-1(F) or HSV-1(vCPc0) and mock treated. The amounts of the proteins varied. This was not particularly surprising inasmuch as the amount of proteins encoded by specific genes tends to vary from one cell line to another.
(ii) The same three proteins were readily detected in Vero cells or 143TK− cells exposed to MG132 at 3 h after infection with either HSV-1(F) or HSV-1(vCPc0). In contrast, in rabbit skin cells infected with the HSV-1(vCPc0) mutant, none of the three proteins accumulated in detectable amounts and, in HEp-2 cells, only ICP0 was readily detected (Fig. 9B and D, lane 6). In both rabbit skin cells and HEp-2 cells infected with HSV-1(F), there was a decrease in the amount of US11 protein that accumulated in MG132-treated cells. We noted that in Vero cells, the amount of accumulating protein was higher in MG132-treated than that in untreated infected cells. This phenomenon was not observed in other infected cell lines.
We conclude from this series of experiments that the arrest in the expression of HSV-1(vCPc0) genes in cells exposed to MG132 at 3 h after infection is cell type dependent.
ICP0 intron 1 sequences expressed in trans do not complement HSV-1(vCPc0) in rabbit skin cells exposed to MG132.
The results of the studies described in the preceding section showed that partial restoration of α0 genomic sequences restored the capacity of HSV-1(vCPc0) to express its genes in rabbit skin cells exposed to MG132 at 3 h after infection. The major difference between HSV-1(vCPc0) and the partially restored HSV-1(vCPc0)R was the presence of intron 1. An earlier study has shown that the intron 1 RNA is transported into the cytoplasm, where it persists for the duration of infection (7). A central question is whether the expression of intron 1 from DNA sequences delivered in trans alters the phenotype of cells infected with HSV-1(vCPc0). To resolve this question, the α0-intron 1 sequences of HSV-1(F), HSV-1 (17), and HSV-1(KOS) were each cloned in baculoviruses as described in Materials and Methods and the legend to Fig. 1B. To test the expression of the introns, replicate cultures of rabbit skin cells grown in 25-cm2 flasks were exposed to the recombinant baculoviruses or HSV-1(F) and maintained as described in Materials and Methods. After 18 h of incubation at 37°C, cells were harvested and their RNA was extracted, separated in 1% formaldehyde agarose gels, transferred to membranes, and hybridized to 32P-labeled α0-intron 1 sequences. The results (Fig. 10) show that intron 1 RNA sequences were readily detected in the infected cells. It is of interest that, although the quantities of intron RNA differed significantly [HSV-1 (17) intron 1 was barely visible], all sequences exhibited a similar electrophoretic mobility that was slightly slower (lanes 1 through 3) than that of the authentic intron 1 sequences expressed by HSV-1(F) in rabbit skin cells (lane 5).
FIG. 10.
Expression of α0-intron 1 RNA sequences by recombinant baculoviruses. Cultures in 25-cm2 flasks of rabbit skin cells were either mock infected or infected with 5 PFU of HSV-1(F) per cell (lane 5) or exposed to recombinant baculoviruses encoding α0-intron 1 sequences of HSV-1(F) (lane 3), HSV-1(KOS) (lane 2), or HSV-1 (17) (lane 1) at MOIs of 40, 25, or 10, respectively. Cultures were harvested at 18 h after infection. Total RNA was extracted by using Trizol reagent (Gibco BRL). Aliquots of 7.5 μg of total RNA were separated on 1% formaldehyde agarose gels, transferred to membranes, and hybridized with 32P-labeled α0-intron 1-specific fragment (Fig. 1B, line 2). The bound probe was visualized by autoradiography.
In the next series of experiments, we tested the effects of intron 1 RNA according to the experimental design depicted in Fig. 11A. Briefly, replicate cultures of rabbit skin cells were exposed to baculoviruses encoding the ICP0 intron 1 sequences of HSV-1(F), HSV-1(KOS), or HSV-1 (17). At 8 h after exposure to the baculoviruses, cells were infected with HSV-1(vCPc0). At 3 h after HSV infection, the cultures were replenished with medium with or without MG132. The results (Fig. 11B and C) were as follows. In the absence of MG132, ICP0 or US11 protein accumulated in equivalent amounts in cells with or without preexposure to baculoviruses expressing the introns (lanes 9 through 14). In the presence of MG132, the levels of both proteins were dramatically reduced and we could not differentiate between the levels of proteins accumulating in cells uninfected (lane 3) or infected with a baculovirus lacking introns (lane 7) from those infected with baculoviruses containing introns (lanes 4 through 6). The exception was that, in cells infected with baculovirus containing the HSV-1(KOS) intron, there was a further reduction in the accumulation of both the ICP0 and US11 proteins (lane 5).
FIG. 11.
Effect of expression of α0-intron 1 sequences delivered in trans on the expression of α0 and US11 genes in HSV-1(vCPc0) infection in the presence or absence of MG132. (A) Experimental design. Replicate cultures in 25-cm2 flasks of rabbit skin cells were either mock infected (exposed to insect cell medium [lanes 3 and 10]) or infected with baculoviruses expressing the α0-intron 1 sequence from HSV-1 strains F, KOS, or 17 at MOIs of 40, 25, or 10, respectively (lanes 4 through 6 and 11 through 13) or baculovirus lacking introns at a MOI of 30 (lanes 7 and 14). Cells were maintained at 37°C in Dulbecco's modified Eagle's medium supplemented with 5% newborn calf serum and 7 mM sodium butyrate (Sigma). At 8 h after infection, the cells were infected with 5 PFU of HSV-1(vCPc0) per cell. In addition, replicate cultures not previously exposed to baculoviruses (or insect cell medium) were mock infected (exposed to 199V medium [lanes 1 and 8]) or infected with HSV-1(vCPc0) (lanes 2 and 9) at this time. The cells were maintained in 199V medium supplemented with 7 mM sodium butyrate. Three hours later, the medium of one set of cultures was replaced with fresh medium (C, lanes 8 through 14) whereas a replicate set was replenished with medium supplemented with 10 μM MG132 (B, lanes 1 through 7). The cells were harvested at 24 h after infection with HSV-1(vCPc0). (B and C) Photographs of immunoblots of electrophoretically separated proteins from MG132-treated (B) and untreated (C) cells. Proteins were solubilized in disruption buffer and electrophoretically separated on 11% denaturing polyacrylamide gels, transferred to nitrocellulose sheets, and reacted with monoclonal antibodies to ICP0 and US11.
We conclude that the ICP0 intron 1 sequences delivered in trans had no effect on the accumulation of ICP0 or US11 protein. The inhibitory effects of HSV-1(KOS) sequences are probably not significant since they were not matched in cells exposed to the introns of other viruses (lanes 11 through 13) and no effects were noted in cells exposed to the HSV-1(KOS) intron 1 sequence.
HSV-1 blocks apoptosis induced by MG132 early in infection.
The shutoff of protein synthesis induced by MG132 in rabbit skin cells raised the possibility that the drug causes damage to the treated cells that is blocked or reversed by infection with wild-type virus. One hypothesis that is easily testable is that MG132 induces apoptosis and that infection with wild-type virus blocks apoptosis induced by MG132 just as it blocks programmed cell death induced by sorbitol, Fas ligand, and other exogenous inducers (16). The two series of experiments detailed below were carried out to test this hypothesis.
In the first series, replicate cultures of rabbit skin cells grown in 25-cm2 flasks were mock infected or exposed to 10 PFU of HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R per cell. As illustrated diagramatically in Fig. 12A, cells were exposed to 10 μM MG132 at 3 h, harvested at 24 h after infection, and processed for the presence of fragmented cellular DNA as described in Materials and Methods. The results (Fig. 12B and B′) were as follows. Fragmented cellular DNA was observed in lysates of mock-infected cells and cells infected with the HSV-1(vCPc0) mutant treated with MG132 (panels B and B′, lanes 5 and 7) but not in lysates of any of the untreated, mock-infected, or infected cells or of cells infected with either wild-type virus or repaired HSV-1(vCPc0)R treated with MG132. We conclude from this series of experiments that the exposure of rabbit skin cells to MG132 causes programmed cell death resulting in the fragmentation of cellular DNA and that the wild-type virus and the repaired recombinant virus blocked programmed cell death whereas the HSV-1(vCPc0) mutant did not.
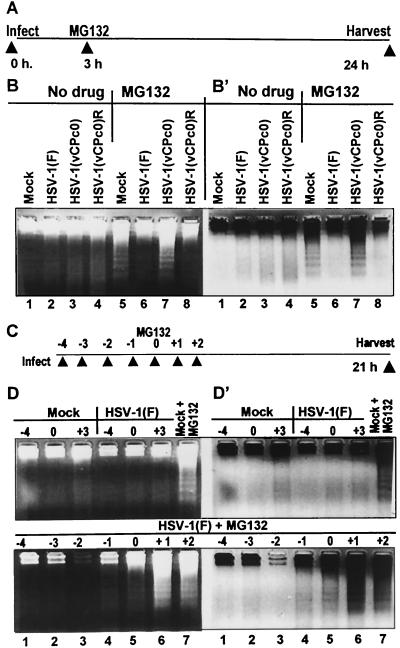
FIG. 12.
HSV-1 blocks apoptosis induced by MG132. (A and B) Wild-type and repaired viruses block apoptosis induced by MG132. (A) Experimental design. Replicate cultures in 25-cm2 flasks of rabbit skin cells were either mock infected or infected with 10 PFU of HSV-1(F), HSV-1(vCPc0), or HSV-1(vCPc0)R per cell. At 3 h after infection, the medium of one set of cultures was replaced with fresh medium (lanes 1 through 4) whereas that of the second set was replenished with medium supplemented with 10 μM MG132 (lanes 5 through 8). The cells were harvested at 24 h after infection. (B and B′) Photographs of positive and negative images of ethidium bromide-stained low-molecular-weight DNA from mock-infected and HSV-1-infected cells. Cells were harvested and processed as described in Materials and Methods. DNA was separated in 1.5% agarose gels containing 0.5 μg of ethidium bromide/ml. Oligonucleosomal DNA fragments were visualized by UV light transillumination. Photographs were taken with the aid of Eagle Eye II (Stratagene), and both positive and reverse images are shown to help visualize bands of fragmented cellular DNA. (C and D) Minimal interval between time of infection and time of addition of MG132 necessary to block fragmentation of cellular DNA. (C) Experimental design. Replicate cultures of rabbit skin cells grown in 25-cm2 flasks were exposed to MG132 at a fixed time (zero time). The cultures were infected with 10 PFU per cell of HSV-1(F) either 4, 3, 2, or 1 h before exposure to the drug, simultaneously with the addition of the drug, or at 1 or 2 h after exposure to the drug (D and D′, lower panels). Cells were harvested and processed as described in Materials and Methods. Replicate cultures either mock infected or infected with HSV-1(F) virus and maintained in medium without MG132 (D and D′, top panels, lanes 1 through 6) were processed in a similar manner. (D and D′) Photographs of positive and negative images of ethidium bromide-stained low-molecular-weight DNA from mock-infected and HSV-1(F)-infected cells. Cells were harvested and processed as described in Materials and Methods. DNA was separated and visualized as described above.
The objective of the second series of experiments was to determine the minimal interval between expression of wild-type virus and time of addition of MG132 necessary to block apoptosis. The design of these experiments is illustrated diagramatically in Fig. 12C. Briefly, replicate cultures of rabbit skin cells grown in 25-cm2 flasks were exposed to MG132 at a fixed time (zero time). The cultures were infected with 10 PFU per cell of HSV-1(F) either 4, 3, 2, or 1 h before exposure to the drug, simultaneously with the addition of the drug, or at 1 or 2 h after exposure to the drug. The cells were harvested and processed as described in Materials and Methods.
The results (Fig. 12D and D′) were as follows. (i) As shown in the upper set of D and D′ panels, there was no detectable fragmented DNA in lysates of mock-infected or wild-type-virus-infected cells (lanes 1 through 6). As expected, MG132 induced fragmentation of cellular DNA in mock-infected cells exposed to MG132 (lanes 7).
(ii) There was no detectable DNA fragmentation in lysates of cells infected with wild-type virus at 4, 3, 2, or 1 h before exposure to MG132 (Fig. 12, lower panels D and D′, lanes 1 through 4). We noted a slight accumulation of fragmented DNA in lysates of cells infected in the presence of MG132 (Fig. 12, lower panels D and D′, lanes 5) and extensive fragmentation of DNA in lysates exposed to the drug before infection (Fig. 12, lower panels D and D′, lanes 6 and 7).
We conclude from this series of experiments that MG132 precludes the expression of wild-type virus genes necessary to block apoptosis in rabbit skin cells exposed to the drug even 1 h before infection.
DISCUSSION
We report on two series of experiments that lead to an expansion of our understanding of the role of the α0 gene in the replication of HSV-1. The first series described in this report showed that, in some cell lines infected with the HSV-1(vCPc0) mutant, there was a delay of many hours in the viral gene expression and particularly in the accumulation of β and γ proteins. This effect was MOI and cell type dependent. Characteristic of this delay is the absence of the posttranslational modification of ICP22. This protein is modified by the US3 and UL13 protein kinases. This observation is consistent with the conclusion that the delayed event was the transition from α to β and γ gene expression.
The second series of studies centered on the effects of MG132, a proteasomal inhibitor, on viral gene expression. In rabbit skin cells treated before infection, wild-type virus gene expression was delayed in an MOI-dependent fashion. In cells treated with the drug 3 h after infection, viral gene expression was not significantly affected by the drug. Treatment of cells with MG132 at 3 h after infection with the HSV-1(vCPc0) mutant resulted in the accumulation of α proteins only. Again, in this instance, the effect was cell type dependent and ICP22 was not posttranslationally processed. Additional evidence concerning the blocked event emerged from the proapoptotic activity of MG132. MG132 induced apoptosis in rabbit skin cells treated with the drug before infection with wild-type virus and in cells treated with the drug 3 h after infection with the HSV-1(vCPc0) mutant. Apoptosis was blocked in cells treated with MG132 added 3 h after infection with wild-type virus; this was concurrent with the expression of the β and γ genes (19, 20). In the studies carried out in this and other laboratories, the antiapoptotic genes expressed by HSV-1 belong to the β or γ kinetic groups (2, 3, 16, 30, 50).
The conclusion that the two phenomena are related is based on the evidence that restoration of the intron 1 sequences to the HSV-1(vCPc0) mutant abolished the delay in the transition from α to β and γ gene expression and also enabled β and γ gene expression in rabbit skin cells treated with MG132 added 3 h after infection. The evidence recited above leads to two key conclusions. First, a proteasome-dependent step was required for the transition from α to β and γ gene expression. This step occurred very early on in infection. Second, this step requires the expression of an α0 gene containing intron 1.
The implications of these two conclusions are as follows. (i) The requirement for a proteasome-dependent step for the transition from α to β and γ gene expression is satisfied by a function mapping in the α0 gene. Evidence from this and other laboratories has firmly established that ICP0 is a ubiquitin ligase involved in the proteasome-dependent degradation of several cellular proteins (6, 13-15, 18, 25, 26, 46). The evidence that a proteasome-dependent step maps to the α0 gene is consistent with the known functions of its products.
(ii) The observation that mutants lacking the α0 gene bypass this requirement in a MOI-dependent manner does not obviate the requirement for a proteasomal activity. The same argument can be made regarding the behavior of the HSV-1(vCPc0) mutant in Vero cells. Thus, it is conceivable that, in cell lines in which there is no apparent requirement for a proteasome-dependent step, the target proteins are either degraded by other means or absent or that, at the MOI used in the experiments, the template dose-dependent basal level of expression of β or γ genes may be sufficient to overcome the block.
(iii) The HSV-1(vCPc0) mutant differs from Δα0 mutants in that it expresses ICP0 and it replicates to a high titer in all cell lines tested at various MOIs. The delay in the expression of β and γ genes in untreated rabbit skin cells and the absence of β and γ proteins in cells treated with MG132 after infection indicates that ICP0 encoded by the cDNA did not express the proteasome-dependent step required for the efficient transition from α to β and γ gene expression. The α0 gene containing a restored intron 1 sequence performed in a manner similar to that of the wild-type gene. There are two hypotheses that could explain a role for intron 1. The first is based on the observation that intron 1 RNA is stable and accumulates in the cytoplasm of infected cells (7). In this study, we have shown that intron 1 delivered in trans did not complement the HSV-1(vCPc0) mutant. The experiment could be interpreted in one of two ways. First, the intron RNA does not act in trans. A second possibility is that, as is the case with negative experimental results, the RNA was not spliced or was spliced incorrectly and hence it lacked an effective secondary structure. Thus, the RNA accumulating in transduced cells migrated more slowly than the authentic intron 1 mRNA, suggesting that it may not correspond to the authentic product of the splicing of α0 mRNA. The second hypothesis is based on the evidence that infected cells contain alternatively spliced α0 mRNA. This hypothesis predicts that an alternatively spliced mRNA is transiently expressed early in infection and that the expression of this mRNA is critical for the transition from α to β gene expression during the early stages of viral gene expression. This hypothesis is under investigation.
The studies presented in this report indicate that α0 exerts a master regulatory function in the transition from α to β and γ protein synthesis in a cell type-specific manner. The phenotype of the α0 gene is that of a promiscuous transactivator (10, 17, 33). Its only known product, ICP0, is a multifunctional, highly processed protein that interacts with a large number of viral and cellular proteins (40). As noted above, many lines of evidence indicate that one of its major roles is that of a ubiquitin ligase (6, 18, 46). The whole spectrum of the proteins targeted for degradation by ICP0 is not yet known. Studies on the α0 transcripts suggest the existence of alternatively spliced mRNAs (7). The requirement for intron 1 for the proteasome-dependent transition from α to β and γ gene expression raises the possibility that, early in infection, a transient product of the α0 gene encoded by an alternatively spliced mRNA targets a specific protein that blocks this transition. If our hypothesis is found to be tenable, it could provide a clue to the process that leads to the establishment of latent rather than productive infection. For example, failure in making the product of alternatively spliced mRNA could prevent productive infection from ensuing.
Acknowledgments
The studies carried out at the University of Chicago were aided by grants from the National Cancer Institute (CA87661, CA83939, CA71933, CA78766, and CA88860). The studies at Columbia University were supported by grant AI-33952.
REFERENCES
- 1.Ackermann, M., M. Sarmiento, and B. Roizman. 1985. Application of antibody to synthetic peptides for characterization of the intact and truncated α22 protein specified by herpes simplex virus 1 and the R325 α22− deletion mutant. J. Virol. 56:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auber, M., and J. A. Blaho. 1999. The herpes simplex virus type 1 regulatory protein ICP27 is required for the prevention of apoptosis in infected human cells. J. Virol. 73:2803-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auber, M., J. O'Toole, and J. A. Blaho. 1999. Induction and prevention of apoptosis in human HEp-2 cells by herpes simplex virus type 1. J. Virol. 73:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and B. Roizman. 1992. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J. Virol. 66:5621-5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines, J. D., A. P. W. Poon, J. Rovnak, and B. Roizman. 1994. The herpes simplex virus UL15 gene encodes two proteins and is required for cleavage of genomic viral DNA. J. Virol. 68:8118-8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter, K. L., and B. Roizman. 1996. Alternatively spliced mRNAs predicted to yield frame-shift proteins and stable intron 1 RNAs of the herpes simplex virus 1 regulatory gene α0 accumulate in the cytoplasm of infected cells. Proc. Natl. Acad. Sci. USA 93:12535-12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolan, A., M. Arbuckle, and D. J. McGeoch. 1991. Sequence analysis of the splice junction in the transcript of herpes simplex virus type 1 gene UL15. Virus Res. 20:97-104. [DOI] [PubMed] [Google Scholar]
- 9.Ejercito, P., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 10.Everett, R. D. 1984. Transactivation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D. 1991. Construction and characterization of herpes simplex virus type 1 viruses without introns in immediate early gene 1. J. Gen. Virol. 72:651-659. [DOI] [PubMed] [Google Scholar]
- 12.Everett, R. D., A. Cross, and A. Orr. 1993. A truncated form of herpes simplex virus type-1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197:751-756. [DOI] [PubMed] [Google Scholar]
- 13.Everett, R. D., P. Freemont, H. Saitoh, M. Dasso, A. Orr, M. Kathoria, and J. Parkinson. 1998. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J. Virol. 72:6581-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw 110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:566-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galvan, V., and B. Roizman. 1998. Herpes simplex virus 1 induces and blocks apoptosis at multiple steps during infection and protects cells from exogenous inducers in a cell-type dependent manner. Proc. Natl. Acad. Sci. USA 95:3931-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelman, I. H., and S. Silverstein. 1985. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc. Natl. Acad. Sci. USA 82:5265-5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagglund, R., C. Van Sant, P. Lopez, and B. Roizman. 2002. Herpes simplex virus 1 infected cell protein 0 contains two E3 ubiquitin ligase sites specific for different E2 ubiquitin conjugating enzymes. Proc. Natl. Acad. Sci. USA 99:631-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi, Y., R. Bruni, and B. Roizman. 1997. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J. Virol. 71:1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi, Y., M. Tanaka, A. Yokoymama, G. Matsuda, K. Kato, H. Kagawa, K. Hirai, and B. Roizman. 2001. Herpes simplex virus 1 α regulatory protein ICP0 functionally interacts with cellular transcription factor BMAL1. Proc. Natl. Acad. Sci. USA 98:1877-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawaguchi, Y., C. Van Sant, and B. Roizman. 1997. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopardi, R., P. L. Ward, W. O. Ogle, and B. Roizman. 1997. Association of herpes simplex virus regulatory protein ICP22 with transcriptional complexes containing EAP, ICP4, RNA polymerase II, and viral DNA requires posttranslational modification by the UL13 protein kinase. J. Virol. 71:1133-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maul, G. G., and R. D. Everett. 1994. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J. Gen. Virol. 75:1223-1233. [DOI] [PubMed] [Google Scholar]
- 26.Maul, G. G., H. H. Guldner, and J. G. Spivack. 1993. Modification of discrete nuclear domains induced by herpes simplex virus type-1 immediate early gene-1 product (ICP0). J. Gen. Virol. 74:2679-2690. [DOI] [PubMed] [Google Scholar]
- 27.Meredith, M., A. Orr, M. Elliott, and R. D. Everett. 1995. Separation of sequence requirements for HSV-1 Vmw110 multimerisation and interaction with a 135-kDa cellular protein. Virology 209:174-187. [DOI] [PubMed] [Google Scholar]
- 28.Meredith, M., A. Orr, and R. D. Everett. 1994. Herpes simplex virus type 1 immediate-early protein Vmw110 binds strongly and specifically to a 135-kDa cellular protein. Virology 200:457-469. [DOI] [PubMed] [Google Scholar]
- 29.Mocarski, E. S., and B. Roizman. 1982. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell 31:89-97. [DOI] [PubMed] [Google Scholar]
- 30.Munger, J., A. V. Chee, and B. Roizman. 2001. The US3 protein kinase blocks apoptosis induced by the d120 mutant of herpes simplex virus 1 at a premitochondrial stage. J. Virol. 75:5491-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Natarajan, R., S. Deshmane, T. Valyi-Nagy, R. D. Everett, and W. W. Fraser. 1991. A herpes simplex virus type 1 mutant lacking the ICP0 introns reactivates with normal efficiency. J. Virol. 65:5569-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogle, W. O., and B. Roizman. 1999. Functional anatomy of herpes simplex virus 1 overlapping genes encoding infected-cell protein 22 and US1.5 protein. J. Virol. 73:4305-4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Hare, P., and G. S. Hayward. 1985. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J. Virol. 53:751-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Panagiotidis, C. A., E. K. Lium, and S. J. Silverstein. 1997. Physical and functional interactions between herpes simplex virus immediate-early proteins ICP4 and ICP27. J. Virol. 71:1547-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry, L. J., F. J. Rixon, R. D. Everett, M. C. Frame, and D. J. McGeoch. 1986. Characterization of the IE110 gene of herpes simplex virus type 1. J. Gen. Virol. 67:2365-2380. [DOI] [PubMed] [Google Scholar]
- 36.Poffenberger, K. L., and B. Roizman. 1985. A noninverting genome of a viable herpes simplex virus 1: presence of head-to-tail linkages in packaged genomes and requirements for circularization after infection. J. Virol. 53:587-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poon, A. P. W., and B. Roizman. 1993. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J. Virol. 67:4497-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poon, A. P. W., W. O. Ogle, and B. Roizman. 2000. Posttranslational processing of infected cell protein 22 mediated by viral protein kinases is sensitive to amino acid substitutions at distant sites and can be cell-type specific. J. Virol. 74:11210-11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roizman, B., and D. M. Knipe. 2001. The replication of herpes simplex viruses, p. 2399-2459. In D. M. Knipe, P. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Field's virology, 4th ed. Lippincott-Williams and Wilkins, New York, N.Y.
- 41.Roller, R. J., and B. Roizman. 1992. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. J. Virol. 66:3624-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmon, B., and J. D. Baines. 1998. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of UL 15-encoded proteins with B capsids requires at least the UL6, UL17, and UL28 genes. J. Virol. 72:3045-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 44.Spatz, S. J., E. C. Nordby, and P. C. Weber. 1996. Mutational analysis of ICP0R, a transrepressor protein created by alternative splicing of the ICP0 gene of herpes simplex virus type 1. J. Virol. 70:7360-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spatz, S. J., E. C. Nordby, and P. C. Weber. 1997. Construction and characterization of a recombinant herpes simplex virus type 1 which overexpresses the transrepressor protein ICP0R. Virology 228:218-228. [DOI] [PubMed] [Google Scholar]
- 46.Van Sant, C., R. Hagglund, P. Lopez, and B. Roizman. 2001. The infected cell protein 0 of herpes simplex virus 1 dynamically interacts with proteasomes, binds and activates the cdc34 E2 ubiquitin conjugating enzyme and possesses in vitro E3 ubiquitin ligase activity. Proc. Natl. Acad. Sci. USA 98:8815-8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber, P. C., and B. Wigdahl. 1992. Identification of dominant-negative mutants of the herpes simplex virus type 1 immediate-early protein ICP0. J. Virol. 66:2261-2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weber, P. C., J. J. Kenny, and B. Wigdahl. 1992. Antiviral properties of a dominant negative mutant of the herpes simplex virus type-1 regulatory protein-ICP0. J. Gen. Virol. 73:2955-2961. [DOI] [PubMed] [Google Scholar]
- 49.Weber, P. C., S. J. Spatz, and E. C. Nordby. 1999. Stable ubiquitination of the ICP0R protein of herpes simplex virus type 1 during productive infection. Virology 253:288-298. [DOI] [PubMed] [Google Scholar]
- 50.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11791. [DOI] [PMC free article] [PubMed] [Google Scholar]