Abstract
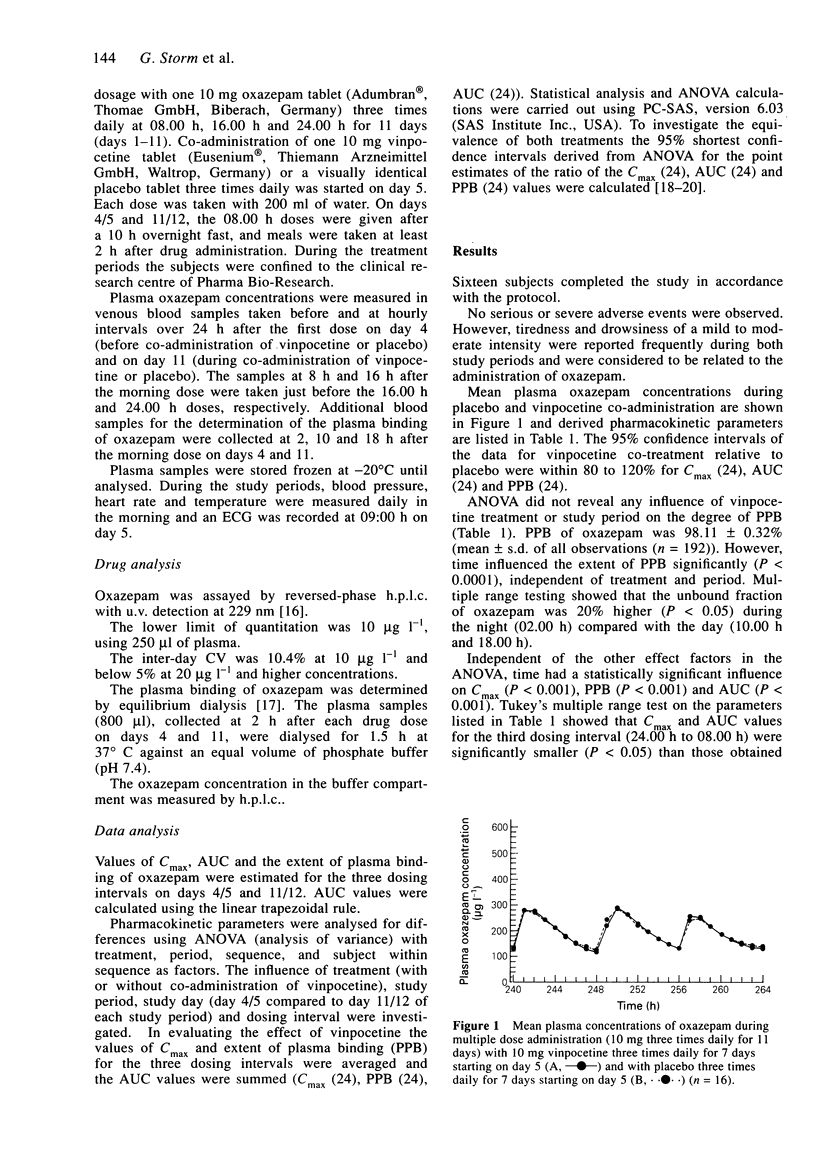
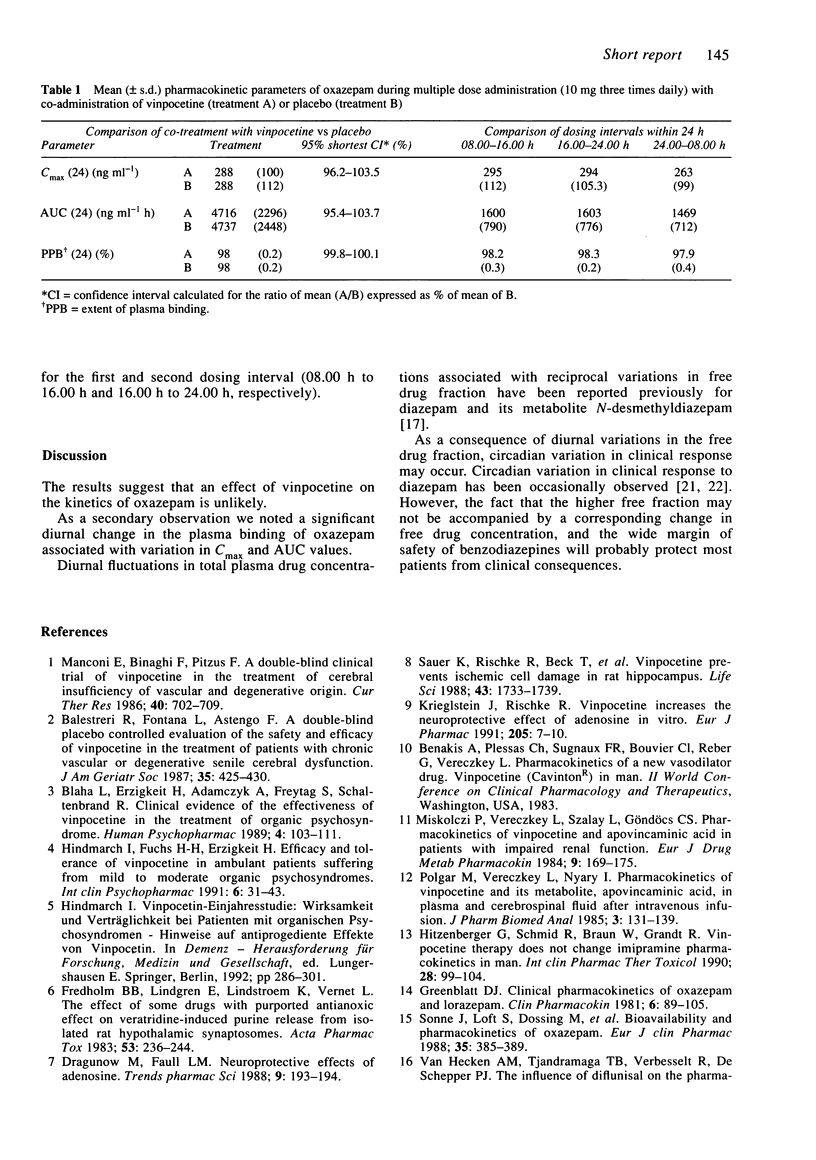
The influence of multiple doses of vinpocetine (10 mg three times daily) on the steady state plasma concentrations of oxazepam (10 mg three times daily) was studied in 16 healthy subjects. The mean (+/- s.d.) AUC (ng ml-1h-1) of oxazepam over 24 h during combined treatment was 4716 +/- 2296 and for oxazepam treatment alone it was 4737 +/- 2448 (95% confidence intervals for ratio of means = 95.4-103.7%). The degree of plasma protein binding of oxazepam was 98.11 +/- 0.32% and was not affected by vinpocetine. Independent of vinpocentine treatment a significant diurnal change in the plasma binding of oxazepam was observed; the free drug fraction was 20% higher during the night than during the day. Cmax and AUC values based on total oxazepam in plasma were 10% lower during the night. The results indicate a lack of influence of vinpocetine on oxazepam kinetics. Diurnal changes in the plasma binding of oxazepam probably have no clinical consequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird E. S., Hailey D. M. Delayed recovery from a sedative: correlation of the plasma levels of diazepam with clinical effects after oral and intravenous administration. Br J Anaesth. 1972 Aug;44(8):803–808. doi: 10.1093/bja/44.8.803. [DOI] [PubMed] [Google Scholar]
- Balestreri R., Fontana L., Astengo F. A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc. 1987 May;35(5):425–430. doi: 10.1111/j.1532-5415.1987.tb04664.x. [DOI] [PubMed] [Google Scholar]
- Dragunow M., Faull R. L. Neuroprotective effects of adenosine. Trends Pharmacol Sci. 1988 Jun;9(6):193–194. doi: 10.1016/0165-6147(88)90079-x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Lindgren E., Lindström K., Vernet L. The effect of some drugs with purported antianoxic effect on veratridine-induced purine release from isolated rat hypothalamic synaptosomes. Acta Pharmacol Toxicol (Copenh) 1983 Sep;53(3):236–244. doi: 10.1111/j.1600-0773.1983.tb01131.x. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981 Mar-Apr;6(2):89–105. doi: 10.2165/00003088-198106020-00001. [DOI] [PubMed] [Google Scholar]
- Hindmarch I., Fuchs H. H., Erzigkeit H. Efficacy and tolerance of vinpocetine in ambulant patients suffering from mild to moderate organic psychosyndromes. Int Clin Psychopharmacol. 1991 Spring;6(1):31–43. doi: 10.1097/00004850-199100610-00005. [DOI] [PubMed] [Google Scholar]
- Hitzenberger G., Schmid R., Braun W., Grandt R. Vinpocetine therapy does not change imipramine pharmacokinetics in man. Int J Clin Pharmacol Ther Toxicol. 1990 Mar;28(3):99–104. [PubMed] [Google Scholar]
- Krieglstein J., Rischke R. Vinpocetine increases the neuroprotective effect of adenosine in vitro. Eur J Pharmacol. 1991 Nov 19;205(1):7–10. doi: 10.1016/0014-2999(91)90762-f. [DOI] [PubMed] [Google Scholar]
- Miskolczi P., Vereczkey L., Szalay L., Göndöcs C. S. Pharmacokinetics of vinpocetine and apovincaminic acid in patients with impaired renal function. Eur J Drug Metab Pharmacokinet. 1984 Apr-Jun;9(2):169–175. doi: 10.1007/BF03189621. [DOI] [PubMed] [Google Scholar]
- Naranjo C. A., Sellers E. M., Giles H. G., Abel J. G. Diurnal variations in plasma diazepam concentrations associated with reciprocal changes in free fraction. Br J Clin Pharmacol. 1980 Mar;9(3):265–272. doi: 10.1111/j.1365-2125.1980.tb04836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár M., Vereczkey L., Nyáry I. Pharmacokinetics of vinpocetine and its metabolite, apovincaminic acid, in plasma and cerebrospinal fluid after intravenous infusion. J Pharm Biomed Anal. 1985;3(2):131–139. doi: 10.1016/0731-7085(85)80016-9. [DOI] [PubMed] [Google Scholar]
- Sauer D., Rischke R., Beck T., Rossberg C., Mennel H. D., Bielenberg G. W., Krieglstein J. Vinpocetine prevents ischemic cell damage in rat hippocampus. Life Sci. 1988;43(21):1733–1739. doi: 10.1016/0024-3205(88)90485-7. [DOI] [PubMed] [Google Scholar]
- Schuirmann D. J. A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm. 1987 Dec;15(6):657–680. doi: 10.1007/BF01068419. [DOI] [PubMed] [Google Scholar]
- Sonne J., Loft S., Døssing M., Vollmer-Larsen A., Olesen K. L., Victor M., Andreasen F., Andreasen P. B. Bioavailability and pharmacokinetics of oxazepam. Eur J Clin Pharmacol. 1988;35(4):385–389. doi: 10.1007/BF00561369. [DOI] [PubMed] [Google Scholar]
- Steinijans V. W., Diletti E. Statistical analysis of bioavailability studies: parametric and nonparametric confidence intervals. Eur J Clin Pharmacol. 1983;24(1):127–136. doi: 10.1007/BF00613939. [DOI] [PubMed] [Google Scholar]
- van Hecken A. M., Tjandramaga T. B., Verbesselt R., de Schepper P. J. The influence of diflunisal on the pharmacokinetics of oxazepam. Br J Clin Pharmacol. 1985 Sep;20(3):225–234. doi: 10.1111/j.1365-2125.1985.tb05065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


