Abstract
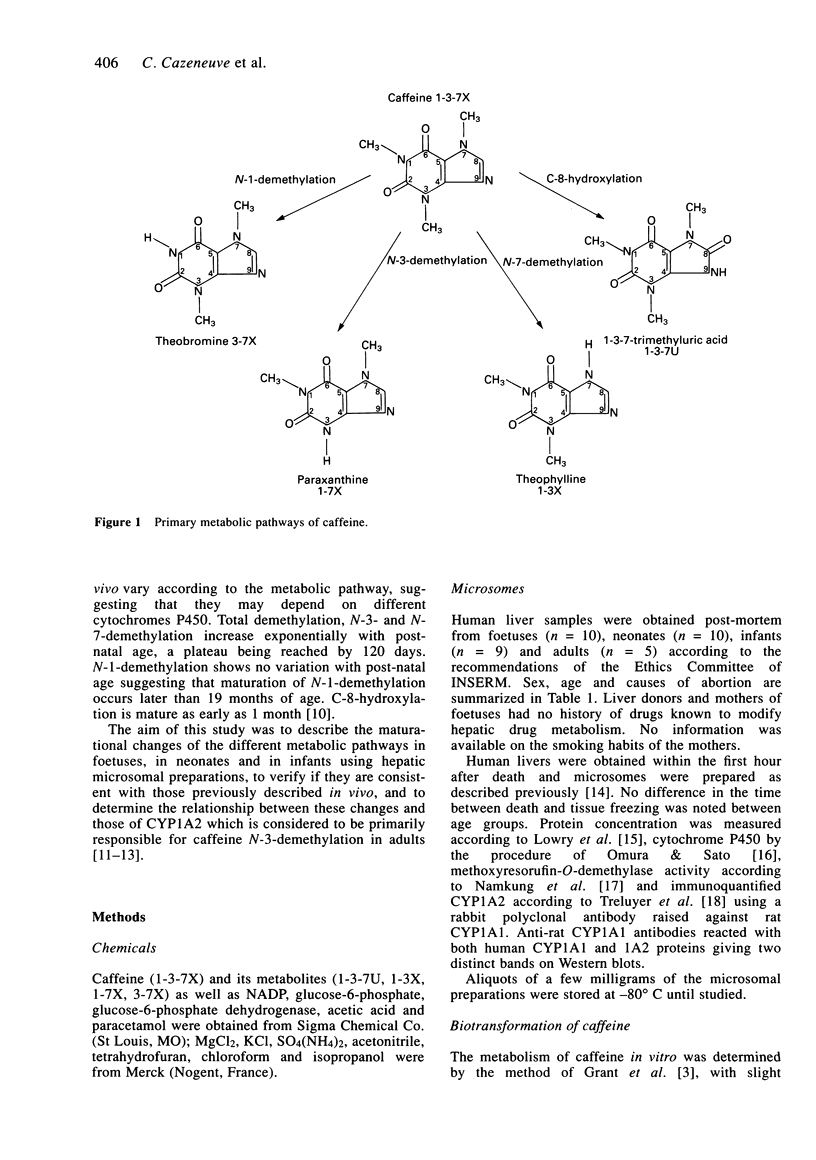
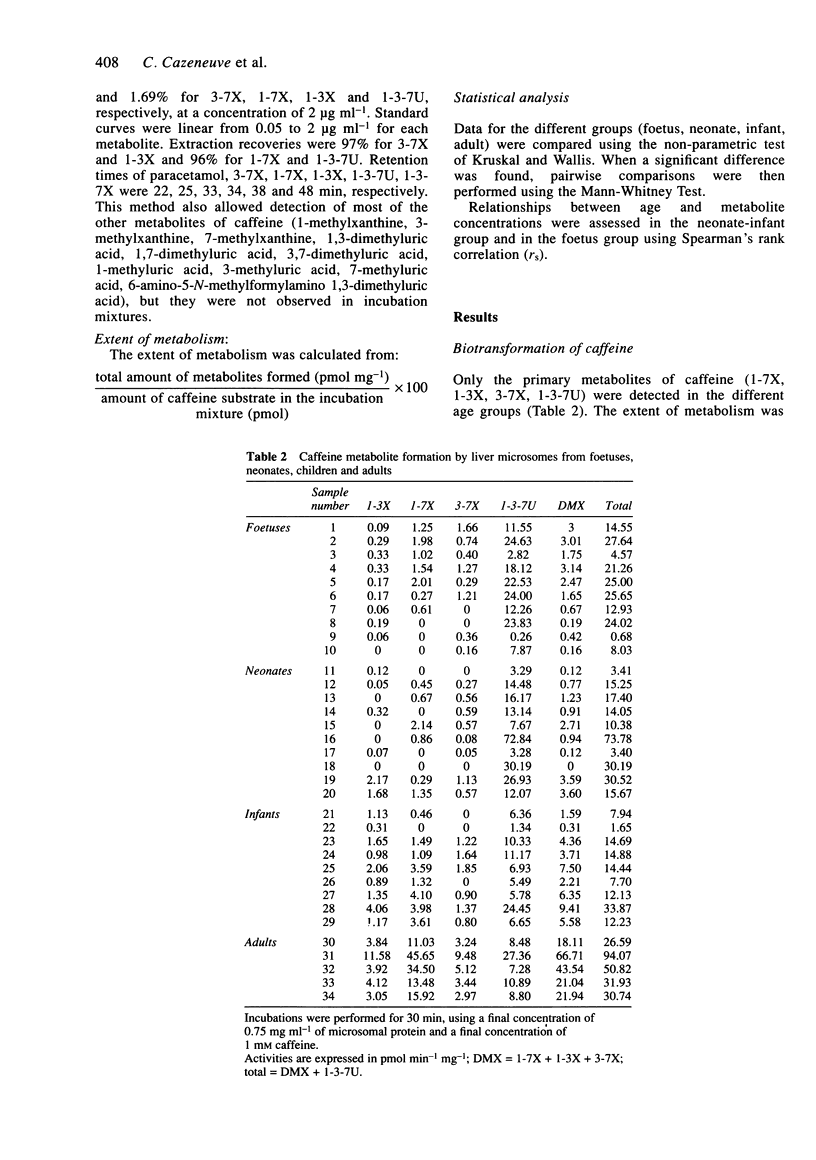
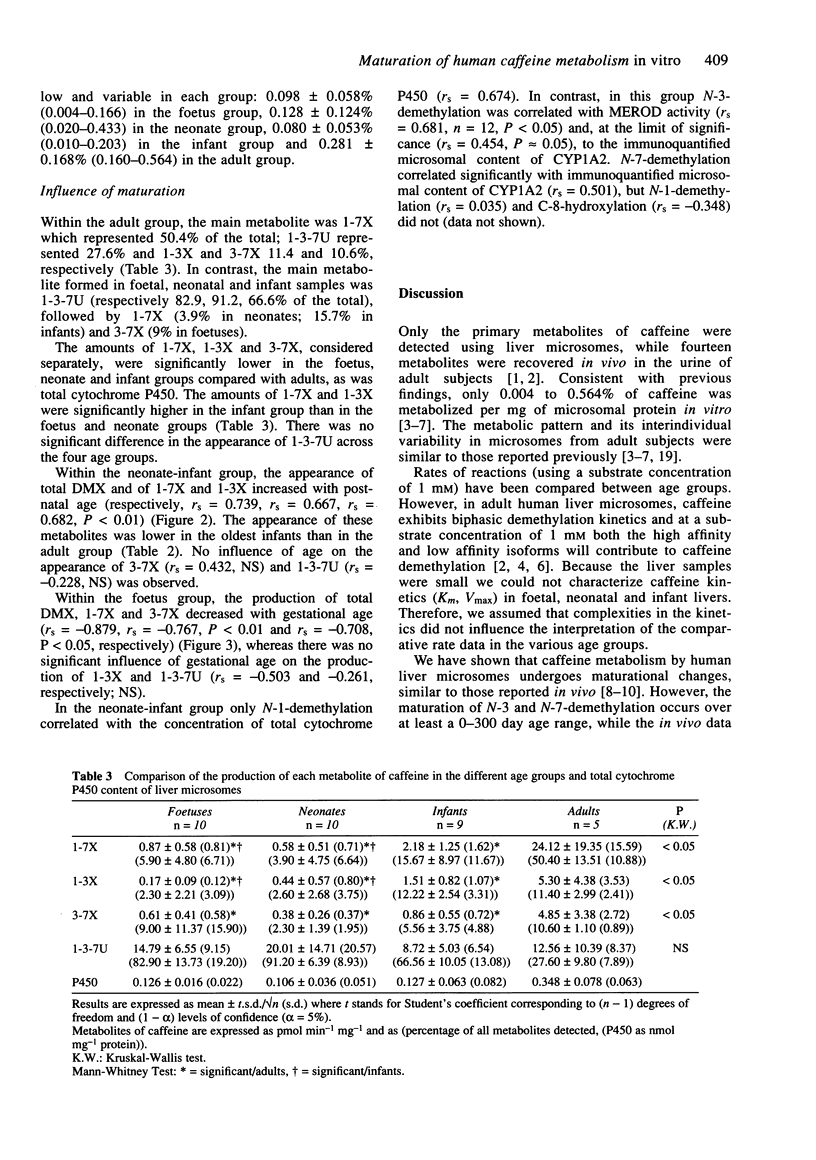
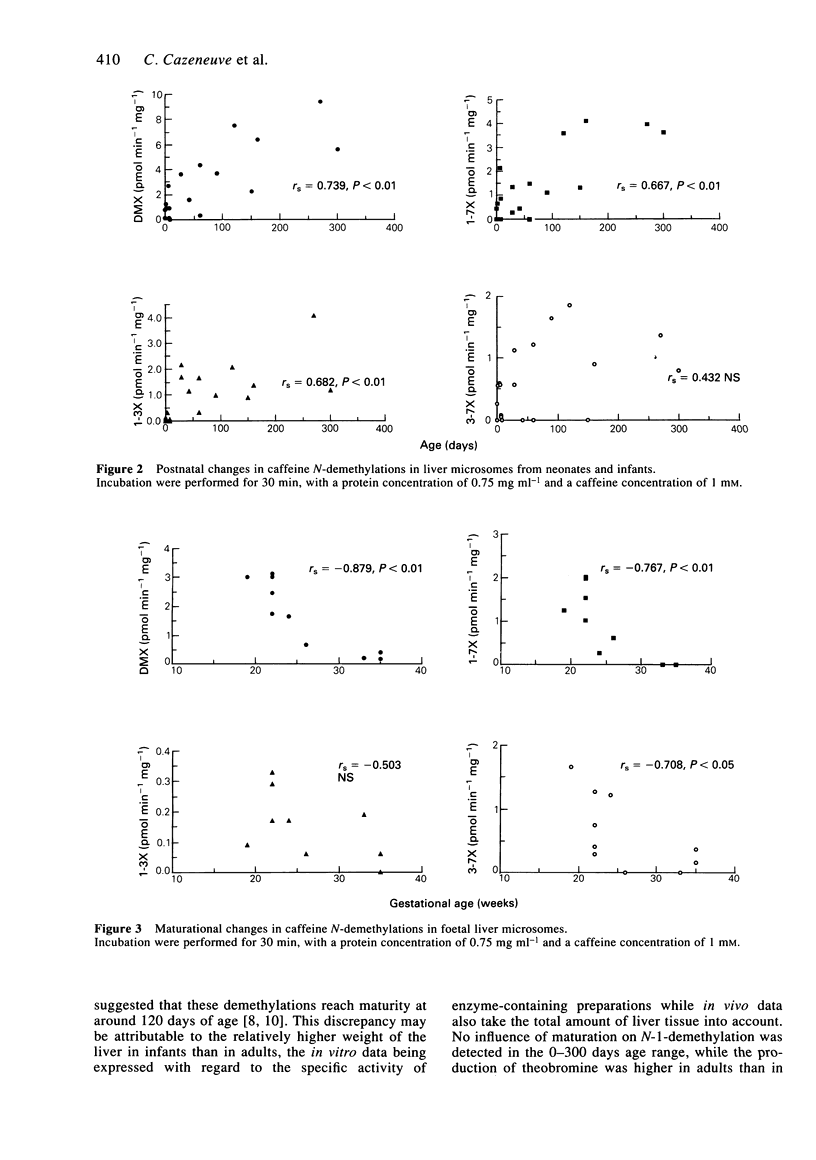
1. Caffeine metabolism was studied in human liver microsomes from foetuses (n = 10), neonates (n = 10), infants (n = 9) and adults (n = 5). Caffeine and its metabolites, 1-3-7-trimethyluric acid, paraxanthine, theophylline and theobromine, were assayed by h.p.l.c. Methoxyresorufin-O-demethylase activity (MEROD) was determined and immunoquantifiable levels of CYP1A2 were measured. 2. The formation of the dimethylxanthines by N-3, N-7 or N-1-demethylation was significantly less in foetuses, neonates and infants than in adults, as shown previously in vivo. The formation of 1-3-7-trimethyluric acid (C-8-hydroxylation) was not significantly different between age groups. The production of total dimethylxanthines, paraxanthine and theophylline increased significantly with age within the neonate-infant group over at least the 0-300 day range (rs = 0.739, 0.667, 0.682, respectively). These data differ from those reported in vivo which suggested that N-3 and N-7-demethylations matured at about 120 days. The difference in maturational profiles of each metabolic pathway suggests that the reactions depend on different isoenzymes. The delay in the maturation of N-1 compared with N-3 and N-7-demethylation is in agreement with previous in vivo data. 3. In the neonate-infant group, only N-3-demethylation correlated with both MEROD activity (rs = 0.681; P < 0.05) and CYP1A2 microsomal concentration (rs = 0.454; P approximately 0.05), suggesting that, as in adults, this reaction depends on CYP1A2. 4. In the foetal samples, the production of total dimethylxanthines, paraxanthine and theobromine decreased significantly (rs = -0.879, -0.767, -0.708, respectively) with increasing gestational age.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthou F., Flinois J. P., Ratanasavanh D., Beaune P., Riche C., Guillouzo A. Evidence for the involvement of several cytochromes P-450 in the first steps of caffeine metabolism by human liver microsomes. Drug Metab Dispos. 1991 May-Jun;19(3):561–567. [PubMed] [Google Scholar]
- Berthou F., Ratanasavanh D., Alix D., Carlhant D., Riche C., Guillouzo A. Caffeine and theophylline metabolism in newborn and adult human hepatocytes; comparison with adult rat hepatocytes. Biochem Pharmacol. 1988 Oct 1;37(19):3691–3700. doi: 10.1016/0006-2952(88)90402-9. [DOI] [PubMed] [Google Scholar]
- Berthou F., Ratanasavanh D., Riche C., Picart D., Voirin T., Guillouzo A. Comparison of caffeine metabolism by slices, microsomes and hepatocyte cultures from adult human liver. Xenobiotica. 1989 Apr;19(4):401–417. doi: 10.3109/00498258909042282. [DOI] [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Grant D. M., Inaba T., Kalow W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome(s) P-450 in human liver microsomes. Drug Metab Dispos. 1987 Mar-Apr;15(2):237–249. [PubMed] [Google Scholar]
- Carrier O., Pons G., Rey E., Richard M. O., Moran C., Badoual J., Olive G. Maturation of caffeine metabolic pathways in infancy. Clin Pharmacol Ther. 1988 Aug;44(2):145–151. doi: 10.1038/clpt.1988.129. [DOI] [PubMed] [Google Scholar]
- Cresteil T., Beaune P., Kremers P., Celier C., Guengerich F. P., Leroux J. P. Immunoquantification of epoxide hydrolase and cytochrome P-450 isozymes in fetal and adult human liver microsomes. Eur J Biochem. 1985 Sep 2;151(2):345–350. doi: 10.1111/j.1432-1033.1985.tb09107.x. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Campbell M. E., Tang B. K., Kalow W. Biotransformation of caffeine by microsomes from human liver. Kinetics and inhibition studies. Biochem Pharmacol. 1987 Apr 15;36(8):1251–1260. doi: 10.1016/0006-2952(87)90078-5. [DOI] [PubMed] [Google Scholar]
- Grant D. M., Tang B. K., Kalow W. Variability in caffeine metabolism. Clin Pharmacol Ther. 1983 May;33(5):591–602. doi: 10.1038/clpt.1983.80. [DOI] [PubMed] [Google Scholar]
- Kalow W. Variability of caffeine metabolism in humans. Arzneimittelforschung. 1985;35(1A):319–324. [PubMed] [Google Scholar]
- Kitada M., Kamataki T., Itahashi K., Rikihisa T., Kanakubo Y. Significance of cytochrome P-450 (P-450 HFLa) of human fetal livers in the steroid and drug oxidations. Biochem Pharmacol. 1987 Feb 15;36(4):453–456. doi: 10.1016/0006-2952(87)90350-9. [DOI] [PubMed] [Google Scholar]
- Kitada M., Kamataki T., Itahashi K., Rikihisa T., Kato R., Kanakubo Y. Purification and properties of cytochrome P-450 from homogenates of human fetal livers. Arch Biochem Biophys. 1985 Aug 15;241(1):275–280. doi: 10.1016/0003-9861(85)90383-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ladona M. G., Park S. S., Gelboin H. V., Hammar L., Rane A. Monoclonal antibody directed detection of cytochrome P-450 (PCN) in human fetal liver. Biochem Pharmacol. 1988 Dec 15;37(24):4735–4741. doi: 10.1016/0006-2952(88)90345-0. [DOI] [PubMed] [Google Scholar]
- Namkung M. J., Yang H. L., Hulla J. E., Juchau M. R. On the substrate specificity of cytochrome P450IIIA1. Mol Pharmacol. 1988 Nov;34(5):628–637. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Pons G., Blais J. C., Rey E., Plissonnier M., Richard M. O., Carrier O., d'Athis P., Moran C., Badoual J., Olive G. Maturation of caffeine N-demethylation in infancy: a study using the 13CO2 breath test. Pediatr Res. 1988 Jun;23(6):632–636. doi: 10.1203/00006450-198806000-00021. [DOI] [PubMed] [Google Scholar]
- Pons G., Carrier O., Richard M. O., Rey E., d'Athis P., Moran C., Badoual J., Olive G. Developmental changes of caffeine elimination in infancy. Dev Pharmacol Ther. 1988;11(5):258–264. doi: 10.1159/000457700. [DOI] [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Murray B. P., Murray S., Segura J., de la Torre R., Davies D. S. Furafylline is a potent and selective inhibitor of cytochrome P450IA2 in man. Br J Clin Pharmacol. 1990 Jun;29(6):651–663. doi: 10.1111/j.1365-2125.1990.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero F., de la Torre R., Boobis A. R., Murray S., Segura J. Assay of caffeine metabolism in vitro by human liver microsomes using radio-high-performance liquid chromatography. J Pharm Biomed Anal. 1990;8(8-12):783–787. doi: 10.1016/0731-7085(90)80121-5. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Molowa D. T., Guzelian P. S. Identification of a cytochrome P-450 in human fetal liver related to glucocorticoid-inducible cytochrome P-450HLp in the adult. Biochem Pharmacol. 1988 Aug 1;37(15):3053–3055. doi: 10.1016/0006-2952(88)90299-7. [DOI] [PubMed] [Google Scholar]


