Abstract
To get an insight into Borna disease virus (BDV) epidemiology, an isolated flock of approximately 25 sheep within the region of Southeast Germany to which the disease is endemic was investigated over a 3-year observation period. BDV-specific antibodies and RNA in peripheral blood mononuclear cells were detected in 12.5 (year 1), 11.5 (year 2), and 19.4% (year 3) and 1.6 (year 1), 0 (year 2), and 14.9% (year 3) of the animals, respectively. BDV persisted in asymptomatic sheep for up to 2 years. Significantly higher numbers of antibody-positive animals were detected seasonally in spring and early summer, the times when usually most of the clinical cases of Borna disease occur. In spring of the third year, numbers of antibody-positive and viral-RNA-positive animals increased significantly despite their having no obvious clinical symptoms. The removal of all antibody- and RNA-positive animals from the flock did not reduce the prevalence of BDV infections in the following year. During a 3-month observation period of three antibody-positive animals, viral RNA was repeatedly detected by reverse transcription-PCR in nasal secretions, saliva, and conjunctival fluids. Sequence analysis revealed clustered nucleotide exchanges among sheep BDV p24 genomes, which differed at five positions from the clustered nucleotide exchanges seen in horse BDV p24 genomes.
Borna disease virus (BDV) is an unsegmented negative-strand RNA virus that causes a nonpurulent encephalomyelitis leading to neurologic and behavioral abnormalities in several vertebrate species, including horses, sheep, cattle, goats, rabbits, cats, and dogs (16, 28, 47). Due to the unique genetic and biological features which involve replication and transcription in the nucleus, RNA splicing, and overlap of open reading frames and transcription units (13, 14, 39), BDV was classified in a new family, Bornaviridae, within the order Mononegavirales.
Clinical disease in sheep reminiscent of Borna disease (BD) in horses was first reported by Walther at the end of the last century (46). Subsequent infection experiments using brain homogenates from diseased sheep caused inflammatory reactions and clinical symptoms in rabbits (3). Since then, BDV infections in sheep were reported from Germany, Switzerland, Lichtenstein, Italy, China, and Japan (11, 19, 20, 28, 33, 45). Naturally occurring BD, however, still seems to be confined to certain areas of central Europe to which it is endemic, despite the unrestricted trade of animals. In these areas, BD in sheep is diagnosed mainly with a seasonal accumulation of diseased animals between March and September, a feature that has not changed since the beginning of the last century (11, 16; T. Vahlenkamp, A. Konrath, and H. Müller, unpublished data). The number of diseased animals differs each year, but no correlation between the numbers of diseased horses and sheep has been seen in disease-endemic areas (16). Clinical signs vary from behavioral changes to severe neurological disorders reflecting the inflammatory reactions in the CNS. BD symptoms generally begin with a short prodromal stage of depression and anorexia. This is followed by overt disease, characterized by somnolence, ataxia, dysphagia, and multiple neuronal deficits. Some sheep recover from these disease signs (32), but usually the course of the disease is progressive over 1 to 3 weeks and affected animals are euthanatized because of their poor prognosis. Animals of all age groups acquire the disease. Histological and immunohistochemical investigations of brains were performed from naturally (10, 33) and experimentally (3, 22, 29, 31) infected animals. Postmortem investigation of naturally infected sheep showed that CD4+ and CD8+ cells are found in the perivascular, parenchymal, and meningeal infiltrates. In general, CD4+ cells outnumber CD8+ cells in perivascular infiltrates, whereas CD8+ cells spread more readily into the neuroparenchyma. Macrophages and B cells are less often seen (10). Despite great variations in the degree of inflammatory reactions, the immunopathogenesis of BD in naturally infected animals seems to be caused by a T-cell-dependent immune reaction which has also been documented in experimentally infected mice and rats (4, 17, 26, 34).
BDV epidemiology is not well understood. This includes the mode of transmission, virus reservoirs, persistence of the infection, and seasonal occurrence of the disease (16, 28, 42). The majority of naturally acquired infections remain clinically unapparent. In horses, antibody prevalence among healthy animals in Germany has been reported to be approximately 10%, which increases to more than 20% in areas to which the disease is endemic (24, 37). Infectious virus has been reported to be incidentally isolated from nasal and lacrimal secretions as well as saliva of diseased horses, and the presence of viral RNA was reported in these secretions in asymptomatic, antibody-positive horses (24, 38). In some stables repeated outbreaks of BD are seen over several years, and sometimes numerous animals in one stable develop BD within a period of months (10, 33). Detailed epidemiologic studies with horses, however, are difficult to perform due to the frequent contacts between animals during sportive competitions and trade.
We performed a 3-year follow-up study with an isolated flock of sheep located within a BDV-endemic region. Investigating the prevalence of BDV-specific antibodies and RNA in the peripheral blood, we found seasonal activations of BDV infections in spring and early summer. Persistently infected, asymptomatic animals were found to shed BDV in nasal and conjunctival fluids as well as saliva, but not in urine, as determined by reverse transcription-PCR (RT-PCR). The removal of all antibody- and RNA-positive animals from the flock after the second year of observation did not reduce the prevalence of BDV infections in the following year. Analysis of the BDV genome sequence derived from the brains of diseased sheep revealed high homologies to the BDV reference strains V and He/80, both originally derived from horses with BD. Clustered nucleotide exchanges in brain-derived sheep BDV p24 sequences, however, differed at several positions from the clustered nucleotide exchanges in horse-derived BDV p24 sequences.
MATERIALS AND METHODS
Animals.
The flock consisted of approximately 25 moorland sheep (breed: Heidschnucke). The year before the study started, six animals of the flock died due to immunohistochemically confirmed BDV infections. During the entire observation period of 3 years, the animals were kept on the farm without contacts with other animals known to be susceptible to BDV infection. During winter the animals were kept indoors with hay and concentrated food. The latter was given supplementarily over the entire breeding season. During spring and summer, all animals were kept on three grazing paddocks directly adjacent to the farm. No foreign livestock was brought into the flock. One male animal was kept within the flock for natural reproduction. Lambs were usually slaughtered at 5 to 6 months of age. In total, seven or eight blood samples were taken from 18 to 26 animals each in the months of May, July, and October. We investigated in total 15 lambs born to serologically positive mothers (animals no. 2, 12 [first year], 7, 10, 19, 24, 25, and 26 [3rd year]) and four lambs born to viral RNA-positive mothers (animals no. 14, 18, and 19 [third year]) for the presence of antibodies and viral RNA in the blood. In order to investigate whether the removal of all antibody- and RNA-positive animals from the flock reduced the prevalence of BDV infections in the following year, we removed these animals after the second year of observation.
Detection of BDV-specific antibodies.
Blood samples were investigated for the presence of BDV-specific antibodies using an indirect immunofluorescence assay (IFA) (23). Serum dilutions were reacted with acetone-fixed infected and uninfected Madin-Darby canine kidney (MDCK) cells. Cells were analyzed by fluorescence microscopy for specific intranuclear reactions. Antibody titers of ⩾20 were defined as positive. A flock of 45 healthy sheep with no history of BD was investigated prior to the study to verify the IFA. None of the sheep sera was found positive for BDV-specific antibodies.
Virus isolation.
Brain suspensions were prepared as described recently (17). Briefly, brain sections were subjected to Dounce homogenization in Glasgow modified Eagle's medium containing 2% fetal calf serum as 10% (wt/vol) suspensions. After three (15-s) ultrasonic pulses, the material was clarified by centrifugation at 4°C for 10 min at 1,000 × g, and supernatants were stored at −70°C. Coculture experiments were performed using rabbit embryonic brain (REB) cells or oligodendroglia (oligo) cells. Tenfold dilutions of the homogenized brain suspensions were cocultured with suspensions of REB or oligo cells. Cells were cultured for 10 passages and analyzed for specific antigen after each passage by IFA.
Isolation and detection of viral RNA.
Swab samples taken from the eyes, nose, and saliva were immediately stored in guanidinium isothiocyanate. RNA was extracted according to the method of Chomczynski and Sacchi (12). Brain samples were stored immediately at −70°C. RNA was isolated using the RNeasy Mini kit (Qiagen) or according to the method of Chomczynski and Sacchi (12). Blood (3 ml) was collected from the animals by jugular venipuncture using EDTA. The isolation of RNA from at least 3 × 106 peripheral blood mononuclear cells (PBMC) was performed using the QIAamp RNA blood mini kit (Qiagen).
The BDV p40-specific RT-PCR was performed as described recently (44). The BDV p24-specific primers used to investigate the swab and urine samples were BDV p24 s (5′-AGCTAGTGACGGAGCTGG-3′, nucleotides (nt) 1501 to 1518 [9]), BDV p24 as (5′-ATGCGCGGAGGTGCAGGA-3′, nt 1822 to 1805), BDV p24 nested s (5′-CGCATCGAGGCAGGGTTTGA-3′, nt 1578 to 1597), and BDV p24 nested as (5′-CGGCGGTTGATGCGTAGAGG-3′, nt 1773 to 1754). Amplification was performed using the same temperature profile as described for the BDV p40-specific primers (44). The sensitivity for both the BDV-p24-specific (unpublished) and BDV-p40-specific (44) RT-PCR has been determined to be between 1 to 10 RNA molecules by the use of in vitro-synthesized RNA standards from different laboratories. We performed the RT-PCR under strict conditions, with local separation of RNA isolation, preparation of the reaction buffers, amplification, and product analysis. Each RT-PCR was routinely screened for contaminations using RT-dependent amplification controls as well as negative reagent controls. All these controls remained negative. The samples were coded and randomly analyzed at the end of each year.
Histological and immunohistochemical examination.
Brain samples of the euthanatized animals (bulbus olfactorius, frontal cortex, hippocampus, cerebellum, and medulla oblongata) were fixed in 4% nonbuffered formalin and embedded in paraffin. For microscopical examination, sections were stained with hematoxylin-eosin. The presence of BDV antigen was investigated with the peroxidase-antiperoxidase method using the monoclonal antibody Bo 18 and rabbit polyclonal sera directed against recombinant BDV p24 and p40 antigens, kindly provided by J. Richt (University of Giessen, Giessen, Germany).
In situ hybridization.
Two digoxigenin-labeled probes were generated from the genome sequence of animal no. 9. The BDV p24-specific probe (length, 195 bp) was amplified using BDV p24 nested s and BDV p24 nested as primers. The BDV p40-specific probe (length, 280 bp) was generated using BDV p40 s (5′-TTACGGGGAAAAGACGA-3′, nt 407 to 423 [9]) and BDV p40 as (5′-TTAGTAGAGACAACACAAAGGAG-3′, nt 687 to 665) primers. Formalin-fixed sections were treated twice with Roti-Histol (Roth, Karlesruhe, Germany) and isopropanol. After rehydration, slides were treated for 15 min at 70°C with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), incubated for 10 min at 37°C with proteinase K (10 μg/ml in 50 mM Tris-HCl, pH 7.6), and washed with aqua bidest. BDV p24- and p40-specific probes were resuspended in 10 μl of herring sperm DNA (10 mg/ml) and 14 μl of formamide and mixed with hybridization solution (86 μl of formamide, 40 μl of 20× SSC, 4 μl of 50× Denhardt's solution, 40 μl of 25× dextran sulfate, 2 μl of aqua bidest). After denaturation for 10 min at 95°C, slides were hybridized overnight at 33°C. Slides were washed twice with 2× SSC and once at 45°C with 0.1× SSC. After incubation with buffer 1 (0.1 M Tris-HCl [pH 7.4], 0.15 M NaCl), slides were equilibrated at 37°C in binding buffer (820 μl of buffer 1, 50 μl of 2% bovine serum albumin, 30 μl of 10% Triton X-100). Slides were briefly rinsed with binding buffer and incubated for 1 h at 37°C with alkaline phosphatase-conjugated antidigoxigenin diluted 1:100 in binding buffer. Slides were washed twice with buffer 1 and once with buffer 2 (0.1 M Tris-HCl [pH 9.5], 0.1 M NaCl, 0.05 M MgCl2). nitroblue tetrazolium-5-bromo-4-chloro-3-indolylphosphate (Roche) was subsequently used as the substrate.
Sequence analysis.
The nucleotide sequence (3,694 bp; corresponding to nt 54 to 3747 of the reference strain V [9]) of the viral genome encoding BDV p40, p10, p24, p16, and p56 genome regions derived from the brain of animal no. 9 was analyzed. Genome regions were amplified using the Titan One Tube RT-PCR System (Roche) and cloned into the plasmid pCR 2.1-Topo (Invitrogen). The nucleotide sequences were determined using an ABI PRISM 377 sequencer (Perkin-Elmer). Sequence alignments were performed using DNA Star software.
Statistical analysis.
The chi-square test for nonbalanced, observational data was used to compare the antibody and viral RNA prevalences at the different bleeding time points. Differences were considered significant at P values of <0.05.
Nucleotide sequence accession number.
BDV genome sequences determined here were submitted to GenBank under the accession numbers listed in Tables 3 and 4.
TABLE 3.
Comparative sequence analysis of horse (strains V and He/80) and sheep BDV isolates with reference to BDV S-589a
| Isolate and/or host | No. of substitionsb for:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p40
|
p10
|
p24
|
p16
|
p56
|
||||||
| nt | aa | nt | aa | nt | aa | nt | aa | nt | aa | |
| Strain V | 18 (1.6) | 1 (0.3) | 6 (2.3) | 2 (2.3) | 9 (1.5) | 4 (2) | 12 (2.9) | 1 (0.7) | 35 (2.3) | 7 (1.4) |
| He/80 | 56 (5.4) | 3 (0.8) | 5 (1.9) | 2 (2.3) | 12 (2) | 2 (1) | 14 (3.3) | 0 | 54 (3.6) | 8 (1.6) |
| Sheepc (S1) | NAh | NA | NA | NA | 14 (2.3) | 2 (1) | NA | NA | NA | NA |
| Sheepd (S2) | NA | NA | NA | NA | 4 (0.6) | 3 (1.5) | NA | NA | NA | NA |
| Sheepe (S6) | NA | NA | NA | NA | 5 (0.8) | 4 (2) | NA | NA | NA | NA |
| Sheep 1f | NA | NA | NA | NA | 16 (2.6) | 1 (0.5) | NA | NA | NA | NA |
| Sheepg | 42 (3.8) | 3 (0.8) | NA | NA | 17 (2.8) | 1 (0.5) | 13 (2.9) | 0 | 60 (3.9) | 4 (0.8) |
GenBank accession number AY066023; from sheep no. 9.
Numbers in parentheses indicate divergence as a percentage of the total sequence. nt, nucleotides; aa, amino acids.
Accession no. U94883.
Accession no. U94884.
Accession no. U94885.
Sequence derived from the work of Binz et al. (5).
NA, not available.
TABLE 4.
Nonrandom distribution of nucleotide exchanges in the BDV p24 coding region
| Species | Isolate or accession no. | Sequence for nucleotide positiona:
|
||||||
|---|---|---|---|---|---|---|---|---|
| 1383-1388 | 1476-1481 | 1491-1496 | 1656-1667 | 1695-1700 | 1707-1712 | 1749-1754 | ||
| * | * | * | * * | * | * | * | ||
| Horse | S67507 | GCA CTG | CTG TCG | CTT ATC | GAC AGC ATC AAG | GAT CGC | AAG ACA | GAC CTC |
| U94864 | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| U94868 | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| WT-1b | ... ... | ... ... | ... ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| 1-2c | ... ... | ... ... | ..A ... | ..T ... ... ... | ... ... | ... ... | ... ... | |
| 2-1c | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| 3c | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| 4c | ... T.. | ... ... | ..A ... | ..T ... ... ... | ... ... | ... ... | ... ... | |
| Sheep | U94883 | ... ... | ... ... | ... ... | ... ... ... ... | ... ... | ..A ... | ..T ... |
| U94884 | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| U94876 | ... ... | ..A ... | ... ... | ... ... ... ..A | ..C ... | ..A ... | ..T ... | |
| U94885 | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| AY066023 | ... T.. | ... ... | ..A ... | ... ... ... ... | ... ... | ... ... | ... ... | |
| 1c | ... ... | ..A ... | ... ... | ... ... ... ..A | ..C ... | ..A ... | ..T ... | |
According to Briese et al. (9). All available completely sequenced BDV p24 genome regions derived from the brains of infected horses and sheep were analyzed. The nonrandomly distributed, clustered nucleotide exchanges at positions 1386, 1478, 1493, 1658, 1667, 1697, 1709, and 1751 are marked by asterisks.
Schneider et al. (40).
Binz et al. (5).
RESULTS
Clinical signs.
In July of the second year, animal no. 9 developed clinical signs of BD with ataxia being the most prominent one. The animal was 15 months old and was euthanatized after 1 week due to the rapid progression of the disease. With the exception of this animal, none of the animals irrespective of detectable antibodies in the plasma and/or viral RNA in the PBMC showed clinical signs of BD. Also, animal no. 2 did not show signs of BD. This animal showed an increase in titer of antibody within 2.5 months from 40 to 160 and was also found to be positive for viral RNA in the PBMC in the second bleeding.
Detection of BDV-specific antibodies.
The prevalence of antibodies among the animals in the three following years was 12.5, 11.5, and 19.4% with the highest titers of antibody being 160, measured in animals no. 2 and 24 (Table 1). The results from October of the second year were not included, because only selected animals were investigated. A significantly higher number of antibody-positive animals (P = 0.031) were detected in the samples taken in spring (May) and early summer (July), the seasons in which most clinical cases are diagnosed, than towards the ends of the years (October). In particular, in May of the third year, the number of antibody-positive animals was significantly higher (P = 0.005) than for all other bleedings. Comparing the samples taken in May and July, three out of four antibody-positive animals in the first year of observation showed an increase in antibody titer. In the third year of observation, six out of nine animals showed a decrease in antibody titer between the samples taken in May and those taken in July. Among the lambs born to serologically positive mothers and those born to viral RNA-positive mothers, only one lamb born to antibody-positive animal no. 26 was antibody positive beyond the third month of age.
TABLE 1.
Follow-up of BDV-specific antibodies in plasma and viral RNA in cells of the peripheral blood over 3 years
| Sheep | Results for year:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
2
|
3
|
||||||||||||||
| May
|
July
|
October
|
July
|
October
|
May
|
July
|
October
|
|||||||||
| Aba | RNAb | Ab | RNA | Ab | RNA | Ab | RNA | Ab | RNA | Ab | RNA | Ab | RNA | Ab | RNA | |
| 1 | NDc | ND | − | − | ND | ND | − | − | ND | ND | 80 | − | 20 | − | − | − |
| 2 | 40 | − | 160 | + | • | • | • | • | • | • | • | • | • | • | • | • |
| 3 | − | − | − | − | − | − | − | − | ND | ND | − | − | ND | ND | − | − |
| 4 | − | − | − | − | − | − | − | − | • | • | • | • | • | • | • | • |
| 5 | − | − | − | − | − | − | − | − | ND | ND | − | − | ND | ND | − | − |
| 6 | − | − | − | − | ND | ND | − | − | ND | ND | 20 | + | 20 | − | − | − |
| 7 | •d | • | − | − | − | − | − | − | ND | ND | 20 | − | ND | ND | − | − |
| 8 | • | • | − | − | − | − | − | − | ND | ND | − | − | ND | ND | − | − |
| 9 | • | • | − | − | − | − | − | (BD)e | • | • | • | • | • | • | • | • |
| 10 | − | − | − | − | − | − | − | − | ND | ND | 20 | − | − | − | − | − |
| 11 | − | − | − | − | − | − | − | − | ND | ND | − | − | − | − | • | • |
| 12 | 40 | − | 20 | − | 20 | − | 20 | − | 20 | − | • | • | • | • | • | • |
| 13 | − | − | 80 | − | • | • | • | • | • | • | • | • | • | • | • | • |
| 14 | − | − | − | − | − | − | − | − | ND | ND | − | + | − | − | − | − |
| 15 | − | − | − | − | − | − | − | − | ND | ND | − | − | − | − | − | − |
| 16 | • | • | • | • | • | • | • | • | ND | ND | 20 | + | 20 | − | − | − |
| 17 | − | − | − | − | − | − | − | − | ND | ND | − | − | − | − | • | • |
| 18 | − | − | − | − | − | − | − | − | ND | ND | − | + | − | − | − | − |
| 19 | − | − | − | − | − | − | − | − | ND | ND | 20 | + | − | − | − | − |
| 20 | − | − | − | − | − | − | − | − | ND | ND | − | + | − | − | − | − |
| 21 | − | − | − | − | − | − | − | − | ND | ND | − | − | − | − | − | − |
| 22 | − | − | 20 | − | − | − | − | − | ND | ND | − | − | − | − | − | − |
| 23 | • | • | • | • | 20 | − | 40 | − | 20 | − | • | • | • | • | • | • |
| 24 | • | • | • | • | − | − | − | − | ND | ND | 160 | − | 20 | − | − | − |
| 25 | • | • | • | • | − | − | − | − | ND | ND | 20 | − | − | − | − | − |
| 26 | • | • | − | − | − | − | − | − | ND | ND | 20 | − | − | − | • | • |
| 27 | • | • | • | • | • | • | 40 | − | (10) | − | • | • | • | • | • | • |
| 28 | − | − | − | − | − | − | − | − | ND | ND | − | − | − | − | − | − |
| 29 | • | • | • | • | − | − | − | − | ND | ND | − | + | − | − | − | + |
| 30 | • | • | • | • | • | • | • | • | ND | ND | − | + | ND | ND | − | − |
| 31 | • | • | • | • | • | • | • | • | ND | ND | − | + | − | − | − | − |
| 32 | • | • | • | • | • | • | • | • | ND | ND | − | − | − | − | − | − |
Ab, Antibody titers were measured by IFA using serial plasma dilutions on persistently BDV-infected MDCK cells. Antibody titers of ≥20 were defined as positive. Titer of antibody for animal no. 27 in October of the second year is given in parentheses.
RNA, The presence of BDV p40 RNA was determined as previously described (44).
ND, not done.
•, animal not yet born or tested or removed from the flock or slaughtered.
(BD), animal was euthanatized due to clinical signs of BD.
Animal no. 12 remained serologically positive over two years and was thereafter removed with animals no. 23 and 27 from the flock to be analyzed for viral shedding. Animal no. 9 was euthanatized due to BD. At the time point of euthanasia, antibodies could not be detected in either serum or cerebrospinal fluid.
Detection of viral RNA.
Similar to the high numbers of antibody-positive samples in May and July, more positive RT-PCR results were also obtained among the samples taken in spring and early summer than among the samples taken in October (Table 1). For the number of viral RNA-positive samples, however, this was not statistically significant (P = 0.13). Interestingly, in May of the third year the number of viral RNA-positive samples increased significantly (P < 0.001) compared to all other bleedings. At this time point also the highest number of antibody-positive animals were detected in the flock. Only three out of nine (33%) RT-PCR positive animals were also positive for BDV-specific antibodies. In July of the first year, animal no. 2 showed a titer of antibody of 160 and was also positive for viral RNA. Unfortunately, this animal was removed from the flock without notice.
In total, 15 lambs born to serologically positive mothers and four lambs born to viral RNA-positive mothers were investigated for the presence of viral RNA in the peripheral blood. None of the lambs was found positive for viral RNA in the PBMC.
In Table 2, the RT-PCR results from the swabs (eye, nose, and saliva) and urine samples are summarized. Viral RNA was detected in all three animals among swab samples, but never in urine. Most of the positive results were obtained from the nose, especially in the samples from animal no. 12. One of these samples was positive for both BDV p24 and p40 coding sequences, whereas the other positive samples were either BDV p24 or p40 specific.
TABLE 2.
Examination of three asymptomatic (seropositive) sheep for the presence of viral RNA in secretions and excretions over a period of 2 and 3 months
| Sheep | Month | Titer of antibody ina:
|
Presence of viral RNA inb:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Plasma | Liquor | PBMC | Urine | Saliva | Nasal secretion | Lacrimal fluid | ||
| 27 | 0 | (10) | − | −/− | −/− | −/− | −/+ | |
| 1 | NDc | ND | ND | −/− | −/− | −/− | −/− | |
| 2 | (10) | − | − | −/− | −/− | +/− | −/− | |
| 3 | ND | ND | ND | −/− | +/− | −/− | −/− | |
| 12 | 0 | 20 | − | − | −/− | −/− | +/+ | −/− |
| 1 | ND | ND | ND | −/− | −/− | +/− | −/− | |
| 2 | (10) | − | − | −/− | +/− | +/− | −/− | |
| 3 | ND | ND | ND | −/− | −/− | −/− | +/− | |
| 23 | 0 | 20 | − | − | −/− | −/− | −/− | −/− |
| 1 | ND | ND | ND | −/− | −/− | −/− | −/− | |
| 2 | (10) | − | − | −/− | −/− | −/− | +/− | |
Antibody titers were measured by IFA using serial plasma dilutions on persistently BDV-infected MDCK cells. Titers below the defined threshold of 20 are in parentheses.
The detection of BDV p40 and BDV p24 RNA is given as p40/p24.
ND, not done.
Histological and immunohistochemical examination.
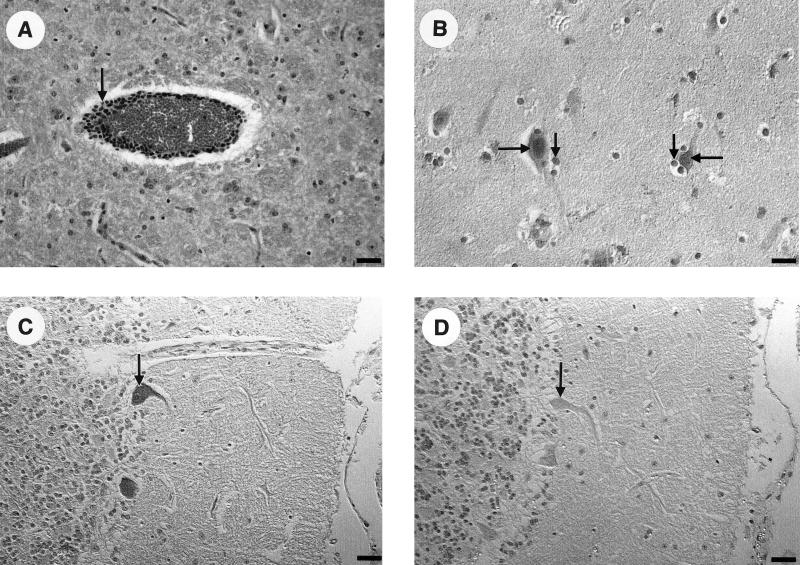
The immunohistochemical and histological results of the brain sections from sheep no. 9 showed a mild nonsuppurative polioencephalomyelitis dominated by mild to moderate perivascular inflammatory reactions, including cuffs of lymphocytes, mainly in the frontal cortex (Fig. 1A ) as well as in the hippocampus. Whereas only a few neurons stained positive for BDV p24 antigen in the frontal cortex, numerous neuronal cells in the hippocampus and Purkinje cells in the cerebellum were found to be infected (Fig. 1B and C). Despite the examination of several brain sections (bulbus olfactorius, frontal cortex, hippocampus, cerebellum, medulla oblongata), immunohistochemical examinations of the three asymptomatic, serologically positive animals no. 12, 23, and 27 showed no clear positive reaction with the polyclonal anti-BDV p24 or anti-BDV p40 rabbit sera. Only in sheep no. 12 was a weak reaction observed with the BDV p24-specific antiserum (data not shown).
FIG. 1.
Histological and immunohistochemical examination of paraffin sections derived from the brain of sheep no. 9. Mild to moderate perivascular cuffs of lymphocytes (arrow) are seen in hematoxylin-eosin-stained sections of the frontal cortex (A). Using anti-BDV p24 rabbit serum, infected neuronal cells and Purkinje cells were detected immunohistochemically in the hippocampus (B) and the ganglion cell layer of the cerebellum (C), respectively. The smaller arrows in Fig. 1B show mild satellitosis. No reaction was observed using a negative rabbit serum as shown for the cerebellum (D). Nomarski interference contrast. Bar = 50 μm.
In situ hybridization.
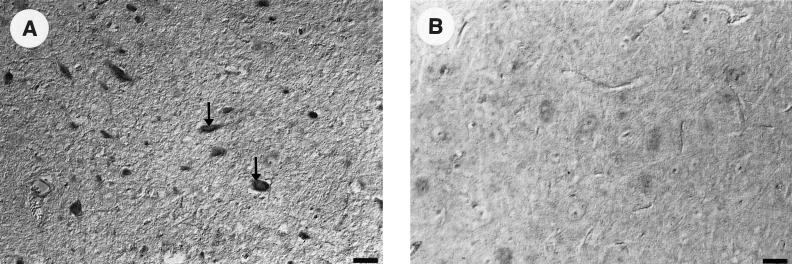
The brain sections obtained from animal no. 12 were further investigated. This animal showed a weak immunohistochemical reaction in the brain and was serologically positive over 2 years, and several swab samples of this animal were positive for viral RNA. As shown in Fig. 2A, in situ hybridization was positive in the brain stem by using both the BDV p24- and BDV p40-specific probes. No reaction was observed when using these probes on brain stem sections derived from a negative, uninfected sheep (Fig. 2B).
FIG. 2.
In situ hybridization using paraffin sections from the brain stem of sheep no. 12. Infected ganglia were detected using BDV-p24- and BDV-p40-specific probes (A). No reaction was seen in the sections derived from the brain stem of a negative control animal (B). Nomarski-Interferenzkontrast. Bar = 50 μm.
Sequence analysis.
Nucleotide sequence analysis of the BDV p40, p10, p24, gp18, and gp57 encoding regions derived from the viral genome of sheep no. 9 revealed homologies to the BDV reference strains V and He/80 between 97.1 and 98.5% and 94.6 and 98.1%, respectively (Table 3). The highest sequence homologies were seen within the BDV p40 and BDV p16 coding regions, with differences in the deduced amino acids of only 0 to 0.8%. Viruses from sheep did not show a higher degree of similarity to each other than to the reference strains, which were originally derived from diseased horses. The deduced amino acid sequence of sheep BDV p56 (from sheep no. 9) revealed mutations with respect to the horse-derived BDV strains V (P→L [at position 3], V→A [17], S→R [60], S→N [68], T→A [220], A→V [234], S→G [296]) and He/80 (S→F [at position 7], Q→R [21], S→R [60], S→N [68], S→P [242], K→R [243], R→K [245], M→V [282]). Except for the mutations at position 296 (BDV strain V) and 282 (BDV He/80), all mutations are located within the N-terminal part of BDV p56, which is involved in receptor recognition and virus entry (35).
Alignment of all completely sequenced BDV p24 coding regions derived from the brains of diseased horses and sheep revealed that most nucleotide exchanges in both animal species are nonrandomly distributed and cluster at certain nucleotide positions. In Table 4 the clustered nucleotide exchanges at positions 1386, 1478, 1493, 1658, 1667, 1697, 1709, and 1751 are shown. Whereas at most positions in the BDV p24 coding region the nucleotide exchanges are at the same sequence positions among genomes derived from horse and from sheep (nt 1386, 1400, 1493, 1535, 1559, 1565, 1574, 1613, 1794, 1851, and 1859; in Table 4 exemplified by the pattern at positions 1386 and 1493), nucleotide exchanges cluster at some positions with respect to their species. At position 1658, nucleotide exchanges seem to occur preferentially in horses, whereas at positions 1478, 1667, 1697, 1709, and 1751, exchanges are seen only in sheep-derived sequences. These nucleotide exchanges are either purine (G/A) or pyrimidine (T/C) exchanges.
Sequence analysis of the RT-PCR products derived from the PBMC and the swab samples confirmed the specificity of the amplified cDNA. The RT-PCR products (p40) from the PBMC of animal no. 2 and those (p24 and p40) from the nose swab samples from animal no. 12 revealed nucleotide sequences identical to the sequence of animal no. 9.
DISCUSSION
We investigated a flock of approximately 25 sheep, which did not have contact with other sheep or livestock during the entire observation period of 3 years. The antibody prevalence differed significantly between individual bleedings and also between the years' seasons. Higher prevalences of BDV infection were detected in spring and early summer. The RT-PCR results also showed a higher prevalence of BDV in the peripheral blood in spring and early summer. This, however, was not statistically significant (P = 0.13). In May of the third year, the number of viral RNA-positive samples differed significantly (P < 0.001) from the other bleedings. The increase in the number of positive RNA samples coincides with the significant increase of antibody-positive animals at this time point. Sixty percent of the animals were positive in May of the third year by either of the infection markers, versus 4.5 to 20% of the animals at the other bleeding times. Both infection markers correlated by their significant increase, but there was no correlation between the markers in the individual animals. Only three of the nine RT-PCR positive animals (33%) also had BDV-specific antibodies at this time point. The accumulated detection of antibodies and viral RNA in the peripheral blood in spring and early summer are reminiscent of the seasonal and epidemic outbreaks of the disease, which are reported for sheep (11, 33). These markers, however, do not seem to be sufficient to monitor BDV epidemiology. The removal of all antibody- and RNA-positive animals in the second year of observation did not result in a lower incidence of BDV infections in the following year. A marked decrease in antibody titers within 2 to 3 months was observed in animals no. 1, 24, and 27. In naturally BDV-infected animals, antibodies are generally of low titer and are not permanently present (16, 32). This is in contrast to a number of acute viral infections, which often result in prolonged (lifelong) production of virus-specific antibodies (1, 15). Concurrent with the resolution of the acute infection, virus-specific plasma cells rapidly decline in the spleen and plasma cells appear in the bone marrow, which becomes the major site of long-term high-affinity antibody production (41, 43). Plasma cells generated in that late phase of an immune reaction represent a successful humoral immune response, and therefore a regulatory function of antigen in humoral immune responses has been postulated (2, 30) in which the generation of long-lived plasma cells in the bone marrow depends on the clearance of antigen. In persistent infections, the continuous presence of antigen probably does not evoke the generation of long-lived plasma cells unless the antigen is eventually cleared (30). A significant part of antibody titers in persistent infections might therefore be derived from short-lived plasma cells, which are continuously generated by the ongoing immune reaction. The rapid turnover rate of short-lived plasma cells generated within germinal centers of the spleen and lymph nodes and the short half-life of immunoglobulin G, usually in the order of days to, at most, a few weeks (8, 25), might explain the decrease of antibody titers measured in the animals no. 1, 24, and 27. Recently Bode et al. (7) reported BDV antigens (bound to PBMC and free in plasma) and circulating immunocomplexes (CIC) to be the most reliable markers for BDV infections in animals and men. This is of particular interest, since plasma antigenemia paralleled by high levels of CIC reflects high burdens of viral proteins that cannot be complexed by antibodies. The present study was initiated before the recent description of CIC and BDV antigens in the plasma of infected animals. Whether the frequently observed absence of antibodies or their presence at low titers in naturally infected sheep (10, 16, 24, 32) are due to the presence of plasma antigens and CIC has to be the focus of further investigations. Plasma antigenemia and CIC might also explain the detection of viral RNA in the absence of detectable antibodies, a condition found only in natural BDV infections (44).
In the first year of observation, three out of four antibody titers increased between the bleedings in May and July. In the third year of observation, most of the antibody-positive animals (six out of nine) showed decreasing titers between these times of bleeding. It is unknown whether this points to a similar time point of BDV exposure/activation or reflects the general immune responsiveness within the flock, since plasma antigens and CIC might influence these titers of antibody. In most animals, BDV-specific antibodies were detected only temporarily. Only two animals had detectable antibody titers over a period of 1 and 2 years. In situ hybridization verified the presence of BDV in the brain of the animal seropositive for 2 years. Obviously, this animal was a persistently infected BDV carrier. The animal was 8 years old and never showed clinical signs of BD. Since BDV-infected cells are nonuniformly distributed in the brains of infected animals (4, 10, 27) and even in animals with overt disease sometimes difficult to detect (10, 32), this animal might not have been the only persistently infected animal in the flock. The events that trigger the transition of BDV infection to immunopathological reactions and likewise the factors that eventually lead to its resolution are at present unknown and impose further investigations. Also, upon experimental infection using different infection routes, only about half of the sheep developed disease (29).
Some cases of vertical transmission of BDV have been reported (6, 21). Because BDV-specific antibodies are transferred with the colostrum to the suckling lambs, serum antibodies within the first 3 months of age are not indicative of BDV infections. We investigated in total 15 lambs born to serologically positive mothers and four lambs born to viral RNA-positive mothers for the presence of antibodies and viral RNA in the blood. None of the lambs was found positive for viral RNA. Only one lamb was antibody-positive beyond the third month of age. As evidenced by animal no. 9, BDV infections cannot always be detected in the blood, but these data seem to show that vertical transmission of BDV is not a common route of infection. Further investigations should include the analysis of plasma antigenemia and CIC.
In contrast to urine, the analysis of swab samples (conjunctival and nasal fluids, saliva) from three asymptomatic, seropositive animals revealed the presence of viral RNA in these secretions. One nasal swab sample (animal no. 12) was positive for both BDV p24 and p40 RNA. The detection of viral RNA in swab samples has also been reported from seropositive, asymptomatic horses (38). Herzog et al. (24) reported the isolation of infectious virus from swab samples and lacrimal and parotic glands of diseased horses. In experimentally infected sheep, Heinig et al. (22) reported the isolation of infectious virus from nasal secretions. The infection, however, was also performed by the intranasal route. We did not succeed in isolating BDV from the swab samples, but obviously, in naturally infected sheep as well, BDV is shed in nasal and lacrimal secretions and transmission most likely occurs through open nerve endings of the nasal and pharyngeal mucosa. Transmission of BDV from sheep to sheep might be possible but does not seem to occur frequently. In search of a possible virus reservoir, we also investigated mice and the cat on the farm, but we did not detect viral RNA or BDV-specific antibodies.
One animal died due to BD. The localized inflammatory response in the CNS around blood vessels (Fig. 1) and the rapid progression of the disease within 1 week point to an acute infection, which might explain why BDV-specific antibodies could not yet be detected. In the brain, numerous Purkinje cells in the cerebellum and a few cells in the frontal cortex were found infected. A remarkably similar distribution of the inflammatory reaction and distribution of viral antigen has been described by Caplazi and Ehrensperger (10) for a diseased horse. Coculture experiments using brain homogenates and REB cells or oligo cells (H. Ludwig, personal communication) gave transient positive IFA results after the second and third passage, but we did not succeed in isolating the virus in cell culture.
Sequence analysis of BDV amplified from the brain of this animal revealed a close phylogenetic relationship to the reference strains V and He/80. Their genome sequences differed by less than 5.4%. The N-terminal part of BDV p56 has been shown to be involved in receptor recognition and virus entry (35). The C-terminal part encoding the furin-cleaved gp43 has been shown to be implicated in pH-dependent fusion after internalization of the virion by endocytosis (18, 36) Analysis of the horse-derived (strain V, He/80) and sheep-derived BDV p56 sequences (U94878, BDV S-589) suggests a selective pressure on the N-terminal part of the glycoprotein, since the C-terminal part is much more conserved. Viruses from the horses did not show a higher degree of similarity to each other (40) than to the sheep-derived virus, raising the assumption of a single animal source from which the various animals acquire the infection (42). A rodent reservoir has been discussed, but so far, all attempts to identify BDV in a rodent species have failed (Vahlenkamp, Konrath, and Müller, unpublished data). Adaptation to neuronal receptors might also contribute to the high level of conservation of the BDV genome among different mammalian species. However, assuming a high degree of viral adaptation, one should expect small changes in the viral RNA among different animal species. We aligned the nucleotides of all completely sequenced BDV p24 encoding regions derived from the brains of infected horses and sheep and analyzed the clustered, nonrandom distribution of nucleotide exchanges (42). Among eight horse and six sheep sequences derived from the brains of the infected animals, mutations at 11 positions clustered at the same positions in horse- and sheep-derived sequences. At five positions (1478, 1667, 1697, 1709, and 1751), however, nucleotide exchanges were observed only in the sheep-derived sequences. At position 1658, exchanges seem to occur preferentially in infected horses. The reason why mutations at these positions are highly preferred over mutations at other positions of the viral genome is unknown. With the exception of the nucleotide exchange at position 1851, none of the mutations results in amino acid substitutions. Most probably, functional constraints on secondary or tertiary RNA structures do exist which restrict sequence alterations to certain nucleotide positions (42). Differences in the clustered nucleotide exchanges as observed between sheep and horse sequences might possibly explain the difficulties in adapting BDV from infected sheep to cell cultures, which has also been described by other investigators (32).
Acknowledgments
We are grateful to Gerald Schusser, Albrecht Uhlig, and Astrid Grosche at the Faculty of Veterinary Medicine (University of Leipzig, Leipzig, Germany) for their help and generously making available animal facilities. Excellent technical assistance was provided by Monika Herold and Marita Wipplinger. We thank Hanns Ludwig (University of Berlin, Berlin, Germany) for the attempts at sheep BDV isolation and Erning Li (North Carolina State University) for statistical analysis. We also thank the shepherd of the flock for his kind support during the three years of investigation.
REFERENCES
- 1.Ahmed, R. 1992. Immunological memory against viruses. Semin. Immunol. 4:105-109. [PubMed] [Google Scholar]
- 2.Bachmann, M. F., T. M. Kundig, H. Hengartner, and R. M. Zinkernagel. 1994. Regulation of IgG antibody titers by the amount persisting of immune-complexed antigen. Eur. J. Immunol. 24:2567-2570. [DOI] [PubMed] [Google Scholar]
- 3.Beck, A., and H. Frohböse. 1926. Die enzootische Encephalitis des Schafes. Vergleichende experimentelle Untersuchungen über die seuchenhafte Gehirnrückenmarksentzündung der Pferde und Schafe. Arch. Tierheilkd. 54:84-110. [Google Scholar]
- 4.Bilzer, T., O. Planz, W. I. Lipkin, and L. Stitz. 1995. Presence of CD4+ and CD8+ T cells and expression of MHC class I and MHC class II antigen in horses with Borna disease virus-induced encephalitis. Brain Pathol. 3:223-230. [DOI] [PubMed] [Google Scholar]
- 5.Binz, T., J. Lebelt, H. Niemann, and K. Hagenau. 1994. Sequence analyses of the p24 gene of Borna disease virus in naturally infected horse, donkey and sheep. Virus Res. 34:281-289. [DOI] [PubMed] [Google Scholar]
- 6.Bode, L., and H. Ludwig. 2001. Borna disease virus—a threat for human mental health? p. 269-310. In G. L. Smith, W. L. Irving, J. W. McCauley, and D. J. Rowlands (ed.), Society for General Microbiology, Symposium 60. Cambridge University Press, Cambridge, United Kingdom.
- 7.Bode, L., P. Reckwald, W. E. Severus, R. Stoyloff, R. Ferszt, D. E. Dietrich, and H. Ludwig. 2001. Borna disease virus-specific circulating immune complexes, antigenemia, and free antibodies—the key marker triplet determining infection and prevailing in severe mood disorders. Mol. Psych. 4:481-491. [DOI] [PubMed] [Google Scholar]
- 8.Brambell, F. W. R., W. A. Hemmings, and I. G. Morris. 1964. A theoretical model of γ-globulin catabolism. Nature 203:1352. [DOI] [PubMed] [Google Scholar]
- 9.Briese, T., A. Schneemann, A. J. Lewis, Y. S. Park, S. Kim, H. Ludwig, and W. I. Lipkin. 1994. Genomic organisation of Borna disease virus. Proc. Natl. Acad. Sci. USA 91:4362-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplazi, P., and F. Ehrensperger. 1998. Spontaneous Borna disease in sheep and horses: immunophenotyping of inflammatory cells and detection of MHC-I and MHC-II antigen expression in Borna encephalitis lesions. Vet. Immunol. Immunopathol. 6:203-220. [DOI] [PubMed] [Google Scholar]
- 11.Caplazi, P., K. Melzer, R. Goetzmann, A. Rohner-Cotti, V. Bracher, K. Zlinszky, and F. Ehrensperger. 1999. Borna disease in Switzerland and in the principality of Liechtenstein. Schweiz. Arch. Tierheilkd. 141:521-527. (In German.) [PubMed] [Google Scholar]
- 12.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 13.Cubitt, B., C. Oldstone, and J. C. de la Torre. 1994. Sequence and genome organization of Borna disease virus. J. Virol. 68:1382-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De la Torre, J. C. 1994. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J. Virol. 68:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doherty, P. C., W. Allan, M. Eichenberger, and S. R. Carding. 1992. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu. Rev. Immunol. 10:123-151. [DOI] [PubMed] [Google Scholar]
- 16.Dürrwald, R., and H. Ludwig. 1997. Borna disease virus (BDV), a (zoonotic?) worldwide pathogen. A review of the history of the disease and the virus infection with comprehensive bibliography. Zentbl. Veterinarmed. B 44:147-184. [DOI] [PubMed] [Google Scholar]
- 17.Enbergs, H. K., T. W. Vahlenkamp, A. Kipar, and H. Müller. 2001. Experimental infection of mice with Borna disease virus (BDV): replication and distribution of the virus after intracerebral infection. J. Neurovirol. 7:272-277. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Dunia, D., B. Cubitt, and J. C. de la Torre. 1998. Mechanism of Borna disease virus entry into cells. J. Virol. 72:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagiwara, K., S. Kawamoto, H. Takahashi, Y. Nakamura, T. Nakaya, T. Hiramune, C. Ishihara, and K. Ikuta. 1997. High prevalence of Borna disease virus infection in healthy sheep in Japan. Clin. Diagn. Lab. Immunol. 4:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagiwara, K., M. Asakawa, L. Liao, W. Jiang, S. Yan, J. Chai, Y. Oku, K. Ikuta, and M. Ito. 2001. Seroprevalence of Borna disease virus in domestic animals in Xinjiang, China. Vet. Microbiol. 80:383-389. [DOI] [PubMed] [Google Scholar]
- 21.Hagiwara, K., N. Momiyama, H. Taniyama, T. Nakaya, H. Tanaka, R. Kirisawa, H. Iwai, and K. Ikuta. 2000. Detection of Borna disease virus in a pregnant mare and her fetus. Vet. Microbiol. 72:207-216. [DOI] [PubMed] [Google Scholar]
- 22.Heinig, A. 1964. Zur experimentellen Infektion der Pferde und Schafe mit dem Virus der Bornaschen Krankheit. Arch. Exp. Vet. Med. 18:753-766. [Google Scholar]
- 23.Herzog, S., and R. Rott. 1980. Replication of Borna disease virus in cell cultures. Med. Microbiol. Immunol. 168:153-158. [DOI] [PubMed] [Google Scholar]
- 24.Herzog, S., K. Frese, J. Richt, and R. Rott. 1994. Ein Beitrag zur Epizootiologie der Bornaschen Krankheit beim Pferd. Wien. Tierärztliche Monatsschrift 81:374-379.2382445 [Google Scholar]
- 25.Junghans, R. P. 1997. IgG biosynthesis: no “immunoregulatory feedback.” Blood 10:3815-3818. [PubMed] [Google Scholar]
- 26.Kao, M., H. Ludwig, and G. Gosztonyi. 1984. Adaptation of Borna disease virus to the mouse. J. Gen. Virol. 65:1845-1849. [DOI] [PubMed] [Google Scholar]
- 27.Lebelt, J., and K. Hagenau. 1996. Die Verteilung des Bornavirus in natürlich infizierten Tieren mit klinischer Erkrankung. Berliner und Münchner Tierärztliche Wochenschrift 109:178-183. [PubMed] [Google Scholar]
- 28.Ludwig, H., and L. Bode. 2000. Borna disease virus: new aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Tech. Off. Int. Epizoot. 19:259-288. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig, H., and M. Kao. 1990. Borna disease in sheep, p. 529-538. In Z. Dinter and B. Morein (ed), Virus infections of ruminants. Elsevier, Amsterdam, The Netherlands.
- 30.Manz, R. A., and A. Radbruch. 2002. Plasma cells for a lifetime? Eur. J. Immunol. 32:923-927. [DOI] [PubMed] [Google Scholar]
- 31.Matthias, D. 1954. Der Nachweis von latent infizierten Pferden, Schafen und Rindern und deren Bedeutung als Virusreservoir bei der Bornaschen Krankheit. Arch. Exp. Vet. Med. 8:506-511. [Google Scholar]
- 32.Metzler, A., F. Ehrensperger, and K. Danner. 1979. Borna-virusinfektion bei Schafen: Verlaufsuntersuchungen nach spontaner Infektion, unter besonderer Berücksichtigung der Antikörperkinetik im Serum und Liquor Cerebrospinalis. Schweiz. Arch. Tierheilk. 121:37-48. [Google Scholar]
- 33.Metzler, A., U. Frei, and K. Danner. 1976. Virologically confirmed outbreak of Borna's disease in a Swiss herd of sheep. Schweiz. Arch. Tierheilkd. 118:483-492. [PubMed] [Google Scholar]
- 34.Narayan, O., S. Herzog, K. Frese, H. Scheefers, and R. Rott. 1983. Behavioral disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science 220:1401-1403. [DOI] [PubMed] [Google Scholar]
- 35.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de la Torre. 2001. N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richt, J. A., T. Fürbringer, A. Koch, I. Pfeuffer, C. Herden, I. Bause-Niedrig, and W. Garten. 1998. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richt, J. A., A. Grabner, and S. Herzog. 2000. Borna disease in horses. Vet. Clin. N. Am. Equine Pract. 3:579-595. [DOI] [PubMed] [Google Scholar]
- 38.Richt, J. A., S. Herzog, K. Haberzettl, and R. Rott. 1993. Demonstration of Borna disease virus-specific RNA in secretions of naturally infected horses by polymerase chain reaction. Med. Microbiol. Immunol. 182:293-304. [DOI] [PubMed] [Google Scholar]
- 39.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, P. A., Briese, T., Zimmermann, W., Ludwig, H., and W. I. Lipkin. 1994. Sequence conservation in field and experimental isolates of Borna disease virus. J. Virol. 68:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slifka, M. K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 43.Tew, J. G., R. M. DiLosa, G. F. Burton, M. H. Kosco, L. I. Kupp, A. Masuda, and A. K. Szakal. 1992. Germinal centers and antibody production in bone marrow. Immunol. Rev. 126:99-122. [DOI] [PubMed] [Google Scholar]
- 44.Vahlenkamp, T. W., H. K. Enbergs, and H. Müller. 2000. Experimental and natural Borna disease virus infections: presence of viral RNA in cells of the peripheral blood. Vet. Microbiol. 76:229-244. [DOI] [PubMed] [Google Scholar]
- 45.Waelchli, R. O., F. Ehrensperger, A. Metzler, and C. Winder. 1985. Borna disease in a sheep. Vet. Rec. 117:499-500. [DOI] [PubMed] [Google Scholar]
- 46.Walther, F. 1899. Gehirn-Rückenmarksentzündung bei Pferden und. Schafen in der Amtshauptmannschaft Borna. Mitteilungen aus den Berichten der Bezirksthierärzte auf das Jahr 1899. Ber. Veterinärwesen Königr. Sachsen 44:80.15276976 [Google Scholar]
- 47.Zwick, W. 1939. Bornasche Krankheit und Enzephalomyelitis der Tiere, p. 254-354. In E. Gildenmeister, E. Haagen, and O. Waldmann (ed.), Handbuch der Viruskrankheiten, vol 2. Fischer-Verlag, Jena, Germany. [Google Scholar]