Abstract
Hepatitis B virus X protein (pX) is implicated in hepatocarcinogenesis by an unknown mechanism. Employing a cellular model linked to pX-mediated transformation, we investigated the role of the previously reported Stat3 activation by pX in hepatocyte transformation. Our model is composed of a differentiated hepatocyte (AML12) 3pX-1 cell line that undergoes pX-dependent transformation and a dedifferentiated hepatocyte (AML12) 4pX-1 cell line that does not exhibit transformation by pX. We report that pX-dependent Stat3 activation occurs only in non-pX-transforming 4pX-1 cells and conclude that Stat3 activation is not linked to pX-mediated transformation. Maximum Stat3 transactivation requires Ser727 phosphorylation, mediated by mitogenic pathway activation. Employing dominant negative mutants and inhibitors of mitogenic pathways, we demonstrate that maximum, pX-dependent Stat3 transactivation is inhibited by the p38 mitogen-activated protein kinase (MAPK)-specific inhibitor SB 203580. Using transient-transreporter and in vitro kinase assays, we demonstrate for the first time that pX activates the p38 MAPK pathway only in 4pX-1 cells. pX-mediated Stat3 and p38 MAPK activation is Ca2+ and c-Src dependent, in agreement with the established cellular action of pX. Importantly, pX-dependent activation of p38 MAPK inactivates Cdc25C by phosphorylation of Ser216, thus initiating activation of the G2/M checkpoint, resulting in 4pX-1 cell growth retardation. Interestingly, pX expression in the less differentiated hepatocyte 4pX-1 cells activates signaling pathways known to be active in regenerating hepatocytes. These results suggest that pX expression in the infected liver effects distinct mitogenic pathway activation in less differentiated versus differentiated hepatocytes.
Chronic infection by the hepatitis B virus (HBV) is the leading cause of liver cancer in humans (59). The World Health Organization has estimated that 400 million people are chronic HBV carriers worldwide, destined to develop liver cancer in the next 30 to 40 years. The 16.5-kDa HBV X protein, termed pX or HBx, is a regulatory protein required for viral replication (17, 69) and is also implicated in hepatocellular carcinoma development, acting as a weak oncogene (14).
A pivotal activity of pX in the hepatocyte is activation of cellular Src (31). pX-mediated c-Src activation occurs via pX-mediated Ca2+ release and is required for HBV replication (10, 32). Considering the effect of pX expression on hepatocyte physiology, pX-mediated c-Src activation is also central in the activation of the mitogenic Ras-Raf-mitogen-activated protein kinase (MAPK) (7, 23) and JNK (6) pathways and likely activation of Stat3 (35, 62). The downstream events resulting from pX-mediated activation of these signaling cascades include transcriptional activation of AP-1 (6, 7, 23, 41), NF-κB (37, 50), CREB/ATF (3, 4, 65), and Stat3-responsive (35, 62) cis-acting elements, leading to transcriptional deregulation of cellular gene expression (34, 54), cell cycle deregulation (5, 9, 34), oncogenic transformation (29, 54, 55), and apoptosis (18, 51, 52). However, the role of each signaling pathway activated by pX in hepatocyte physiology is only now beginning to be understood (34, 54, 55). By the employment of an immortalized, AML12-derived, hepatocyte cell line (3pX-1) expressing pX conditionally, a cellular model linking pX to hepatocyte transformation has been established (54). Recent studies demonstrated the causal role of pX-dependent, sustained activation of the Ras-Raf-MAPK pathway in hepatocyte transformation (55). By contrast, pX-mediated, sustained JNK activation occurs selectively in an AML12-derived, dedifferentiated hepatocyte cell line (4pX-1), in which conditional pX expression does not effect oncogenic transformation (54). In this study, we have employed our 3pX-1 and 4pX-1 cell lines (34, 54, 55) to define the role of the reported pX-mediated Stat3 activation in hepatocyte transformation (35, 62).
Stat3 is one of the signal transducers and activators of transcription (STATs), originally identified to mediate interferon and cytokine signaling (22, 49). STATs are latent cytoplasmic transcription factors, activated by tyrosine phosphorylation (12), a requirement for dimerization via reciprocal phosphotyrosine-SH2 interactions. Following dimerization, STATs translocate to the nucleus to effect transcription of responsive genes. STATs control fundamental biological processes, including cell differentiation, proliferation, apoptosis, and development (13, 20, 26, 28, 53, 68). Regarding liver physiology, it is established that Stat3 activation by interleukin 6 (IL-6) is required for liver regeneration following partial hepatectomy (20).
In addition, Stat3 has been shown to participate in oncogenic transformation in cells transformed by v-src, v-Ab1, and various other oncogenes and tumor viruses (11, 56, 67). Constitutive Stat3 activation occurs with high frequency in a wide variety of human tumors, including leukemias, lymphomas, and breast, ovarian, and melanoma tumors (11). Maximal Stat3 transcriptional activation involves the combinatorial effect of mitogenic cascades, which converge on Stat3 activation by phosphorylating the Ser727 residue (57). In v-src-transformed NIH 3T3 cells, maximal Stat3 transactivation and oncogenic transformation are mediated by JNK and p38 MAPK phosphorylation of Ser727 (57).
Based on the role of constitutive Stat3 activation in v-Src-mediated oncogenic transformation (11, 56, 67), we have employed a well-characterized cellular model of 3pX-1 and 4pX-1 cell lines (34, 54, 55) to investigate the role of Stat3 activation by pX (35, 62) in hepatocyte transformation. Interestingly, we demonstrate that pX effects selective Stat3 activation in the dedifferentiated hepatocytes of the 4pX-1 cell line, which does not become oncogenically transformed by pX. These results support that Stat3 activation is not involved in pX-mediated hepatocyte transformation. We also investigated combinatorial effects of mitogenic pathways in maximally activating the transactivation potential of Stat3 in 4pX-1 cells. We report for the first time that pX activates the p38 MAPK pathway only in the dedifferentiated hepatocyte 4pX-1 cell line and that the activated p38 MAPK regulates maximal Stat3 transactivation by phosphorylation of Ser727. Importantly, activation of both Stat3 and p38 MAPK is dependent on Ca2+ release and c-Src activation by pX. Although the role of Stat3 activation by pX in 4pX-1 cells remains to be determined, the pX-dependent activation of p38 MAPK activates the G2/M checkpoint by phosphorylation of Cdc25C, resulting in a pX-dependent growth delay, characteristic of the 4pX-1 cell line (34).
MATERIALS AND METHODS
Cell culture.
Cell lines 3pX-1 and 4pX-1, derived from AML12 cells, were propagated as described earlier (34, 54, 55). Transient-transfection assays were carried out in triplicate in 3.5-cm-diameter plates, employing Fugene 6 reagent (Roche Molecular Biochemicals). CHOP-10 transreporter assays (Stratagene) included 100 ng of pFA-CHOP and 100 ng of pFR-luciferase. Stat3 reporter assays employed 100 ng of pLucTKS3, 100 of ng pMν-src, and 400 ng of Stat3S727A for both cell lines. DNAs were added onto cells in medium containing 10% fetal bovine serum (FBS) and 5 μg of tetracycline/ml. Following 8 h of incubation, cells were washed three times with phosphate-buffered saline and were incubated in medium containing 10% FBS ±5 μg of tetracycline/ml and ± 25 μM PD 98059 or ± 10 μM SB 203580 or ± 3 μM PP2, as indicated. Luciferase assays were performed as described previously (4, 34, 35).
DNA-protein binding assays were performed with 32P-radiolabeled SIE oligonucleotide probe (56) and nuclear extracts from 3pX-1 and 4pX-1 cells, prepared as described earlier (56).
Immunoprecipitation and immunoblotting assays.
For Stat3 immunoprecipitations, cells were grown in 10% FBS medium ± 5 μg of tetracycline/ml and were collected in 1× radioimmunoprecipitation assay (RIPA) buffer (1× phosphate-buffered saline, pH 7.4; 1% NP-40; 0.5% deoxycholic acid; 0.1% sodium dodecyl sulfate [SDS]; 2.5 mM EGTA; 10% glycerol; 20 mM β-glycerolphosphate; 1 mM Na3VO4 and 1 mM NaF; 10 μg each of pepstatin, leupeptin, and aprotinin per ml; 5 mM p-nitrophenyl phosphate [PNPP]; and 1 mM phenylmethylsulfonyl fluoride [PMSF]). Whole-cell extract (WCE; 250 μg) was immunoprecipitated with Stat3 antibody (Cell Signaling Technology, Inc.) at 4°C overnight, followed by addition of 30 μl of 50% protein A-Sepharose slurry (Sigma) for 1 h at 4°C. Immunoprecipitates were washed twice with 1× RIPA buffer, once with buffer A (20 mM Tris, pH 7.5; 150 mM NaCl; 1 mM EDTA; a 1 mM concentration each of Na3VO4, NaF, and PMSF; and 5 mM PNPP), and once with buffer B (20 mM Tris, pH 7.5; 1 mM EDTA; a 1 mM concentration each of Na3VO4, NaF, and PMSF; and 5 mM PNPP). Immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis and Western blots by enhanced chemiluminescence detection (Amersham Pharmacin Biotech).
In vitro kinase assays.
For p38 MAPK assays, cells were maintained in 10% Dulbecco's modified Eagle's medium-F12 ± 5 μg of tetracycline/ml. Cells were lysed in 1× RIPA buffer at 4°C for 30 min. WCE (150 μg) was mixed with protein A-immobilized phospho-p38 MAPK (Thr180/Tyr182) monoclonal antibody at 1:20 dilution (Cell Signaling Technology, Inc.) at 4°C overnight. Immunoprecipitates were washed twice with 1× RIPA buffer and twice with kinase buffer (25 mM Tris, pH 7.5; 5 mM β-glycerolphosphate; 2 mM dithiothreitol; 0.1 mM Na3VO4; and 10 mM MgCl2). Immunoprecipitates were resuspended in 20 μl of kinase buffer supplemented with 200 μM ATP and 2 μg of glutathione transferase (GST)-ATF2 as substrate (Cell Signaling Technology, Inc.) at 30°C for 30 min. The kinase reaction mixture was analyzed by SDS-polyacrylamide gel electrophoresis followed by Western blots to detect phosphorylated ATF2.
Western blot analyses with phospho-Tyr705-Stat3 and phospho-ATF2 (Thr71) antibodies (Cell Signaling Technology, Inc.) were used to detect phosphorylated Stat3 and ATF2, respectively. For detecting total Stat3 and p38 MAPK, WCE (10 μg) from each time point was analyzed by Western blot analyses employing total Stat3 and p38 antibody (Cell Signaling Technology, Inc.), respectively. For detecting phosphorylated and nonphosphorylated Cdc25C, WCE (30 μg) was analyzed by Western blots employing phospho-Cdc25C (Ser216) and total Cdc25C antibodies (Cell Signaling Technology, Inc.), respectively. Densitometric scanning was performed by the OPTIMAS 6.1 software, and statistical analyses were performed, where indicated, by the Prism software.
RESULTS
Selective pX-mediated Stat3 activation in 4pX-1 cells.
The study by Lee and Yun (35) was the first to report pX-dependent activation of Stat3 in a mouse hepatoma cell line (Hepa 1-6). They suggested that this pX-dependent Stat3 activation is linked to hepatocyte transformation. In our study we employ the two conditional (Tet-off), pX-expressing 3pX-1 and 4pX-1 cell lines, which provide a comparative cellular model linking pX expression to oncogenic transformation (54). 3pX-1 cells are differentiated AML12 hepatocytes (54, 66), which become oncogenically transformed by pX expression following tetracycline removal. The 4pX-1 cell line is a dedifferentiated AML12 hepatocyte cell line in which pX expression does not effect cellular transformation (54).
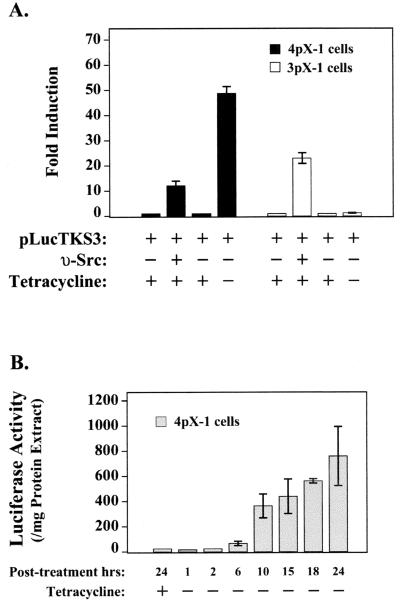
To define the role of the reported Stat3 activation by pX (35, 62) in hepatocyte transformation, we first determined by transient-transfection assays whether pX expression by tetracycline removal leads to Stat3 activation in the two cell lines. A Stat3-responsive reporter construct, pLucTKS3 (56), containing multimerized Stat3-responsive binding sites upstream from the minimal thymidine kinase promoter, was transiently transfected in 3pX-1 and 4pX-1 cells as a function of pX expression. We observe a pronounced and selective pX-dependent expression from the pLucTKS3 reporter in 4pX-1 cells (Fig. 1A). Importantly, in both 3pX-1 and 4pX-1 cell lines, cotransfected v-src expression vector results in pLucTKS3 reporter expression, supporting that both cell lines have functional Stat3 cascades mediating v-Src signaling (Fig. 1A).
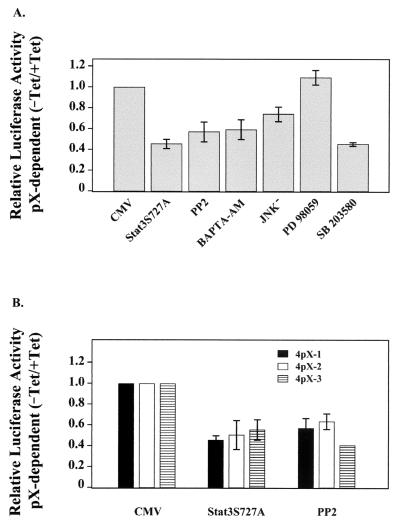
FIG. 1.
Selective pX-mediated Stat3 activation in 4pX-1 cells. (A) Transient transfections in 3pX-1 and 4pX-1 cells of pLucTKS3 reporter (100 ng) ± 5 μg of tetracycline/ml and cotransfections of pLucTKS3 plasmid ± 100 ng of pMv-src plasmid or 100 ng of empty vector as indicated. (B) Transient transfections of pLucTKS3 reporter (100 ng) in 4pX-1 cells. Cells were harvested for luciferase measurement at the indicated times following pX expression.
To assess the kinetics of pX-dependent expression from the pLucTKS3 reporter in 4pX-1 cells, we measured luciferase activity as a function of pX expression, in the interval 1 to 24 h after tetracycline removal. A progressive increase in reporter gene expression is observed starting at 6 h and reaching a maximum by 24 h following pX expression (Fig. 1B). These observations suggest a selective, pX-dependent activation of Stat3 in 4pX-1 cells, occurring 6 to 24 h following pX expression.
pX-dependent Stat3 DNA binding and tyrosine phosphorylation in 4pX-1 cells.
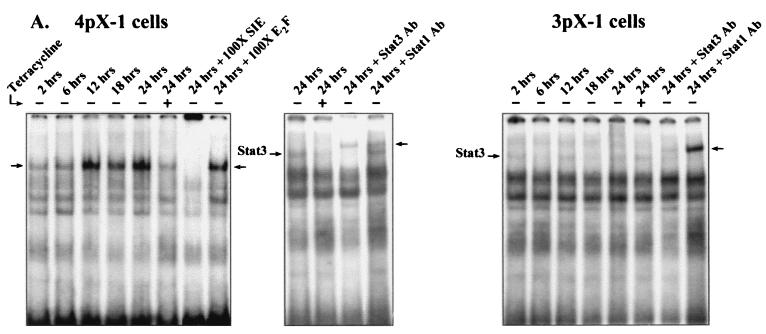
To directly demonstrate pX-dependent, selective activation of Stat3 in 4pX-1 cells, we measured the kinetics of pX-dependent Stat3 DNA-binding activity and Stat3 tyrosine phosphorylation in the two cell lines. Nuclear extracts from 3pX-1 and 4pX-1 cells were prepared (56) in a time course after pX expression. DNA-protein binding assays employed the SIE binding site (56), a high-affinity site that binds both Stat1 and Stat3 (67). DNA-binding assays employing 4pX-1 nuclear extracts demonstrate the pX-dependent appearance of an SIE binding protein complex (Fig. 2A) displaying a progressive increase in its formation, starting at 6 h and reaching a maximum by 24 h following pX expression. Competition assays show that this DNA-protein complex is sequence specific, since it is competed in the presence of a 100-fold-excess SIE binding site DNA but is not competed in a 100-fold-excess E2F binding site DNA (Fig. 2A). Moreover, supershift assays employing the Stat3- and Stat1-specific antibodies demonstrate that this pX-dependent DNA-protein complex is supershifted by Stat3 antibody but not by Stat1 antibody. These experiments conclusively demonstrate that pX promotes the appearance of Stat3 DNA-binding activity in 4pX-1 cells. By contrast, DNA-binding assays employing nuclear extracts from 3pX-1 cells demonstrate that pX expression does not activate Stat3 in 3pX-1 cells (Fig. 2A).
FIG. 2.
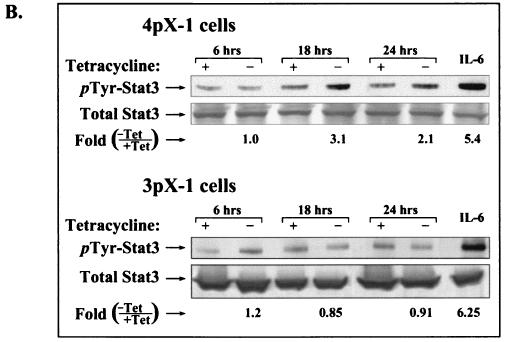
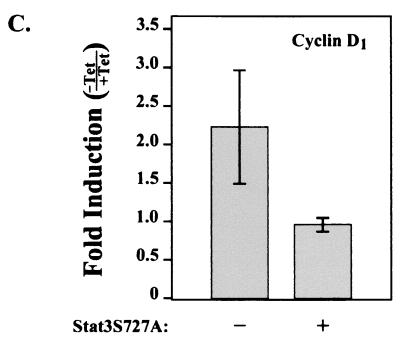
pX-dependent Stat3 DNA binding and tyrosine phosphorylation in 4pX-1 cells. (A) Shown are results of DNA-protein binding assays employing 32P-radiolabeled SIE DNA probe and nuclear extracts (38) from 4pX-1 cells and 3pX-1 cells, prepared in a time course following pX expression. 4pX-1 nuclear extracts were used in DNA-protein binding assays with the SIE probe, in the presence of 100-fold-excess competitor oligonucleotides or Stat3 and Stat1 antibodies, as indicated. (B) WCE (250 μg) from 4pX-1 and 3pX-1 cells isolated at the indicated times ± tetracycline treatment was immunoprecipitated with total Stat3 antibody, followed by Western blot analyses employing phosphotyrosine-specific Stat3 antibody. Treatment of cells with IL-6 was the positive control. Quantitation was performed by digital densitometry using the OPTIMAS 6.1 software. pX-dependent pTyr phosphorylation in 4pX-1 cells is statistically significant (P < 0.007). (C) Real-time quantitative PCR monitoring cyclin D1 expression (34). Total RNA was isolated from 4pX-1 cells grown ± 5 μg of tetracycline/ml for 20 h and from 4pX-1 cells transiently transfected with dn Stat3 (Stat3S727A) ± 5 μg of tetracycline/ml for 20 h. Quantitation was performed relative to the internal control glyceraldehyde-3-phosphate dehydrogenase, as described earlier (34).
The DNA-binding activity of Stat3 requires phosphorylation of tyrosine residue 705. Reciprocal phosphotyrosine-SH2 interactions participate in Stat3 dimerization, a prerequisite for its translocation to the nucleus (68). Thus, Stat3 exhibiting DNA-binding activity (Fig. 2A) is tyrosine phosphorylated. Accordingly, we assessed the appearance of pX-dependent tyrosine phosphorylation of Stat3 in 3pX-1 and 4pX-1 cells (Fig. 2B). We performed Western blot assays using Stat3 phosphotyrosine-specific antibodies and Stat3 immunoprecipitates derived from nuclear extracts from 3pX-1 and 4pX-1 cells. Figure 2B demonstrates that pX-dependent Stat3 tyrosine phosphorylation occurs selectively in 4pX-1 cells and is detectable at 18 and 24 h of pX expression. Collectively, these results (Fig. 2) support that pX expression selectively increases both the DNA-binding activity and tyrosine phosphorylation of Stat3 in 4pX-1 cells in a time frame that is in agreement with the functional reporter assay (Fig. 1B).
To demonstrate that this pX-dependent Stat3 activation in 4pX-1 induces the expression of endogenous Stat3-responsive genes, we examined the expression of cyclin D1. Earlier studies (48) demonstrated that transcription of cyclin D1 in response to ν-Src expression is mediated by Stat3. Moreover, studies (34) demonstrated the pX-dependent induction of cyclin D1 in 4pX-1 cells, occurring within 6 h and lasting 24 h following pX expression. Real-time PCR monitoring cyclin D1 expression was performed as described earlier (34), with RNA samples isolated from 4pX-1 cells expressing pX for 20 h with or without cotransfection of a dominant negative (dn) Stat3 expression vector (57). The results demonstrate (Fig. 2C) that expression of dn Stat3 partially inhibits pX-dependent cyclin D1 RNA transcription, showing that the pX-dependent Stat3 activation deregulates cellular gene expression in 4pX-1 cells.
Effect of mitogenic pathways on pX-dependent Stat3 activation.
Maximum transcriptional regulation of various STATs requires serine phosphorylation mediated by mitogenic signaling cascades (57, 64). Specifically, maximum Stat3 activation by v-Src, leading to oncogenic transformation, requires phosphorylation of serine residue 727 by the JNK and p38 MAPK pathways (57).
Accordingly, we employed dn mutants and specific inhibitors of the mitogenic pathways to assess the contribution of combinatorial, mitogenic pathway signaling in the pX-mediated Stat3 activation of 4pX-1 cells (Fig. 3A). Earlier studies (55) established that pX expression in 4pX-1 cells effects transient activation of the Ras-Raf-MAPK pathway, lasting approximately 2 h after pX expression, and sustained activation of the JNK pathway, lasting 6 to 15 h after pX expression, suggesting that both of these pX-activated mitogenic cascades regulate Stat3 transactivation.
FIG. 3.
Effect of mitogenic pathways on a pX-dependent Stat3 activation. (A) Transient transfections were carried out for pLucTKS3 reporter (100 ng) in the presence of cotransfected plasmid encoding cytomegalovirus (CMV) empty vector (400 ng), dn Stat3S727A (400 ng), or dn JNK− (400 ng) where indicated. Inhibitor concentration is as follows: 3 μM PP2 (43, 44), 20 μM BAPTA-AM (7), 25 μM PD 98059 (24), and 10 μM SB 203580 (40). (B) Transient transfections of pLucTKS3 (100 ng) ± 5 μg of tetracycline/ml in clonal cell lines 4pX-1, 4pX-2, and 4pX-3 (54) in the presence of cotransfected Stat3S727A (400 ng) or 3 μM PP2 inhibitor.
Initially we used the dn Stat3S727A mutant (57) to verify whether pX-dependent Stat3 activation in 4pX-1 cells requires input of mitogenic signaling cascades for maximal transactivation. Transient transfection of the plasmid encoding Stat3, with Ser727-to-Ala substitution, reduced pX-dependent Stat3 activation by 50%, suggesting that its maximum transactivation is dependent on Ser727 phosphorylation (Fig. 3). Importantly, addition of the c-Src-specific inhibitor PP2 (24, 27) or the cytosolic calcium chelator BAPTA-AM (10) inhibited pX-mediated Stat3 activation by approximately 50%, supporting the central role of the pX-mediated Ca2+ release and c-Src activation in Stat3 activation.
To determine whether the pX-dependent, sustained activation of the JNK pathway (55) mediates Ser727 phosphorylation of Stat3, we transiently expressed the dn JNK− mutant (Fig. 3A). We observe an approximately 25% reduction in pX-dependent Stat3 activation by dn JNK− expression. By contrast, addition of the MEK-1-specific inhibitor PD 98059 had no effect on Stat3 transactivation. Interestingly, treatment of 4pX-1 cells with the p38 MAPK-specific inhibitor SB 203580 (25) reduced pX-dependent Stat3 activation by 50%. These results (Fig. 3) support that both the JNK and p38 MAPK pathways regulate the pX-dependent transactivation of Stat3 in 4pX-1 cells but to different levels. Importantly, these results suggest that pX effects activation of the p38 MAPK pathway.
To demonstrate that this pX-dependent activation of Stat3 in 4pX-1 cells is not due to clonal variation, we investigated the occurrence of pX-dependent Stat3 activation in 4pX-2 and 4pX-3 clonal cell lines derived from the same AML12 clone 4 background as the 4pX-1 cell line (54). Both 4pX-2 and 4pX-3 cell lines demonstrated Stat3 activation similar to 4pX-1 cells (Fig. 3B). Importantly, this Stat3 activation is partially inhibited by expression of the dn Stat3S727A mutant and by treatment with the c-Src inhibitor PP2 (Fig. 3B). Accordingly, these observations show that pX activates the same signaling program in dedifferentiated hepatocytes, modeled by the 4pX-1, 4pX-2, and 4pX-3 clonal cell lines.
pX activates the p38 MAPK pathway in 4pX-1 cells.
Since inhibition of p38 MAPK by addition of SB 203580 resulted in nearly 50% reduction in the pX-mediated Stat3 activation, we investigated further whether pX activates the p38 MAPK pathway. We used a transreporter assay that monitors direct activation of p38 MAPK. This assay is comprised of the Gal4UAs-luciferase reporter plasmid and an expression vector encoding the Gal41-147 DNA-binding domain in fusion with the transactivation domain of the transcription factor CHOP-10. CHOP-10 is activated by phosphorylation by the p38 MAPK (61). We demonstrate that pX expression results in reporter gene expression via the conditional activation of CHOP-10, only in the less differentiated 4pX-1 cells. This pX-dependent activation of CHOP-10-mediated transcription is inhibited by addition of SB 203580. Importantly, this pX-mediated activation of the p38 MAPK pathway (Fig. 4A) is also sensitive to inhibition by the c-Src-specific inhibitor PP2 (24, 27), as well as by the cytosolic calcium chelator BAPTA-AM (10). These results demonstrate that pX activates the p38 MAPK only in the less-differentiated 4pX-1 hepatocyte cells in a Ca2+- and c-Src-dependent manner.
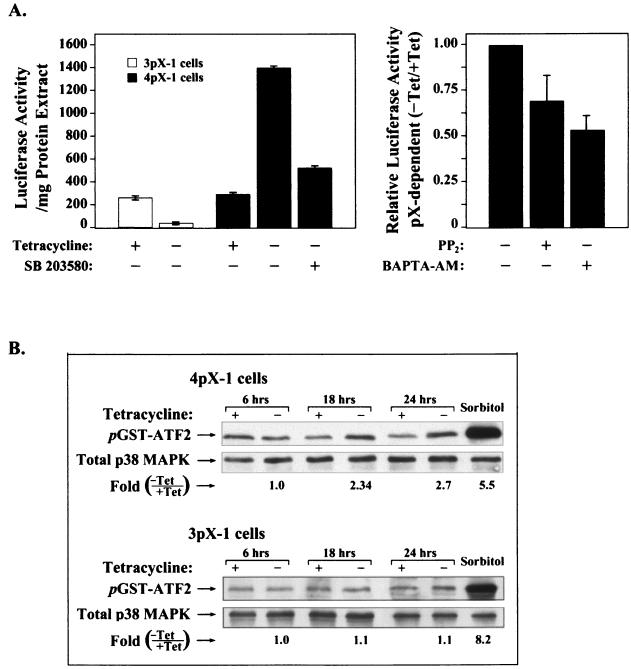
FIG. 4.
pX activates the p38 MAPK pathway in 4pX-1 cells. (A) Transient-transreporter assays employing pFR-luciferase reporter (100 ng) and pFA-CHOP expression (100 ng) plasmids (Stratagene) in 3pX-1 and 4pX-1 cells ± 5 μg of tetracycline/ml. Inhibitor concentration is as follows: 10 μM SB 203580, 3 μM PP2, and 20 μM BAPTA-AM. (B) In vitro p38 immunocomplex kinase assays with WCE (150 μg) from 4pX-1 and 3pX-1 cells, isolated at the indicated times ± 5 μg of tetracycline/ml. GST-ATF2 (2 μg) substrate phosphorylation was monitored by Western blots using phospho-ATF2 antibodies. Sorbitol treatment was the positive control. Quantitation was performed by digital densitometry using the OPTIMAS 6.1 software. pX-dependent ATF2 phosphorylation was statistically significant (P < 0.03).
To directly demonstrate pX-dependent activation of p38 MAPK, we performed immunocomplex kinase assays (19). Cellular extracts were isolated from 3pX-1 and 4pX-1 cells in a time course following pX expression and were immunoprecipitated with phospho-active p38 MAPK antibody. The resulting immunokinase complex was assayed in vitro using GST-ATF2 as the substrate (Fig. 4B). pX-dependent p38 MAPK activation is detected only in the 4pX-1 cell line, occurring after 6 h of pX synthesis (Fig. 4B). These results further confirm that pX activates selectively the p38 MAPK pathway in the less differentiated 4pX-1 hepatocytes.
pX-mediated p38 MAPK activation initiates the G2/M checkpoint in 4pX-1 cells.
We recently reported our studies of the molecular events occurring during pX-dependent cell cycle progression in the 3pX-1 and 4pX-1 cell lines (34). Mitogenic reprogramming of the cell cycle by pX increases pX-dependent G1- and S-phase progression in both cell lines. Interestingly, in 4pX-1 cells, a pX-dependent S-phase pause was observed, linked to the absence of pX-dependent Cdc2 kinase activation. By contrast, in 3pX-1 cells, pX expression effected Cdc2 kinase activation (34).
Activation of Cdc2 kinase is a prerequisite for progression into mitosis (43, 58). This activation requires association of Cdc2 kinase with cyclin B1 (43) and concomitant dephosphorylation of residues Ser14 and Tyr15 by the dual-specificity Cdc25 phosphatases (8, 36, 39, 45, 63). Recent studies have demonstrated the role of p38 MAPK in regulating, directly (15) or indirectly (60), Cdc25 protein phosphatases in response to cellular stress, thereby activating a G2/M mitotic checkpoint and resulting in growth arrest or delay.
Despite pX-dependent transcriptional induction of cdc2 and cyclins B and A in 4pX-1 cells, the absence of pX-dependent Cdc2 kinase activation in 4pX-1 cells (34) suggested an absence of Cdc25 phosphatase activation. Based on the selective, pX-dependent p38 MAPK pathway activation that we observe in 4pX-1 cells (Fig. 4), we tested whether the activated p38 MAPK regulates progression of 4pX-1 cells into mitosis. Initially, we carried out flow cytometry analyses of 4pX-1 cells grown as a function of pX expression for 18 h ± the p38 MAPK-specific inhibitor SB 203580 (Fig. 5A to C). In agreement with previous observations (34), pX expression for 18 h in 4pX-1 cells causes a nearly 40% increase in the number of cells entering S phase (Fig. 5A and B), compared to the number found in the +Tet control. Interestingly, treatment of 4pX-1 cells expressing pX for 18 h with SB 203580 increased by approximately 50% the number of cells that exited from S into G2/M in a pX-dependent manner. These results demonstrate that SB 203580 inhibition of p38 MAPK, which is maximally activated by pX in this 18-h interval (Fig. 4), allows progression of 4pX-1 cells into G2/M, suggesting that pX activation of p38 MAPK inactivates the Cdc25 phosphatase by phosphorylation.
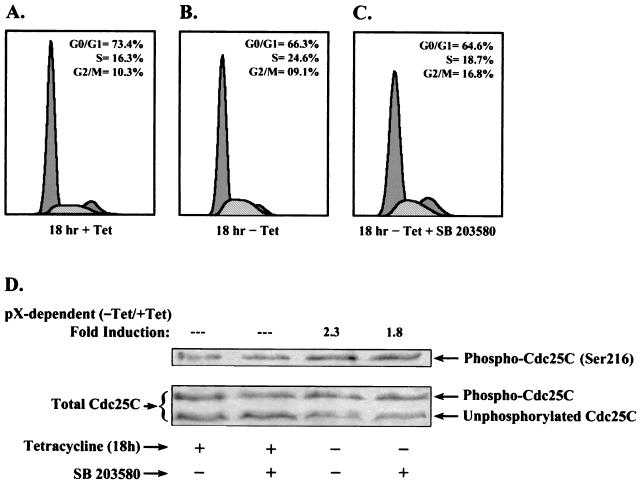
FIG. 5.
pX-mediated p38 MAPK activation initiates G2/M checkpoint in 4pX-1 cells. Flow cytometry analyses of 4pX-1 cells grown for 18 h ± tetracycline addition. Cells were treated with 5 μg of tetracycline/ml (A), not treated with tetracycline (B), or not treated with tetracycline while being treated with 10 μM SB 203580 (C). (D) Western blot analyses of 4pX-1 WCE isolated ± pX for 18 h and ± 10 μM SB 203580. Where indicated, Ser216 phosphorylation of Cdc25C (upper panel) or total Cdc25C (lower panel) was monitored. Quantitation was by the OPTIMAS 6.1 software.
Accordingly, we have employed Western blots using the phospho-specific Cdc25C (Ser216) antibody to monitor pX-dependent Cdc25C phosphorylation at 18 h of pX synthesis ± SB 203580 treatment (Fig. 5D). The results demonstrate a 2.3-fold increase in pX-dependent, Cdc25C phosphorylation, which is sensitive to SB 203580, showing that the pX-mediated activation of the p38 MAPK plays a critical role in initiation of a G2 delay in 4pX-1 cells.
DISCUSSION
By employing a cellular model, linking pX expression to hepatocyte transformation, we have defined the role of the reported pX-dependent activation of Stat3 (35, 62) in the process of pX-mediated transformation.
We demonstrate that pX expression activates Stat3 only in the non-pX-transforming (4pX-1) cell line (Fig. 1). We have used three independent assay methods to demonstrate this selective, pX-dependent, Stat3 activation in 4pX-1 cells: (i) transient reporter assays using the Stat3-responsive reporter pLucTKS3 in 3pX-1 and 4pX-1 cells (Fig. 1); (ii) Stat3 DNA-binding assays using nuclear extracts from 3pX-1 and 4pX-1 cells (Fig. 2A); and (iii) Western blot assays monitoring the appearance of pX-dependent Stat3 tyrosine phosphorylation (Fig. 2B). All three assays demonstrate that pX mediates selective Stat3 activation in the dedifferentiated 4pX-1 hepatocytes, occurring after 6 h of pX expression and reaching maximum activation by 24 h. This selective pX-dependent Stat3 activation in 4pX-1 cells is not due to clonal variation, but it is a characteristic of dedifferentiated hepatocyte cell lines, including the 4pX-2 and 4pX-3 clonal cell lines (Fig. 3B). Importantly, we demonstrate (Fig. 2C) that the pX-mediated transcriptional activation of Stat3 induces the expression of endogenous Stat3-responsive genes. It has recently been shown (34) that 4pX-1 cells express in a pX-dependent manner prolonged levels of cyclin D1 and p21Cip1, genes known to be transcriptionally induced by Stat3 in other cell types (48). Herein we show (Fig. 2C) that the induction of cyclin D1 observed in 4pX-1 cells (34) is partially inhibited by expression of the dn Stat3 mutant (Stat3S727A). These observations indicate that pX-mediated Stat3 activation alters endogenous gene expression in the dedifferentiated 4pX-1 cells.
The observed pX-mediated Stat3 activation in 4pX-1 cells is dependent on Ca2+ release and c-Src activation also known to be effected by pX (10, 31, 32), evidenced by the inhibitory effect of BAPTA-AM and PP2 treatment, respectively (Fig. 3A). These results provide further support for the principle that the pX-mediated Ca2+ release and c-Src activation required for viral replication (10, 32) also play a central role in the cellular responses of the hepatocyte to pX (6, 7, 10, 23, 31, 32). Importantly, both 3pX-1 and 4pX-1 cell lines undergo Ca2+-mediated and c-Src-dependent activation of mitogenic pathways by pX (data not shown). In addition, both cell lines activate endogenous Stat3 by cotransfection with oncogenic v-src (Fig. 1A), suggesting that the Stat3 pathway is intact in both cell types. However, the mechanisms contributing to pX-dependent Stat3 activation in 4pX-1 cells and differential Stat3 activation in the two cell lines remain to be deciphered. We interpret the partial Stat3 inhibition effected by PP2, BAPTA-AM, and SB 203580 to mean that both c-Src-dependent and c-Src-independent mechanisms account for the pX-mediated Stat3 activation. For example, the direct interaction between pX and JAK-1, as reported by Lee and Yun (35), represents such a Src-independent mechanism.
Although dedifferentiated hepatocytes, modeled by the 4pX-1, 4pX-2, and 4pX-3 cells, do exhibit Stat3 activation by pX, they do not undergo pX-mediated transformation (54). Thus, we conclude that Stat3 activation is not directly responsible for hepatocyte transformation.
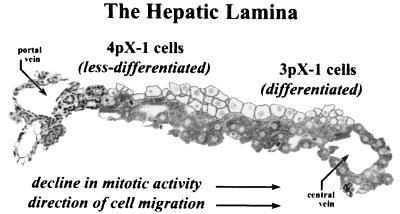
Other studies have linked Stat3 activation to the maintenance of the dedifferentiated state (38) and the continued proliferative capacity of cells (16, 47). Moreover, use of IL-6-deficient mice has shown that activation of Stat3 is essential for liver regeneration (20, 21, 33). Accordingly, we propose that the 4pX-1 cell line, which is less differentiated than the parental AML12 cell line (54) and displays selective pX-dependent JNK (55) and Stat3 activation (Fig. 1 to 3), models the highly mitotic, proliferating hepatocyte, such as those found in the periportal region (40) of regenerating liver (Fig. 6).
FIG. 6.
Less differentiated 4pX-1 cells model periportal, regenerating hepatocytes. Diagram shows the hepatic lamina extending between the portal triad and central vein of the hepatic lobule. Based on selective pX-dependent Stat3, p38 MAPK, and JNK (55) pathway activation, our model proposes that the less differentiated 4pX-1 cell line models periportal, regenerating hepatocytes, whereas 3pX-1 cells model fully differentiated, mature hepatocytes.
Maximal Stat3 transactivation in v-src-transformed fibroblasts involves phosphorylation of Ser727 by JNK and p38 kinases (57). Expression of dn Stat3S727A reduced pX-mediated Stat3 activation by approximately 50% (Fig. 3), indicating the regulatory importance of Ser727 phosphorylation in pX-mediated Stat3 activation. Considering the combinatorial effect of JNK and p38 MAPK pathways in Stat3 transactivation (57), we report herein this novel observation: pX expression activates selectively in 4pX-1 cells the p38 MAPK pathway (Fig. 3 and 4), which significantly regulates Stat3 transactivation. Recent studies (55) demonstrated that, in 4pX-1 cells, pX activates the JNK pathway in a sustained manner, lasting during the 15 h following pX expression. In the present study, use of the dn JNK− mutant reduced pX-dependent Stat3 activation by approximately 25% (Fig. 3), showing that the JNK pathway also participates in regulating Stat3 transactivation. Interestingly, treatment of cells with SB 203580 reduced pX-dependent Stat3 activation by approximately 50% (Fig. 3). These results suggest that pX activates the p38 MAPK pathway and that its activation contributes significantly to regulation of Stat3 transactivation.
pX-dependent activation of p38 MAPK was demonstrated by two types of assays. One type is the transient-transreporter assays employing the transcription factor CHOP-10 transactivation domain in fusion with the DNA-binding domain of Gal41-147 (Strategene). The transcription factor CHOP-10 is known to be one of the downstream effectors of the p38 MAPK pathway (25). We demonstrate (Fig. 4A) that pX activates CHOP-10-mediated reporter expression only in 4pX-1 cells. Importantly, this activation is reduced by 50% by addition of SB 203580, supporting the involvement of the p38 MAPK pathway. The mechanism of this pX-mediated activation of the p38 MAPK pathway involves Ca2+ release and c-Src activation, based on the inhibitory effects of BAPTA-AM and PP2, respectively (Fig. 4A). Thus, the pX-initiated reprogramming of the 4pX-1 cells employs the common mechanism of Ca2+ release and c-Src activation, initially described in HepG2 cells (7, 10, 31, 32). Studies by others have also demonstrated the Ca2+-mediated activation of the p38 MAPK pathway via PYK2 activation (44).
Secondly, we demonstrate direct pX-dependent activation of the p38 MAPK enzyme by immunocomplex kinase assays of cellular extracts (3pX-1 and 4pX-1 cells) in a time course (6 to 24 h) following pX expression, using the phospho-active p38 kinase antibody and GST-ATF2 as the substrate. pX-dependent phosphorylation of ATF2 is detected at 18 and 24 h following pX expression only in 4pX-1 cells (Fig. 4B). Thus, activation of p38 MAPK by pX occurs concurrently with pX-dependent Stat3 activation (Fig. 1B). These kinetic studies of p38 MAPK activation by pX (Fig. 4B) explain the pronounced (50%) regulatory effect of the p38 MAPK pathway on Stat3 activation, evidenced by the inhibitory effect of SB 203580 (Fig. 3A). Likewise, the kinetics of pX-dependent JNK activation in 4pX-1 cells (55) explains the smaller (25%) regulatory effect of the JNK pathway on pX-mediated Stat3 transactivation. In an earlier study, it has been shown that pX-mediated JNK activation occurs 1 to 15 h following pX expression (55); i.e., it precedes or minimally overlaps Stat3 tyrosine phosphorylation and Stat3 DNA-binding activity (Fig. 2).
What is the physiologic relevance of pX-mediated activation of the p38 MAPK pathway in 4pX-1 cells? The p38 MAPK pathway was initially identified in yeast and in mammalian cells as a stress-activated mitogenic pathway (42). Subsequent studies linked its activation to cellular differentiation in various cell types (42). Recent studies have linked p38 MAPK pathway activation to initiation of a G2 delay (46). p38 MAPK was shown to inactivate by phosphorylation, directly (15) or indirectly (60), the dual-specificity phosphatase Cdc25 proteins, Cdc25B and Cdc25C, respectively. Cdc25 activates Cdc2 kinase required for G2/M progression (43, 58), by dephosphorylating Thr14 and Tyr15 (8, 36, 39, 45, 63). In earlier studies (34), it was demonstrated that 4pX-1 cells display a pX-dependent S-phase pause or delay, due to the absence of pX-dependent Cdc2 kinase activation. Considering the known regulatory role of the p38 MAPK pathway in G2/M progression during cellular stress (46), we investigated whether the pX-dependent activation of the p38 MAPK pathway regulates activation of the G2/M checkpoint in our 4pX-1 cells. Our flow cytometry analyses (Fig. 5A and B) demonstrate the pX-dependent S-phase accumulation (40% increase) of 4pX-1 cells at 18 h following pX expression, in agreement with earlier observations (34). Treatment of 4pX-1 cells with SB 203580 increased by approximately 50% the progression of these cells from S into G2/M phase (Fig. 5C), indicating a role for p38 MAPK pathway activation by pX in initiating the G2/M checkpoint.
Western blot analyses using the phospho-specific (Ser216) antibody of Cdc25C demonstrated the pX-dependent phosphorylation of Cdc25C at 18 h following pX expression (Fig. 5D). Importantly, treatment with SB 203580 decreased the phosphorylation of Cdc25C at Ser216 (Fig. 5D) and increased pX-dependent cell cycle progression of 4pX-1 cells (Fig. 5A to C). Taken together, these results (Fig. 5) link the pX-dependent activation of the p38 MAPK pathway to Cdc25 inactivation (Fig. 5D), which in turn mediates the observed pX-dependent S-phase pause of 4pX-1 cells (Fig. 5A to C).
Bulavin et al. (15) have proposed that activation of the p38 MAPK pathway is a cellular stress “sensor” allowing progression of cells into mitosis only after their recovery from stress. Similarly, we propose that expression of viral pX initiates the cellular stress response by activation of the p38 MAPK pathway, inactivating Cdc25 and initiating a G2/M delay. Interestingly, activation of the p38 MAPK plays a physiologically important role in liver, acting as a growth-inhibiting regulator of hepatocyte proliferation during liver development (2). Specifically, high p38 MAPK activity correlates with the temporary hepatocyte growth arrest, known to occur at term and again at the mature stage of liver growth (2). Furthermore, p38 MAPK is activated 24 h after partial hepatectomy (1), suggesting that it functions as a tonic growth inhibitor in hepatocyte growth. Thus, it would appear, the selective pX-dependent activation of Stat3, p38 MAPK, and JNK (55) pathways in 4pX-1 cells recapitulates the signaling program operational in regenerating hepatocytes (40). Interestingly, a recent study demonstrated that, in biopsy samples from chronic HBV patients with cirrhosis, pX-positive cells are preferentially localized at the periportal region of the hepatic lobules and the periphery of cirrhotic nodules (30), i.e., at sites of liver regeneration.
In conclusion, our cellular model (Fig. 6), which is comprised of the less differentiated hepatocytes (modeled by the 4pX-1, 4pX-2, and 4pX-3 cell lines) and the differentiated-hepatocyte 3pX-1 cell line, indicates that pX expression in the infected liver elicits activation of distinct signaling cascades in less differentiated, proliferating hepatocytes, as opposed to what is found in fully differentiated, no longer proliferating hepatocytes. The consequences of these differential cellular responses to pX expression in hepatocyte transformation are presently under investigation.
Acknowledgments
We thank R. Jove for Stat3 reagents (pLucTKS3, Stat3S727A, and ν-src plasmids) and Stat3 protocols and J. Darnell for Stat3 and Stat1 antibodies.
This study was supported by NIDDK grant 44533 to O.M.A.
REFERENCES
- 1.Awad, M. M., and P. A. Gruppuso. 2000. Cell cycle control during liver development in the rat: evidence indicating a role for cyclin D1 posttranscriptional regulation. Cell Growth Differ. 11:325-334. [PubMed] [Google Scholar]
- 2.Awad, M. M., H. Enslen, J. M. Boylan, R. J. Davis, and P. A. Gruppuso. 2000. Growth regulation via p38 mitogen-activated protein kinase in developing liver. J. Biol. Chem. 275:38716-38721. [DOI] [PubMed] [Google Scholar]
- 3.Barnabas, S., and O. M. Andrisani. 2000. Different regions of hepatitis B virus X protein are required for enhancement of bZip-mediated transactivation versus transrepression. J. Virol. 74:83-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnabas, S., T. Hai, and O. M. Andrisani. 1997. The hepatitis B virus X protein enhances the DNA binding potential and transcription efficacy of bZip transcription factors. J. Biol. Chem. 272:20684-20690. [DOI] [PubMed] [Google Scholar]
- 5.Benn, J., and R. J. Schneider. 1995. Hepatitis B virus HBx protein deregulates cell cycle checkpoint controls. Proc. Natl. Acad. Sci. USA 92:11215-11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benn, J., F. Su, M. Doria, and R. J. Schneider. 1996. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-related and c-Jun N-terminal mitogen-activated protein kinases. J. Virol. 70:4978-4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benn, J., and R. J. Schneider. 1994. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc. Natl. Acad. Sci. USA 91:10350-10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Booher, R. N., P. S. Holman, and A. Fattaey. 1997. Myt1 is a cell cycle-regulated kinase that inhibits Cdc2 but not Cdk2 activty. J. Biol. Chem. 272:22300-22306. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard, M., S. Giannakopoulos, E. H. Wang, N. Tanese, and R. J. Schneider. 2001. Hepatitis B virus HBx protein activation of cyclin A-cyclin-dependent kinase 2 complexes and G1 transit via an Src kinase pathway. J. Virol. 75:4247-4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard, M. J., L. Wang, and R. J. Schneider. 2001. Calcium signaling by HBx protein in hepatitis B virus DNA replication. Science 294:2376-2378. [DOI] [PubMed] [Google Scholar]
- 11.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 12.Bromberg, J., and J. E. Darnell, Jr. 2000. The role of STATs in transcriptional control and their impact on cellular function. Oncogene 19:2468-2473. [DOI] [PubMed] [Google Scholar]
- 13.Bromberg, J. F., C. M. Horvath, Z. Wen, R. D. Schreiber, and J. E. Darnell, Jr. 1996. Transcriptionally active Stat1 is required for antiproliferative effects of both interferon alpha and interferon gamma. Proc. Natl. Acad. Sci. USA 93:7673-7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buendia, M. A. 1998. Hepatitis B viruses and cancerogenesis. Biomed. Pharmacother. 52:34-43. [DOI] [PubMed] [Google Scholar]
- 15.Bulavin, D. V., Y. Higashimoto, I. J. Popoff, W. A. Gaarde, V. Basrur, O. Potapova, E. Appella, and A. J. Fornace. 2001. Initiation of a G2/M checkpoint after ultraviolet radiation requires p38 kinase. Nature 411:102-107. [DOI] [PubMed] [Google Scholar]
- 16.Catlett-Falcone, R., T. H. Landowski, M. M. Oshiro, J. Turkson, A. Levitzki, R. Savino, G. Ciliberto, L. Moscinski, J. L. Fernández-Luna, G. Nuñez, W. S. Dalton, and R. Jove. 1999. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 10:105-115. [DOI] [PubMed] [Google Scholar]
- 17.Chen, H.-S., S. Kaneko, R. Girones, R. W. Anderson, W. E. Hornbuckle, B. C. Tennant, P. J. Cote, J. L. Gerin, R. H. Purcell, and R. H. Miller. 1993. The woodchuck hepatitis virus X gene is important for establishment of virus infection in woodchucks. J. Virol. 67:1218-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chirillo, P., S. Pagano, G. Natoli, P. Puri, V. L. Burgio, C. Balsano, and M. Levrero. 1997. The hepatitis B virus X gene induces p53-mediated programmed cell death. Proc. Natl. Acad. Sci. USA 94:8162-8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen, P. 1997. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Cell Biol. 7:353-361. [Google Scholar]
- 20.Cressman, D. E., L. E. Greenbaum, R. A. DeAngelis, G. Ciliberto, E. E. Furth, V. Poli, and R. Taub. 1996. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science 274:1379-1383. [DOI] [PubMed] [Google Scholar]
- 21.Cressman, D. E., R. H. Diamond, and R. Taub. 1995. Rapid activation of the Stat3 transcription complex in liver regeneration. Hepatology 21:1443-1449. [PubMed] [Google Scholar]
- 22.Darnell, J. E., Jr. 1997. STATs and gene regulation. Science 277:1630-1635. [DOI] [PubMed] [Google Scholar]
- 23.Doria, M., N. Klein, R. Lucito, and R. J. Schneider. 1995. The hepatitis B virus is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factors. EMBO J. 14:4747-4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowler, S., L. Montalvo, D. Cantrell, N. Morrice, and D. R. Alessi. 2000. Phosphoinositide 3-kinase-dependent phosphorylation of the dual adaptor for phosphotyrosine and 3-phosphoinositides by the Src family of tyrosine kinase. Biochem. J. 349:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman, J. A., M. P. Lisanti, and P. E. Scherer. 1998. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 273:32111-32120. [DOI] [PubMed] [Google Scholar]
- 26.Fukada, T., M. Hibi, Y. Yamanaka, M. Takahashi-Tezuka, Y. Fujitani, T. Yamaguchi, K. Nakajima, and T. Hirano. 1996. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity 5:449-460. [DOI] [PubMed] [Google Scholar]
- 27.Hanke, J. H., J. P. Gardner, R. L. Dow, P. S. Changelian, W. H. Brissette, E. J. Weringer, B. A. Pollok, and P. A. Connelly. 1996. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J. Biol. Chem. 271:695-701. [DOI] [PubMed] [Google Scholar]
- 28.Hauser, P. J., D. Agrawal, J. Hackney, and W. J. Pledger. 1998. STAT3 activation accompanies keratinocyte differentiation. Cell Growth Differ. 10:847-855. [PubMed] [Google Scholar]
- 29.Höhne, M., S. Schaefer, M. Seifer, M. A. Feitelson, D. Paul, and W. H. Gerlich. 1990. Malignant transformation of immortalized transgenic hepatocytes after transfection with hepatitis B virus DNA. EMBO J. 9:1137-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin, Y. M., C. Yun, C. Park, H. J. Wang, and H. Cho. 2001. Expression of hepatitis B virus X protein is closely correlated with the high periportal inflammatory activity of liver diseases. J. Viral Hepat. 8:322-330. [DOI] [PubMed] [Google Scholar]
- 31.Klein, N. P., and R. J. Schneider. 1997. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signal to Ras. Mol. Cell. Biol. 17:6427-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein, P. N., M. J. Bouchard, L. Wang, C. Kobarg, and R. J. Schneider. 1999. Src kinases involved in hepatitis B virus replication. EMBO J. 18:5019-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovalobich, K., W. Li, R. DeAngelis, L. E. Greenbaum, G. Ciligerto, and R. Taub. 2001. Interleukin-6 protects against Fas-mediated death by establishing a critical level of anti-apoptotic hepatic proteins FLIP, Bcl-2, and Bcl-xL. J. Biol. Chem. 276:26605-26613. [DOI] [PubMed] [Google Scholar]
- 34.Lee, S., C. Tarn, W. H. Wang, S. Chen, R. L. Hullinger, and O. M. Andrisani. 2002. Hepatitis B virus X protein differentially regulates cell cycle progression in X-transforming versus non-transforming hepatocyte (AML12) cell lines. J. Biol. Chem. 277:8730-8740. [DOI] [PubMed] [Google Scholar]
- 35.Lee, Y. H., and Y. Yun. 1998. HBx protein of hepatitis B virus activates Jak1-STAT signaling. J. Biol. Chem. 273:25510-25515. [DOI] [PubMed] [Google Scholar]
- 36.Liu, F., J. J. Stanton, Z. Wu, and H. Piwnica-Worms. 1977. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol. Cell. Biol. 17:571-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lucito, R., and R. J. Schneider. 1992. Hepatitis B virus X protein activates transcription factor NF-κB without a requirement for protein kinase C. J. Virol. 66:983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuda, T., T. Nakamura, K. Nakao, T. Arai, M. Katsuki, T. Keike, and T. Yokota. 1999. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 18:4261-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGowan, C. H., and P. Russell. 1993. Human WEE 1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 12:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michalopoulos, G. K., and M. C. DeFrances. 1997. Liver regeneration. Science 276:60-66. [DOI] [PubMed] [Google Scholar]
- 41.Natoli, G., M. L. Avantaggiati, P. Chirillo, A. Costanzo, M. Artini, C. Balsano, and M. Levrero. 1994. Induction of the DNA-binding activity of c-Jun/c-Fos heterodimers by the hepatitis B virus transactivator pX. Mol. Cell. Biol. 14:989-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nebreda, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 43.Nurse, P. 1990. Universal control mechanism regulating onset of M-phase. Nature 334:503-508. [DOI] [PubMed] [Google Scholar]
- 44.Pandey, P., S. Avraham, S. Kumar, A. Nakazawa, A. Place, L. Ghanem, A. Rana, V. Kumar, P. K. Majumder, H. Avraham, R. J. Davis, and S. Kharbanda. 1999. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J. Biol. Chem. 274:10140-10144. [DOI] [PubMed] [Google Scholar]
- 45.Parker, L. L., and H. Piwnica-Worms. 1992. Inactivation of the p34cdc2-cyclin B complex by the human WEE 1 tyrosine kinase. Science 257:1955-1957. [DOI] [PubMed] [Google Scholar]
- 46.Pearce, A. K., and T. C. Humphrey. 2001. Integrating stress-response and cell-cycle checkpoint pathways. Trends Cell Biol. 11:426-433. [DOI] [PubMed] [Google Scholar]
- 47.Shen, Y., G. Devgan, J. E. Darnell, Jr., and J. F. Bromberg. 2001. Constitutively activated Stat3 protects fibroblasts from serum withdrawal and UV-induced apoptosis and antagonizes the proapoptopic effects of activated Stat1. Proc. Natl. Acad. Sci. USA 98:1543-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinibaldi, D., W. Wharton, J. Turkson, T. Bowman, W. J. Pledger, and R. Jove. 2000. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signaling. Oncogene 19:5419-5427. [DOI] [PubMed] [Google Scholar]
- 49.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 50.Su, F., and R. J. Schneider. 1996. Hepatitis B virus HBx protein activates transcription factor NF-κB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J. Virol. 70:4558-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su, F., and R. J. Schneider. 1997. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc. Natl. Acad. Sci. USA 94:8744-8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su, F., C. N. Theodosis, and R. J. Schneider. 2001. Role of NF-κB and Myc proteins in apoptosis induced by hepatitis B virus HBx protein. J. Virol. 75:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda, K., K. Noguchi, W. Shi, T. Tanaka, M. Matsumoto, N. Yoshida, T. Kishimoto, and S. Akira. 1997. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 94:3801-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tarn, C., M. L. Bilodeau, R. L. Hullinger, and O. M. Andrisani. 1999. Differential immediate early gene expression in conditional hepatitis B virus pX-transforming versus nontransforming hepatocyte cell lines. J. Biol. Chem. 274:2327-2336. [DOI] [PubMed] [Google Scholar]
- 55.Tarn, C., S. Lee, Y. Hu, C. Ashendel, and O. M. Andrisani. 2001. Hepatitis B virus X protein differentially activates RAS-RAF-MAPK and JNK pathways in X-transforming versus non-transforming AML-12 hepatocytes. J. Biol. Chem. 276:34671-34680. [DOI] [PubMed] [Google Scholar]
- 56.Turkson, J., T. Bowman, R. Garcia, E. Caldenhoven, R. P. De Groot, and R. Jove. 1998. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol. Cell. Biol. 18:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turkson, J., T. Bowman, J. Adnane, Y. Zhang, J. Y. Djeu, M. Sekharam, D. A. Frank, L. B. Holzman, J. Wu, S. Sebti, and R. Jove. 1999. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol. Cell. Biol. 19:7519-7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Heuvel, S., and E. Harlow. 1993. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262:2050-2054. [DOI] [PubMed] [Google Scholar]
- 59.Wands, J. R., and H. E. Blum. 1991. Primary hepatocellular carcinoma. N. Engl. J. Med. 325:729-731. [DOI] [PubMed] [Google Scholar]
- 60.Wang, X., C. H. McGowan, M. Zhao, L. He, J. S. Downey, C. Fearns, Y. Wang, S. Huang, and J. Han. 2000. Involvement of the MKK6-p38γ cascade in γ-radiation-induced cell cycle arrest. Mol. Cell. Biol. 20:4543-4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang, X. Z., and D. Ron. 1996. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by MAP kinase. Science 272:1347-1349. [DOI] [PubMed] [Google Scholar]
- 62.Waris, G., K. Huh, and A. Siddiqui. 2001. Mitochondrially associated hepatitis B virus X protein constitutively activates transcription factors STAT-3 and NF-κB via oxidative stress. Mol. Cell. Biol. 21:7721-7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe, N., M. Broome, and T. Hunter. 1995. Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the cell cycle. EMBO J. 14:1878-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wen, Z., Z. Zhong, and J. E. Darnell, Jr. 1995. Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82:241-250. [DOI] [PubMed] [Google Scholar]
- 65.Williams, J. S., and O. M. Andrisani. 1995. The hepatitis B virus X protein targets the basic region-leucine zipper domain of CREB. Proc. Natl. Acad. Sci. USA 92:3819-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, J. C., G. Merlino, and N. Fausto. 1994. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc. Natl. Acad. Sci. USA 91:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu, C. L., D. J. Meyer, G. S. Campbell, A. C. Larner, C. Carter-Su, J. Schwartz, and R. Jove. 1995. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science 269:81-83. [DOI] [PubMed] [Google Scholar]
- 68.Zhong, Z., Z. Wen, and J. E. Darnell, Jr. 1994. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264:95-98. [DOI] [PubMed] [Google Scholar]
- 69.Zoulim, F., J. Saputelli, and C. Seeger. 1994. Woodchuck hepatitis virus X protein is required for viral infection in vivo. J. Virol. 68:2026-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]