Abstract
Dengue virus (DV) primarily infects blood monocytes (MO) and tissue macrophages (Mφ). We have shown in the present study that DV can productively infect primary human MO/Mφ regardless of the stage of cell differentiation. After DV infection, the in vitro-differentiated MO/Mφ secreted multiple innate cytokines and chemokines, including tumor necrosis factor alpha, alpha interferon (IFN-α), interleukin-1β (IL-1β), IL-8, IL-12, MIP-1α, and RANTES but not IL-6, IL-15, or nitric oxide. Secretion of these mediators was highlighted by distinct magnitude, onset, kinetics, duration, and induction potential. A chemokine-to-cytokine hierarchy was noted in the magnitude and induction potential of secretion, and a chemokine-to-cytokine-to-chemokine/Th1 cytokine cascade could be seen in the production kinetics. Furthermore, we found that terminally differentiated MO/Mφ cultured for more than 45 days could support productive DV infection and produce innate cytokines and chemokines, indicating that these mature cells were functionally competent in the context of a viral infection. In addition, DV replication in primary differentiated human MO/Mφ was enhanced and prolonged in the presence of lipopolysaccharide (LPS), and LPS-mediated synergistic production of IFN-α could be seen in DV-infected MO/Mφ. The secretion of innate cytokines and chemokines by differentiated MO/Mφ suggests that regional accumulation of these mediators may occur in various tissues to which DV has disseminated and may thus result in local inflammation. The LPS-mediated enhancement of virus replication and synergistic IFN-α production suggests that concurrent bacterial infection may modulate cytokine-mediated disease progression during DV infection.
Infection with dengue virus (DV) has results ranging widely in severity, from self-limited dengue fever to potentially fatal dengue hemorrhagic fever-dengue shock syndrome, which represents the leading viral hemorrhagic fever in the world, with 500,000 cases annually (20, 21). Virus-specific, serotype-cross-reactive immune responses during secondary DV infection may give rise to more severe forms of dengue hemorrhagic fever-dengue shock syndrome due to antibody-mediated enhancement of DV infection of monocytes/macrophages (MO/Mφ) and/or activation of memory T lymphocytes (22, 23, 31, 50). Both of these mechanisms highlight the important role of antigen-specific immunity. However, only a few patients with secondary infection develop dengue hemorrhagic fever-dengue shock syndrome, and dengue hemorrhagic fever-dengue shock syndrome can also occur in primary DV infection (5, 14, 30, 57, 66). It is likely that dysregulation of certain types of innate and bystander immune activation may play a role in exacerbating disease progression during either primary or secondary DV infection.
MO/Mφ are the prime targets of DV both in vitro and in vivo (22, 23). MO/Mφ comprise heterogeneous subpopulations with distinct properties and functions (18, 19, 65). MO are the blood precursors of tissue Mφ. Circulating MO undergo differentiation and migrate through blood vessel walls to various tissues, where they mature into differentiated resident tissue Mφ. Although the differentiation stage of MO/Mφ is crucial in determining the susceptibility of these cells to productive infection by a number of viruses (25, 29, 48, 59, 61), the definitive influence of MO/Mφ differentiation on DV infection has not been established. In addition, it has recently been shown that human MO-derived dendritic cells (DC) can be infected and activated by DV (39, 67). Since DC can also differentiate from CD14+ MO both in vivo and in vitro (35, 47, 53), it is interesting to see if there is any difference between Mφ and DC in response to DV infection and their potential impact on immunity and pathogenesis.
Cytokines play an important role in the pathogenesis of DV infection (50), and serum levels of certain cytokines are elevated during DV infection (8, 33, 34). However, the physiological roles of plasma and compartmental cytokines might be different (51); it is thus important to investigate the cytokines released from virally infected, differentiated MO/Mφ. Furthermore, it is known that interleukin-12 (IL-12) and alpha interferon (IFN-α) play a crucial role in initiation of Th1-type immune responses and activation of natural killer (NK) cells and cytotoxic T lymphocytes (CTL) during viral infection (6, 7, 15, 60). Although MO/Mφ were thought to be one of the major cell sources of these cytokines, evidence on IL-12 production directly induced by virus infection of human MO/Mφ is not available.
It has been demonstrated that infection with certain viruses impairs the production of cytokines by MO/Mφ in response to bacterial stimulation (1, 9, 13, 26, 36, 37, 56, 58). This mechanism of virally induced, MO/Mφ-mediated immunosuppression may partly account for the detrimental outcome of viral infection followed by a superinfection with bacterial pathogens. On the other hand, bacterial lipopolysaccharide (LPS) was able to regulate virus replication and virally induced cytokine production by human MO/Mφ. We have previously documented a novel interaction among DV, LPS, and CD14 at the surface of primary human MO/Mφ (10). Hence, we asked whether the regulatory effect of LPS and viral impairment of cytokine production could be observed in DV infection of human MO/Mφ.
Appreciable progress in understanding the pathogenesis of DV infection has been made by a number of recent studies on virus-host cell interactions. However, a majority of these studies have focused on DV infection of different types of cancerous cells derived from various animal species, and it is important to understand the interaction of DV with its principal target cell, human MO/Mφ. Because DV is strictly a human pathogen and no appropriate animal model is available, we established an in vitro infection model using primary cultures of human MO/Mφ at different stages of differentiation. We found that DV can productively infect MO/Mφ in all differentiation states and subsequently trigger the secretion of multiple innate cytokines and chemokines. Production of these mediators varied remarkably in magnitude, onset, kinetics, duration, and induction potential and could be regulated by varying the initial infectious dose. Furthermore, LPS could modify both the magnitude and the kinetics of virus replication and cytokine secretion after DV infection of MO/Mφ.
MATERIALS AND METHODS
Virus.
DV strain 16681 (serotype 2), which was initially isolated from a dengue hemorrhagic fever patient, was used for infection. The preparation and titers of virus stocks were described previously (10).
Primary culture of human MO/Mφ.
Peripheral blood MO were isolated from dengue-naïve donors and cultured as described previously (10). Briefly, peripheral blood mononuclear cells were obtained from heparinized whole blood by density gradient centrifugation on Histopaque (Sigma, St. Louis, Mo.) and then incubated in complete alpha minimal essential medium (α-MEM) (Life Technology, Grand Island, N.Y.) containing 10% freshly prepared, heat-inactivated autologous human serum and 2 mM l-glutamine at 37°C with 7.5% CO2 for 2.5 h to allow MO adhesion. Thereafter, the cultures were washed extensively with Hanks' balanced salt solution (HBSS) three times to remove nonadherent cells, and adherent cells were detached by incubating the monolayers with an elution buffer (6 mM EDTA in phosphate-buffered saline supplemented with 5% fetal calf serum). The adherent cells were resuspended in complete α-MEM at a density of 4 × 105 cells per ml and then cultured in 24-well (1 ml/well) or 48-well (0.5 ml/well) tissue culture plates (Costar, Cambridge, Mass.) at 37°C with 7.5% CO2. These purified adherent cells contained more than 95% MO, as described previously (10).
In vitro differentiation of MO into Mφ.
MO were cultured in complete α-MEM containing 10% heat-inactivated autologous human serum in the absence of any stimulus to allow spontaneous differentiation into Mφ of various ages, from less than 1 day to more than 45 days of cultivation. Half of the culture medium was replaced with fresh complete α-MEM every 5 to 6 days. The remaining conditioned medium was essential to promote differentiation and survival of MO/Mφ. The cells grown in this long-term culture system were MO/Mφ and not DC in that they showed the typical MO/Mφ nonspecific esterase staining pattern, possessed no dendritic process, and expressed CD14, CD11b, and CD68, and no CD1a was detected (see Results).
Phagocytosis and cytochemistry.
The phagocytic capacity of MO/Mφ was assessed by incubating the cells with incomplete α-MEM containing yeast cells (108/ml) or latex beads (1.1 μm in diameter; Sigma) at 37°C for 30 min. The cytoplasmic peroxidase and nonspecific esterase activities were analyzed as described previously (65). The production of cytoplasmic superoxide was detected by a nitroblue tetrazolium reduction test.
Infection of MO/Mφ with DV.
MO/Mφ in different stages of differentiation were washed twice with HBSS and then once with incomplete α-MEM to remove heat-inactivated autologous human serum in culture medium. The cells were then infected with DV at a multiplicity of infection (MOI) of 2 to 3 PFU per cell or as indicated. For long-term cultures, we used initial numbers of MO to calculate the MOI. The virus inoculum (about 40 to 60 μl per well of a 48-well plate) was incubated with the cells in serum-free α-MEM (final volume of 0.2 to 0.25 ml) at 37°C for 2.5 h to permit viral adsorption. The culture plates were gently agitated every 15 to 20 min for optimal virus-cell contact. Thereafter, the unabsorbed viruses were removed by washing the cell monolayers once with HBSS and then once with complete α-MEM. The DV-infected and uninfected MO/Mφ cultures were replenished with fresh complete α-MEM and further incubated for 40 to 48 h or the time periods indicated. At the end of incubation, the cell-free supernatants and adherent MO/Mφ (in fresh α-MEM) were harvested separately and stored in aliquots at −70°C until assayed for infectious-virus production and cytokine secretion.
Stimulation of DV-infected MO/Mφ with LPS.
After washing and replenishment with fresh culture medium, LPS (Escherichia coli serotype O55:B5; Sigma) at different concentrations (from 0 to 5 μg/ml) was added to the infected MO/Mφ and maintained in the culture until the end of incubation.
DV titration.
The titers of both extracellular and intracellular DV were determined by plaque assay on BHK-21 cells as previously described (10).
Assay for cytokines and chemokines.
The levels of IL-8, IL-15, MIP-1α, and RANTES in the culture supernatants were measured with enzyme-linked immunosorbent assay kits purchased from R & D (Minneapolis, Minn.). The enzyme-linked immunosorbent assay kits for detection of IL-1β, IL-12 (both p40 subunit and p70 heterodimer), tumor necrosis factor alpha (TNF-α), and IFN-α were obtained from Biosource (Camarillo, Calif.), and the detection reagents for IL-6 and TNF-α were purchased from Genzyme (Cambridge, Mass.). The assays were performed according to the instructions of the manufacturers. Nitric oxide production was assayed by measuring the levels of nitrite in the culture supernatants with the Griess assay.
RESULTS
Morphological changes during MO/Mφ differentiation.
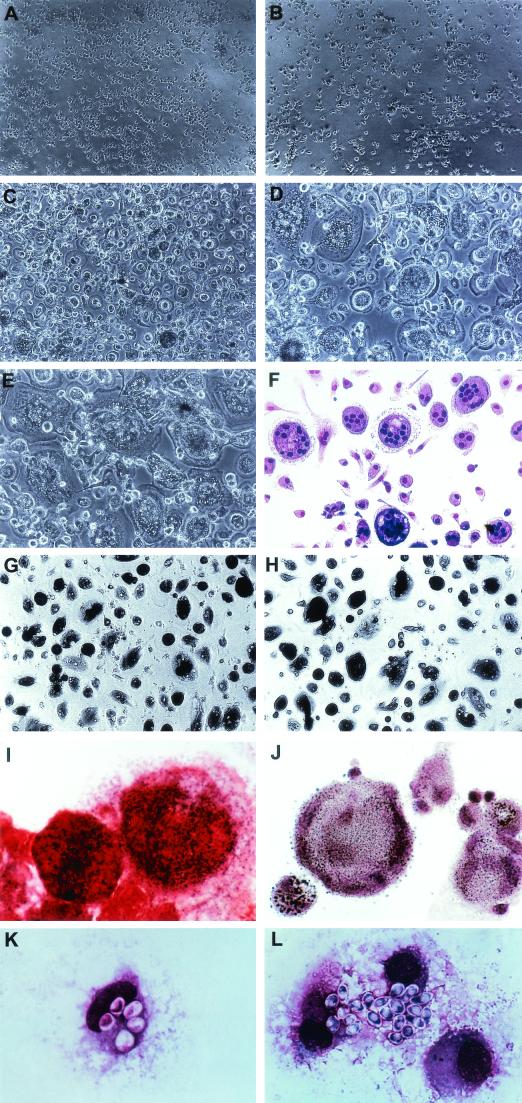
As presented in Fig. 1, freshly isolated MO were small in size and evenly distributed. After 2 days of cultivation, these cells began to enlarge and make contact, with frequent formation of cell clumps. Around 5 to 6 days of culture, the cell monolayers reached subconfluence, and subpopulations of cells began to fuse and form small multinucleated giant cells (MNGC), which contained about 2 to 3 nuclei per cell. After 6 to 7 days, the number of MNGC increased markedly and the cell monolayer reached confluence. Afterwards, the size of MNGC enlarged progressively, and the number of nuclei in a single MNGC increased from 2 to more than 10, although some MO/Mφ remained mononuclear. At about 10 days after culture, huge MNGC which possessed about 40 to 50 nuclei formed and were osteoclast-like in morphology (18, 19, 28).
FIG.1.
Morphological development and enzymatic and phagocytic activity of primary differentiated human MO/Mφ. Cell morphology of MO/Mφ cultured for 1 day (A), 2 to 3 days (B), 7 days (C), 10 days (D and F), 14 days (E), 45 days (G), and 50 days (H) is shown. MO/Mφ grew slowly for the first 4 to 5 days, and after 5 days of culture the cells enlarged rapidly and reached confluence at about day 7 to day 8. MNGC formed at ∼day 5, and huge, osteoclast-like MNGC were generated at ∼10 days of culture. After 6 weeks of culture, the cells decreased in size, and condensed cytoplasm was evident in every single cell as a feature of aging (G and H). Degeneration of MNGC was noticeable after 6 weeks of culture, in which the edge of the MNGC was vague (G) and disappeared with time (H). (F) Liu's stain of MNGC. (I) Nitroblue tetrazolium reduction by MNGC. (J) Nonspecific esterase staining of MNGC. Phagocytosis of yeasts by 9-day-old, differentiated mononuclear MO/Mφ (K) and MNGC (L) is shown. Magnifications: (A to C, G, and H) ×84; (D to F) ×126; (I to L) ×840.
After 2 to 3 weeks of culture, lipid droplets appeared in the cytoplasm of most of the cells and increased in quantity with time. Along with cytoplasmic lipid accumulation, there was a reduction in the size of MNGC and the number of nuclei in a single MNGC. The cell population exhibited heterogeneous morphology throughout the course of differentiation. There was significant donor-to-donor and experiment-to-experiment variability in the speed and extent of cell differentiation and MNGC formation and the duration that the cultures could be maintained (from 30 days to >100 days). The phagocytic and nonspecific esterase activity increased with cell differentiation, while the nitroblue tetrazolium reduction capacity remained the same. Strikingly, peroxidase activity could only be detected in cells cultured for less than 2 days. In addition, cell surface expression of CD14 decreased (from 98% to 80%) and that of CD68 increased (>90%) with cell differentiation. High levels of CD11b and HLA-DR expression were sustained throughout the course of cell differentiation (>90%).
Effects of cell differentiation on DV infection of human MO/Mφ.
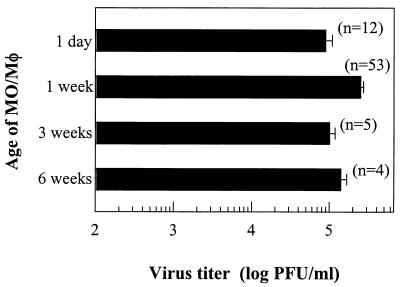
The effect of cell differentiation on the susceptibility of primary human MO/Mφ to DV infection was explored based on infectious-virus production. As shown in Fig. 2, the titers of infectious viruses from cells cultured for 1 day to more than 6 weeks were identical and were approximately 105 PFU per 4 × 105 cells at ∼42 h postinfection. The individual-to-individual and experiment-to experiment variability was invariably minimal (<1 log PFU/4 × 105 cells) with MO/Mφ obtained from ∼20 different donors in more than 50 experiments. These results indicate that human MO/Mφ are fully susceptible to DV infection regardless of the stage of differentiation.
FIG. 2.
Production of infectious DV by primary human MO/Mφ at various differentiation stages. Peripheral blood MO were cultured for 1 day or 1, 3, or >6 weeks, washed, and then infected with DV at an MOI of 2 to 3 PFU per cell in the absence of serum. After 2.5 h of viral adsorption, the cells were washed, and the cultures were further incubated with fresh complete medium for 40 to 48 h. At that time, the culture supernatants were harvested and assayed for infectious-virus production. The results are expressed as the mean ± standard error of pooled data from the number of separate experiments shown in parentheses with cells obtained from up to 20 different donors.
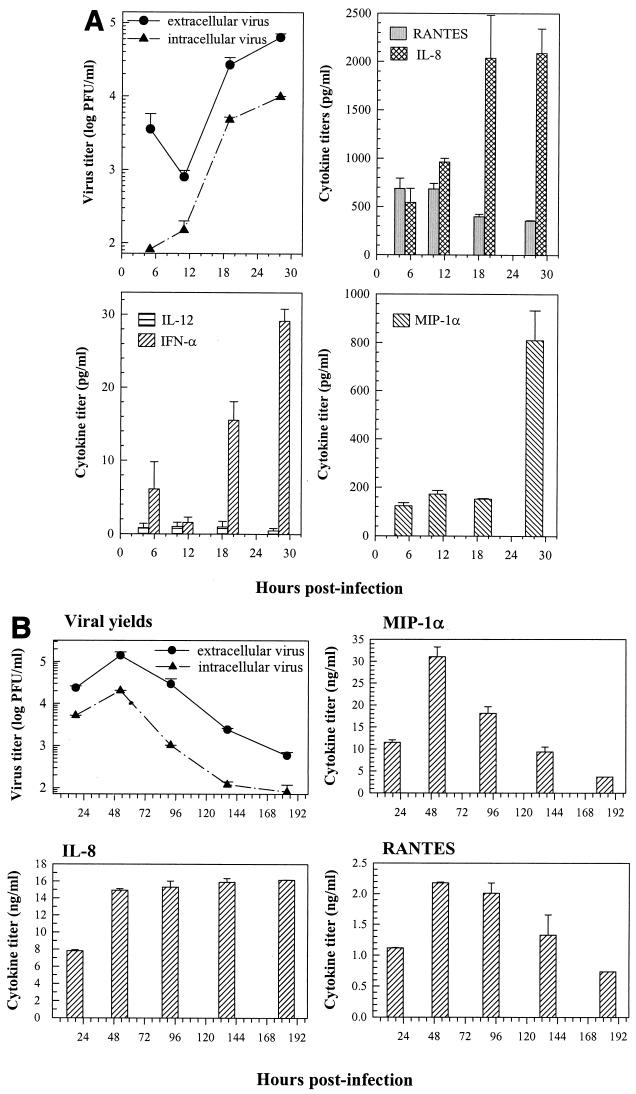
Kinetic studies showed that intracellular and extracellular infectious viruses were synthesized and released after 12 h of infection, followed by a log phase of growth, and peak viral yields were detectable at about 36 to 48 h postinfection (Fig. 3A). The yields of infectious viruses then progressively declined, but infectious virus could still be detected in the culture medium of infected cells even after 1 week of infection (Fig. 3B). No noticeable cell death could be seen after DV infection, based on trypan blue dye exclusion and the MTT assay (not shown), indicating a lack of direct DV-induced cytotoxicity.
FIG. 3.
Short-term (A) and long-term (B) production kinetics of various cytokines and chemokines in 7-day-old human MO/Mφ after DV infection. The cells were cultured and infected as described in the legend to Fig. 2. At the indicated times, the culture supernatants were harvested and assayed for release of infectious viruses, cytokines, and chemokines. The cryolysates of infected MO/Mφ were analyzed for intracellular infectious-virus titers. The results shown in panels A and B were derived separately from one of the representative experiments. Each time point represents the mean ± standard error of results from three independent wells. The levels of these cytokines and chemokines in the supernatants of mock-infected control cultures remained constant before and after infection over time: (A) average values were 462 to 600 pg/ml for IL-8, 336 to 617 pg/ml for RANTES, 82 to 196 pg/ml for MIP-1α, and below the detection limit for IFN-α (<5 pg/ml) and IL-12 (<2 pg/ml); (B) average values were 1.7 to 2.8 ng/ml for MIP-1α, 2.4 to 4.2 ng/ml for IL-8, and 0.32 to 0.35 ng/ml for RANTES.
Secretion of multiple cytokines and chemokines by primary differentiated MO/Mφ after DV infection.
We next explored the profile of cytokines and chemokines generated by DV-infected human MO/Mφ. As shown in Fig. 3 and Table 1, after DV infection of 1-week-old MO/Mφ, secretion of the proinflammatory cytokines IL-1β and TNF-α and the Th1/NK-stimulatory cytokines IL-12 and IFN-α was induced and constitutive secretion of the CXC chemokine IL-8 and the CC chemokines MIP-1α and RANTES was markedly enhanced. Although human MO/Mφ can be stimulated to release IL-6, IL-15, and nitric oxide, we failed to detect these secretory products at any time after DV infection.
TABLE 1.
Dose-dependent release of infectious viruses, cytokines, and chemokines by DV-infected MO/Mφa
| Input MOI (PFU/cell) | Virus yield (PFU/ml) | Mean concn of Pyrogenic cytokines (pg/ml) ± SE
|
Mean concn of Th1/NK-stimulatory cytokines (pg/ml) ± SE
|
Mean concn of CXC and CC chemokines (pg/ml) ± SE
|
||||
|---|---|---|---|---|---|---|---|---|
| TNF-α | IL-1β | IL-12 | IFN-α | IL-8 | MIP-α | RANTES | ||
| 0 (mock) | UD | UD | UD | UD | 1,975.03 ± 109.35 | 112.09 ± 1.33 | 34.52 ± 3.22 | |
| 5 | 4.83 × 105 | 134.99 ± 12.07 | 18.63 ± 1.31 | 339.18 ± 25.97 | 248.17 ± 11.98 | 74,224.81 ± 1136.30 | 19,165.06 ± 135.12 | 813.82 ± 67.90 |
| 0.5 | 1.54 × 105 | 15.46 ± 4.61 | UD | 97.81 ± 3.00 | 68.19 ± 4.06 | 58,406.18 ± 439.15 | 2,095.56 ± 488.74 | 381.30 ± 26.72 |
| 0.05 | 3.28 × 104 | UD | UD | 15.29 ± 2.65 | 9.10 ± 0.49 | 27,045.24 ± 463.80 | 293.02 ± 4.96 | 218.95 ± 9.42 |
| 0.005 | 1.68 × 103 | UD | UD | UD | UD | 9,370.08 ± 339.44 | 134.23 ± 0.70 | 200.65 ± 17.79 |
Seven-day-old MO/Mφ were washed and then either mock infected with C6/36 cell-conditioned medium or infected with DV at an MOI of 5, 0.5, 0.05, or 0.005 PFU per cell. After 2.5 h of viral adsorption, cells were washed twice to remove unabsorbed viruses and then replenished with fresh complete α-MEM. Culture supernatants were collected at 44 h postinfection and analyzed for titers of infectious viruses, cytokines, and chemokines. The results shown were obtained from one representative experiment with cells derived from the same donor for strict comparison among these cytokines and chemokines. Each experimental point represents the results obtained from three independent wells. UD, undetectable (<7 pg/ml).
When the production kinetics was examined (Fig. 3), it was found that the enhanced secretion of IL-8 took place by 18 h postinfection and was sustained after 1 weeks of infection. Significant enhancement of RANTES secretion was observed about 2 days postinfection, peaked by day 4, and then subsided to the normal constitutive level by 8 days postinfection. The augmented production of MIP-1α occurred by 28 h postinfection, peaked at day 2, and then subsided to the constitutive level by 6 days postinfection. Furthermore, no IL-12 induction was observed by 28 h after infection, whereas induction of IFN-α was rapid (<18 h postinfection).
Size of inoculum affects cytokine secretion.
As shown in Table 1, release of infectious progeny viruses and each of the cytokines and chemokines after DV infection was dependent on the initial dose of input virus. Notably, at any MOI, the magnitude of secretion was invariably higher for the chemokines IL-8, MIP-1α, and RANTES, intermediate for IL-12 and IFN-α, and much lower for TNF-α and IL-1β. In parallel, the minimal infectious dose required for induction (or enhancement) of secretion varied remarkably among these mediators. Enhancement of MO/Mφ secretion of IL-8, MIP-1α, and RANTES required only a trace amount of input virus (MOI ≤ 0.005 PFU per cell). Induction of IL-12 and IFN-α required a higher initial virus input (MOI ≤ 0.05), while much higher initial infectious doses were needed for the induction of TNF-α and IL-1β (MOI ≤ 0.5 and 5 for TNF-α and IL-1β, respectively).
In addition, treatment of cells with very low concentrations of LPS triggered the release of large amount of TNF-α, IL-1β, and IL-6 but not IFN-α (data not shown), indicating that the cytokine-chemokine profile observed here is likely to be DV specific and is not merely a general consequence of MO/Mφ activation. Our results clearly demonstrated that MO/Mφ secretion of chemokines (IL-8, MIP-1α, and RANTES) could be induced much more efficiently by DV infection than that of the other cytokines tested. Moreover, DV was also a potent inducer of IL-12 and IFN-α, but was a poor inducer of TNF-α and IL-1β.
Role of terminally differentiated human MO/Mφ in DV infection.
Although the phenotypes and enzymatic activities have been characterized (28, 62-64), little information is available on the functions of terminally differentiated human MO/Mφ, particularly with respect to viral infection and cytokine secretion. The average life span of human MO/Mφ in vivo ranges from 1 to 2 months. We therefore cultured blood-derived MO for 45 days and infected these lipid-laden, fully mature MO/Mφ with DV (Fig. 4). We established a long-term culture system using autologous serum in the culture medium in the absence of any exogenous growth factors, cytokines, or other stimuli that are required for growth, differentiation, and maintenance of other types of leukocytes, including DC. It is known that under such culture conditions, MO/Mφ survive for only weeks.
FIG. 4.
Kinetics of virus replication and cytokine secretion in terminally differentiated human MO/Mφ after DV infection. Peripheral blood MO were cultured with α-MEM containing 10% heat-inactivated autologous human serum that was half-replaced with fresh complete α-MEM every 5 to 6 days to promote MO/Mφ differentiation. After 45 days of culture, the cells were washed and infected with DV at an MOI of 3 PFU per cell. Culture supernatants were harvested at the time points indicated and assayed for infectious-virus production and cytokine secretion. Cell cryolysates were analyzed for intracellular infectious-virus titers. For each time point, three separate wells were prepared and analyzed, and the results are expressed as the mean ± standard error. Some error bars are too small to be seen. The levels of these cytokines and chemokines in the supernatants of mock-infected control cultures remained constant after infection over time. The average values were 2.2 to 3.6 ng/ml for IL-8, 0.14 to 2.16 ng/ml for MIP-1α, 343 to 464 pg/ml for RANTES, and below the detection limit for IL-1β (<2 pg/ml), IL-12 (<2 pg/ml), TNF-α (<2 pg/ml), and IFN-α (<5 pg/ml). NA, not available.
Surprisingly, these terminally differentiated cells were fully susceptible to productive DV infection, releasing large amounts of infectious virions by 24 h postinfection. The viral yields peaked at 36 to 48 h after infection, with titers of 2 × 105 PFU and 3 × 103 PFU per 4 × 105 cells for extracellular and intracellular viruses, respectively. Compared to 1-week-old MO/Mφ, production of intracellular infectious viruses by these terminally differentiated MO/Mφ was only transient and less prominent. In addition, DV infection neither induced cytopathic effect nor affected cell survival.
Most importantly, after DV infection, these terminally differentiated MO/Mφ secreted cytokines and chemokines with a profile and hierarchy similar to those observed in 7-day-old MO/Mφ (Fig. 3 and 4 and Table 1). The peak titers reached ∼90 ng/ml, 25 ng/ml, and 2 ng/ml for IL-8, MIP-1α and RANTES, respectively. On the other hand, the peak titers of IL-12, IFN-α, and TNF-α were between 100 and 200 pg/ml, whereas the peak titer of IL-1β was only about 50 pg/ml. In addition, the time course of secretion was distinct among these cytokines and chemokines and, to some extent, similar to that observed in 7-day-old MO/Mφ (Fig. 3). Induction (or enhancement) of IL-8, MIP-1α, and TNF-α secretion commenced early after infection (≤24 h), followed by RANTES, IL-1β, and IFN-α (≤36 h). Secretion of IL-12 was again slightly delayed (≤48 h). Moreover, secretion of MIP-1α and TNF-α peaked at ∼2 days after infection, coinciding with the peak time of infectious-virus production. Secretion of IL-1β secretion peaked at ∼3 days postinfection, whereas the peak time of RANTES and IFN-α release occurred at ∼4 days after infection. By contrast, secretion of IL-8 and IL-12 did not attain peak levels until 7 days postinfection. Furthermore, secretion of TNF-α, MIP-1α, and RANTES was relatively transient, while the levels of IL-8, IL-12, and IFN-α were sustained at high values even at 14 days after infection, a time more than 1 week after infectious-virus synthesis had ceased. Although secretion of IL-1β declined gradually after 3 days postinfection, the levels remained detectable at the end of the experiment (2 weeks postinfection).
Effect of LPS on DV replication and induction of IFN-α.
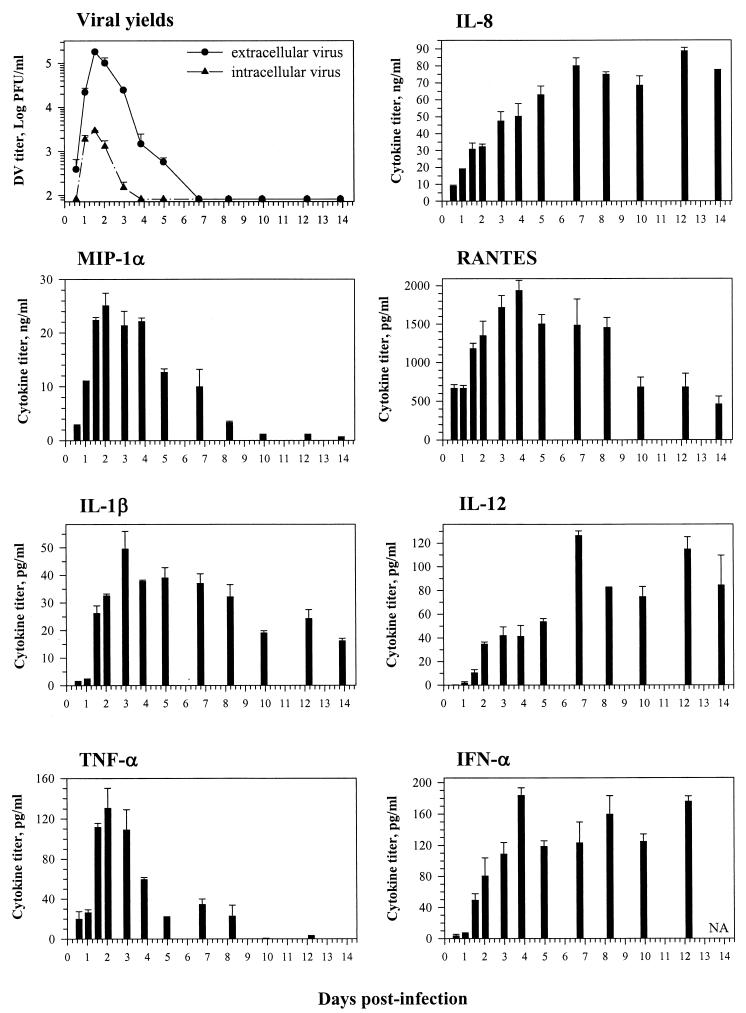
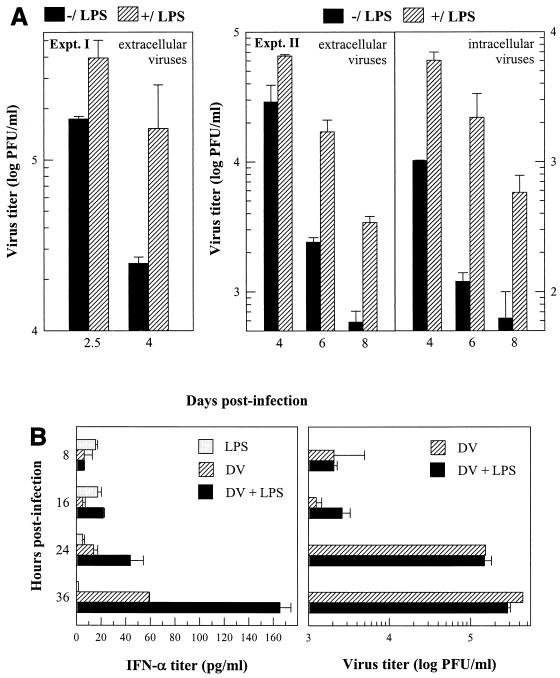
Viral infection of MO/Mφ could be modulated by LPS. Since infection with bacteria can occur during DV infection, we examined the consequences of MO/Mφ infection by DV in the presence of LPS. Primary differentiated, 7-day-old MO/Mφ were infected with DV and then treated with LPS, which remained in the culture throughout the course of infection. The virus titers of LPS-treated and untreated MO/Mφ cultures were similar before 48 h postinfection, the log phase of viral replication (Fig. 5B and data not shown). However, after this point, both intracellular and extracellular viral yields in cultures without LPS dropped more rapidly than those with LPS, resulting in significantly higher viral yields and prolonged duration of viral replication in the LPS-containing cultures (Fig. 5). The cell numbers and viability were similar between the two groups by the end of the experiment.
FIG. 5.
Effects of LPS on DV replication and DV-induced IFN-α production in differentiated human MO/Mφ. Seven-day-old MO/Mφ were washed and then left uninfected or infected with DV at an MOI of 3 PFU per cell. After 2.5 h of viral adsorption, cells were washed and replenished with fresh complete medium. After 6 h of infection, the cultures were either left untreated (−/LPS) or treated with 5 μg of LPS per ml (+/LPS). Culture supernatants were harvested at different time points and analyzed for extracellular infectious-virus and IFN-α titers. Cell cryolysates in fresh α-MEM of the same volume were used for titration of intracellular infectious viruses. (A) LPS enhanced and sustained DV replication at later times postinfection. (B) Enhancing effect of LPS on DV-induced IFN-α secretion at early times postinfection. For each time point, three separate wells were prepared and analyzed, and the results are expressed as the mean ± standard error. Titers of IFN-α in the mock-infected control cultures were below the detection limit (<7 pg/ml). Representative data out of four independent experiments are shown. Some error bars are too small to be seen.
It is known that viral infections can impair MO/Mφ cytokine production in response to LPS stimulation (1, 9, 13, 26, 36, 37, 56, 58). We therefore investigated LPS-stimulated cytokine secretion by DV-infected human MO/Mφ. We found that DV infection did not impair cell responsiveness to LPS stimulation in secretion of the innate cytokines and chemokines tested above. Instead, an additive or synergistic production after LPS stimulation could be seen in DV-infected MO/Mφ cultures (our unpublished observations). More importantly, although LPS per se did not induce MO/Mφ secretion of IFN-α, it markedly enhanced DV-induced secretion of IFN-α from these differentiated cells (Fig. 5B). Such an augmentation occurred shortly after infection (<24 h), a time when the enhancing effect of LPS on DV replication was still not evident (Fig. 5B). These data suggest that the enhancement of DV-induced MO/Mφ IFN-α production by LPS might not result from increased virus replication and does not promptly suppress virus production.
DISCUSSION
In the present study, we examined the production of infectious viruses and secretion of cytokines and chemokines by primary human MO/Mφ after DV infection and explored the modulatory effect of cell differentiation and bacterial LPS. To our knowledge, this report provides the first evidence for direct induction of IL-12 production by human MO/Mφ exposed to a viral pathogen and supports the idea that MO/Mφ are one of the sources for IL-12 during viral infection. Furthermore, we provide new insight into Mφ biology by demonstrating that terminally differentiated human MO/Mφ are capable of supporting virus replication and producing multiple cytokines and chemokines. Thus, these mature cells are physiologically competent in the context of viral infection.
MO/Mφ differentiation and DV infection.
Many studies have shown that cell susceptibility to viral infection is altered during in vitro cultivation of human MO/Mφ (25, 29, 48, 59, 61). It has been reported that immature promonocytic U937 cells were not infected by DV unless they had been provided with a differentiation signal and become Mφ-like prior to DV infection (45). In the present study, we demonstrated that DV could productively infect primary human MO/Mφ regardless of the state of cell differentiation (Fig. 2, 3, and 4). Our observations imply that DV can propagate in differentiated tissue Mφ as well as in blood MO. The noncytopathic, productive DV infection that proceeded with MO/Mφ differentiation, along with the production of intracellular infectious virions, suggested that blood MO may act as a carrier for disseminating this blood-borne virus to different tissues. This proposed mechanism might partly account for the systemic symptoms observed in dengue fever.
Production of innate cytokines and chemokines in a hierarchical manner.
Some reports have demonstrated the production of cytokines or chemokines after in vitro DV infection of different types of cells, including monocytic cells (38, 55), endothelial cells (3), blood MO (2, 32), liver Kuppfer cells (41), a mast cell/basophil line (27), cord blood mononuclear cells (43), and MO-derived DC (39). In the present study, we attempted to obtain an integrated view of the cytokine-chemokine responses of human MO/Mφ to DV infection by using long-term primary cultures and simultaneously comparing the production kinetics and dose responses. More importantly, the potential modifying effects of cell differentiation and a microbial stimulus (LPS) were also explored.
(i) Differential magnitude and kinetics of secretion.
After DV infection of MO/Mφ, various cytokines and chemokines were released with distinct quantities, kinetics, and durations. For a given MOI, the magnitude of IL-8 secretion was highest, followed by that of MIP-1α and RANTES. The levels of IFN-α, IL-12, and TNF-α were intermediate, while increased secretion of IL-1β was only marginal. This hierarchy was the same for both 1-week-old and 45-day-old MO/Mφ (Fig. 3 and 4 and Table 1). Moreover, the production kinetics between these two MO/Mφ cultures was also similar. The onset of IL-8, MIP-1α, and TNF-α secretion occurred earlier than that of RANTES, IL-1β, and IFN-α, while the onset of IL-12 release was slightly delayed. Secretion of MIP-1α, RANTES, IL-1β, and TNF-α peaked at early times after DV infection (≤2 to 4 days) and declined thereafter. By contrast, IL-8, IL-12, and IFN-α persisted at high levels long after the production of infectious viruses and other cytokines and chemokines had ceased or subsided (up to 2 weeks postinfection). This might result from either constant production or stability of these mediators, and our observations warrant further study at the gene expression level.
(ii) Differential induction potential.
We showed that the minimal initial virus input required for DV-induced secretion varied significantly among the cytokines and chemokines of different categories (Table 1). These observations provide several novel implications. First, because IL-8, MIP-1α, and RANTES could readily be induced by small amounts of virus, it is likely that these chemokines can be induced rapidly at the initial phase of DV infection, when the virus load is still low. Second, induction of IL-12 and IFN-α did not require a high MOI, indicating that signals for activation of Th1-type immune responses can be provided by DV-infected MO/Mφ. Finally, it has been a prevailing thought that IL-1β and TNF-α are the key mediators for development of dengue hemorrhagic fever-dengue shock syndrome. Nevertheless, the requirement for high doses of DV to induce small amounts of TNF-α and IL-1β suggests that by the time these two cytokines are detectable (8), the virus load and other cytokines and chemokines had reached pathophysiological levels and had detrimental consequences.
(iii) Secretion by terminally differentiated MO/Mφ.
The immunoregulatory role of terminally differentiated human MO/Mφ was not known, particularly with respect to viral infection. We have shown here that blood MO cultured in vitro for more than 45 days secreted multiple cytokines and chemokines in response to DV infection (Fig. 4). Similarly, it has been reported that DV infection of human liver Kupffer cells, albeit abortive, can result in significant cytokine production (41). The discrepancy in cell permissiveness to DV reflects the functional heterogeneity of MO/Mφ and the complexity of the virus-host relationship.
(iv) Immunopathophysiological significance.(a) Inflammation.
The clinical presentations of dengue fever and dengue hemorrhagic fever-dengue shock syndrome reveal many signs of local inflammation, which may result from extravasation of leukocytes to sites of infection. The onset and peak of chemokine production by differentiated MO/Mφ that occurred at early times after DV infection indicated that these molecules may play a role in early recruitment of different subsets of leukocytes and take part in the early response to viral infection as well as tissue injury (7, 11, 16, 17, 49, 51, 52). Interestingly, endothelial cells can release chemokines after DV infection (3), suggesting a possible cooperative interaction of these two types of cells in leukocyte trafficking by differential production of chemokines.
(b) Th cells and CTL.
It is conceivable that production of IFN-α and IL-12 by DV-infected differentiated MO/Mφ may act to prevent activated effector lymphocytes from death and maintain and/or amplify Th1-type immune activation in vivo even after virus clearance (6, 7, 15, 42, 60). Moreover, RANTES was shown to preferentially recruit and activate T lymphocytes with the memory phenotype (54) and thus might be important for rapid T-cell effector functions during secondary DV infection.
(c) NK cells.
It is known that MIP-1α, RANTES, IFN-α, and IL-12 can induce NK cell activation, chemotaxis, adhesion, and transendothelial migration (6, 7, 17, 49). MIP-1α was also shown to possess dual roles in viral infection by conferring NK cell-mediated protection and tissue inflammation (11, 52). Therefore, regulation of NK cell activity by these mediators may affect DV infection and tissue injury (50).
(d) Hemostasis.
Some of the innate cytokines, such as TNF-α, IFN-α, and MIP-1α, can suppress hemopoiesis (17, 46, 49, 64). Secretion of these mediators by DV-infected MNGC may contribute to the bone marrow suppression observed in dengue fever and dengue hemorrhagic fever-dengue shock syndrome (55).
LPS exacerbates DV infection.
Concurrent bacterial infection during the course of a viral infection usually aggravates disease progression (4, 12, 44). Here we showed that production of infectious DV was enhanced and prolonged in the presence of bacterial LPS, suggesting a potential role for bacterial infection in increasing viral load during DV infection. We have previously demonstrated that treatment of MO/Mφ with LPS prior to or together with but not after viral adsorption markedly inhibited DV infection (10). Together with the data presented here, it is clear that LPS was able to block DV entry but unable to elicit an intracellular antiviral state of human MO/Mφ for DV clearance. By contrast, treatment of MO/Mφ with IFN-γ either prior to or at 6 h after viral adsorption potently suppressed DV replication (our unpublished results). Taken together, these observations point to a unique and novel dichotomous effect of LPS on DV infection of human MO/Mφ that is dependent on the timing of cell exposure to LPS and DV. The questions of how and at what stage(s) LPS acts to enhance DV replication require further investigation.
Notably, although LPS by itself was unable to induce IFN-α production, it potently augmented DV-induced secretion of this IFN at early times after infection and throughout infection. This was consistent with the fact that priming of human MO/Mφ was needed for LPS-stimulated IFN-α production (24). Interestingly, early augmentation of IFN-α was not concomitant with an immediate decrease in DV replication in the LPS-treated cultures (Fig. 5). Given the multiple roles of IFN-α in shaping both innate and adaptive immunity, enhanced IFN-α production by DV-infected MO/Mφ treated with LPS provided a potential pathogenic mechanism whereby bacterial coinfection may modulate immunity and/or immunopathology during DV infection. (12, 44).
Potential distinct roles for Mφ and DC in DV infection.
DC are potent antigen-presenting cells that can initiate immune responses by transporting antigens to secondary lymphoid organs and prime naïve antigen-specific T cells there. It was found that DC could also differentiate from CD14+ MO both in vitro and in vivo (35, 47, 53), suggesting divergent pathways for MO differentiation. The ability of DV to infect both myeloid DC and self-differentiated Mφ indicates that this virus is able to infect “differentiated cells derived from MO origin” (39, 67). Given the fact that DC prefer to migrate to secondary lymphoid organs and MO/Mφ tend to home to multiple various peripheral tissues, it is tempting to speculate that these two types of antigen-presenting cells play distinct roles in DV infection: infected DC undergo maturation and transport dengue virus antigens to secondary lymphoid organs to initiate immunity, whereas MO/Mφ act as a Trojan horse for the virus, spreading to various peripheral organs and mediating inflammatory reactions in situ.
In addition, the consequences of DV infection of MO-derived DC have been described by Libraty et al. (39), who showed weak production of IL-12 at 2 days postinfection. It is interesting that, after infection of the terminally differentiated MO/Mφ with DV, there was a dramatic increase in IL-12 secretion by 7 days postinfection (Fig. 4). The delayed IL-12 production might result partly from the inhibitory effect of certain early cytokines, such as TNF-α (40). Thus, the decline in TNF-α might account for the rapid release of IL-12 at ∼7 days after infection (Fig. 4).
Immunopathogenesis of DV infection.
Based on our findings, a cytokine-mediated inflammation and pathogenic cascade in DV infection are proposed. First, early secretion of chemokines would form a local gradient and specifically recruit various subsets of circulating leukocytes to sites of infection. Meanwhile, these chemokines, together with IL-12, TNF-α, and IL-1β, act locally on endothelial cells to increase vascular permeability and adhesion molecule expression, facilitating blood leukocyte adherence and diapedesis. As a result, increased numbers of inflammatory cells are brought to sites where antigen-bearing tissue Mφ are located. Thereafter, the cellular infiltrates are further influenced by the synergistic effects of cytokines and chemokines released from infected tissue Mφ and undergo functional maturation. More importantly, the long-lasting cytokines, such as IL-12, IFN-α, and IL-1β, may contribute to the differentiation, activation, and maintenance of antigen-specific or bystander CTL, Th1, and NK cells in secondary lymphoid as well as peripheral tissues. These effector cells exert cytotoxic actions and secrete IFN-γ, IL-2, and other mediators that would eventually modify host immune responses.
We suggest that for DV infection, protection or pathogenesis is determined at the interface of innate and adaptive immunity controlled by innate cytokines produced as a consequence of the interaction of DV with its principal target cells, human MO/Mφ.
Acknowledgments
Great thanks are given to Chi-Kuan Ho (Veterans General Hospital [VGH]—Taipei) and Siamon Gordon (Oxford) for reviewing the paper and to all the members of the Laboratory of Hematology at VGH—Taipei for their help and friendship.
This study was supported by research grant VGH 90-406 from the Veterans General Hospital—Taipei, Taipei, Taiwan. Yun-Chi Chen is the Burton Senior Scholar at Oriel College of the University of Oxford.
Footnotes
Yun-Chi Chen dedicates this paper to Sheng-Yuan Wang, who passed away during the preparation of the manuscript, because of his support, encouragement, and education and because he provided the opportunity to study dengue virus and human macrophages.
REFERENCES
- 1.Adler, H., T. W. Jungi, H. Pfister, M. Strasser, M. Sileghem, and E. Peterhans. 1996. Cytokine regulation by virus infection: bovine viral diarrhea virus, a flavivirus, downregulates production of tumor necrosis factor alpha in macrophages in vitro. J. Virol. 70:2650-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R., S. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avirutnan, P., P. Malasit, B. Seliger, S. Bhakdi, and M. Husmann. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 161:6338-6346. [PubMed] [Google Scholar]
- 4.Babiuk, L. A., M. J. Lawman, and H. B. Ohmann. 1988. Viral-bacterial synergistic interaction in respiratory disease. Adv. Virus Res. 35:219-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes, W. J., and L. Rosen. 1974. Fatal hemorrhagic disease and shock associated with primary dengue infection on a Pacific island. Am. J. Trop. Med. Hyg. 23:495-506. [DOI] [PubMed] [Google Scholar]
- 6.Biron, C. A. 1998. Role of early cytokines, including alpha and beta interferons (IFN-alpha/beta), in innate and adaptive immune responses to viral infections. Semin. Immunol. 10:383-390. [DOI] [PubMed] [Google Scholar]
- 7.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi, U. C., R. Agarwal, E. A. Elbishbishi, and A. S. Mustafa. 2000. Cytokine cascade in dengue hemorrhagic fever: implications for pathogenesis. FEMS Immunol. Med. Microbiol. 28:183-188. [DOI] [PubMed] [Google Scholar]
- 9.Chehimi, J., S. E. Starr, I. Frank, A. D'Andrea, X. Ma, R. R. MacGregor, J. Sennelier, and G. Trinchieri. 1994. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J. Exp. Med. 179:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, Y. C., S. Y. Wang, and C. C. King. 1999. Bacterial lipopolysaccharide inhibits dengue virus infection of primary human monocytes/macrophages by blockade of virus entry via a CD14-dependent mechanism. J. Virol. 73:2650-2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook, D. N., M. A. Beck, T. M. Coffman, S. L. Kirby, J. F. Sheridan, I. B. Pragnell, and O. Smithies. 1995. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science 269:1583-1585. [DOI] [PubMed] [Google Scholar]
- 12.Doughty, L. A., K. B. Nguyen, J. E. Durbin, and C. A. Biron. 2001. A role for IFN-αβ in virus infection-induced sensitization to endotoxin. J. Immunol. 166:2658-2664. [DOI] [PubMed] [Google Scholar]
- 13.Dudding, L. R., and H. M. Garnett. 1987. Interaction of strain AD169 and a clinical isolate of cytomegalovirus with peripheral monocytes: the effect of lipopolysaccharide stimulation. J. Infect. Dis. 155:891-896. [DOI] [PubMed] [Google Scholar]
- 14.Eram, S., Y. Setyabudi, T. I. Sadono, D. S. Sutrisno, D. J. Gubler, and J. Sulianti Saroso. 1979. Epidemic dengue hemorrhagic fever in rural Indonesia. II. Clinical studies. Am. J. Trop. Med. Hyg. 28:711-716. [PubMed] [Google Scholar]
- 15.Farrar, J. D., and K. M. Murphy. 2000. Type I interferons and T helper development. Immunol. Today 21:484-489. [DOI] [PubMed] [Google Scholar]
- 16.Furie, M. B., and G. J. Randolph. 1995. Chemokines and tissue injury. Am. J. Pathol. 146:1287-1301. [PMC free article] [PubMed] [Google Scholar]
- 17.Gale, L. M., and S. R. McColl. 1999. Chemokines: extracellular messengers for all occasions? Bioessays 21:17-28. [DOI] [PubMed] [Google Scholar]
- 18.Gordon, S. 1995. The macrophage. Bioessays 17:977-986. [DOI] [PubMed] [Google Scholar]
- 19.Gordon, S., S. Keshav, and L. P. Chung. 1988. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr. Opin. Immunol. 1:26-35. [DOI] [PubMed] [Google Scholar]
- 20.Gubler, D. J. 1998. Dengue and dengue hemorrhagic fever. Clin. Microbiol Rev. 11:480-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubler, D. J., and M. Meltzer. 1999. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 53:35-70. [DOI] [PubMed] [Google Scholar]
- 22.Halstead, S. B. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476-481. [DOI] [PubMed] [Google Scholar]
- 23.Halstead, S. B., and E. J. O'Rourke. 1977. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by nonneutralizing antibody. J. Exp. Med. 146:201-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes, M. P., J. C. Enterline, T. L. Gerrard, and K. C. Zoon. 1991. Regulation of interferon production by human monocytes: requirements for priming for lipopolysaccharide-induced production. J. Leukoc. Biol. 50:176-181. [DOI] [PubMed] [Google Scholar]
- 25.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 27.King, C. A., J. S. Marshall, H. Alshurafa, and R. Anderson. 2000. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J. Virol. 74:7146-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreipe, H., H. J. Radzun, P. Rudolph, J. Barth, M. L. Hansmann, K. Heidorn, and M. R. Parwaresch. 1988. Multinucleated giant cells generated in vitro. Terminally differentiated macrophages with down-regulated c-fms expression. Am. J. Pathol. 130:232-243. [PMC free article] [PubMed] [Google Scholar]
- 29.Krilov, L. R., R. M. Hendry, E. Godfrey, and K. McIntosh. 1987. Respiratory virus infection of peripheral blood monocytes: correlation with ageing of cells and interferon production in vitro. J. Gen. Virol. 68:1749-1753. [DOI] [PubMed] [Google Scholar]
- 30.Kuberski, T., L. Rosen, D. Reed, and J. Mataika. 1977. Clinical and laboratory observations on patients with primary and secondary dengue type 1 infections with hemorrhagic manifestations in Fiji. Am. J. Trop. Med. Hyg. 26:775-783. [DOI] [PubMed] [Google Scholar]
- 31.Kurane, I., and F. A. Ennis. 1994. Cytotoxic T lymphocytes in dengue virus infection. Curr. Top. Microbiol. Immunol. 189:93-108. [DOI] [PubMed] [Google Scholar]
- 32.Kurane, I., and F. A. Ennis. 1988. Production of interferon alpha by dengue virus-infected human monocytes. J. Gen. Virol. 69:445-449. [DOI] [PubMed] [Google Scholar]
- 33.Kurane, I., B. L. Innis, S. Nimmannitya, A. Nisalak, A. Meager, and F. A. Ennis. 1993. High levels of interferon alpha in the sera of children with dengue virus infection. Am. J. Trop. Med. Hyg. 48:222-229. [DOI] [PubMed] [Google Scholar]
- 34.Kurane, I., B. L. Innis, S. Nimmannitya, A. Nisalak, A. Meager, J. Janus, and F. A. Ennis. 1991. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Investig. 88:1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larregina, A. T., A. E. Morelli, L. A. Spencer, A. J. Logar, S. C. Watkins, A. W. Thomson, and L. D. Falo, Jr. 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151-1158. [DOI] [PubMed] [Google Scholar]
- 36.Lechner, F., J. Machado, G. Bertoni, H. F. Seow, D. A. Dobbelaere, and E. Peterhans. 1997. Caprine arthritis encephalitis virus dysregulates the expression of cytokines in macrophages. J. Virol. 71:7488-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, C. H., Y. H. Choi, S. H. Yang, C. W. Lee, S. J. Ha, and Y. C. Sung. 2001. Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology 279:271-279. [DOI] [PubMed] [Google Scholar]
- 38.Lee, D. H., S. S. Tam, S. Benyoucef, D. De Groote, V. Deubel, and P. Wattre. 1996. Enhanced TNF alpha production by monocytic-like cells exposed to dengue virus antigens. Immunol. Lett. 53:115-120. [DOI] [PubMed] [Google Scholar]
- 39.Libraty, D. H., S. Pichyangkul, C. Ajariyakhajorn, T. P. Endy, and F. A. Ennis. 2001. Human dendritic cells are activated by dengue virus infection: enhancement by gamma interferon and implications for disease pathogenesis. J. Virol. 75:3501-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma, X., J. Sun, E. Papasavvas, H. Riemann, S. Robertson, J. Marshall, R. T. Bailer, A. Moore, R. P. Donnelly, G. Trinchieri, and L. J. Montaner. 2000. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J. Immunol. 164:1722-1729. [DOI] [PubMed] [Google Scholar]
- 41.Marianneau, P., A. M. Steffan, C. Royer, M. T. Drouet, D. Jaeck, A. Kirn, and V. Deubel. 1999. Infection of primary cultures of human Kupffer cells by Dengue virus: no viral progeny synthesis, but cytokine production is evident. J. Virol. 73:5201-5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murgue, B., O. Cassar, X. Deparis, M. Guigon, and E. Chungue. 1998. Implication of macrophage inflammatory protein-1α in the inhibition of human haematopoietic progenitor growth by dengue virus. J. Gen. Virol. 79:1889-1893. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen, K. B., and C. A. Biron. 1999. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-gamma production. J. Immunol. 162:5238-5246. [PubMed] [Google Scholar]
- 45.O'Sullivan, M. A., and H. M. Killen. 1994. The differentiation state of monocytic cells affects their susceptibility to infection and the effects of infection by dengue virus. J. Gen. Virol. 75:2387-2392. [DOI] [PubMed] [Google Scholar]
- 46.Pelus, L. M., O. G. Ottmann, and K. H. Nocka. 1988. Synergistic inhibition of human marrow granulocyte-macrophage progenitor cells by prostaglandin E and recombinant interferon-alpha, -beta, and -gamma and an effect mediated by tumor necrosis factor. J. Immunol. 140:479-484. [PubMed] [Google Scholar]
- 47.Randolph, G. J., K. Inaba, D. F. Robbiani, R. M. Steinman, and W. A. Muller. 1999. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity 11:753-761. [DOI] [PubMed] [Google Scholar]
- 48.Rich, E. A., I. S. Chen, J. A. Zack, M. L. Leonard, and W. A. O'Brien. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J. Clin. Investig. 89:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rollins, B. J. 1997. Chemokines. Blood 90:909-928. [PubMed] [Google Scholar]
- 50.Rothman, A. L., and F. A. Ennis. 1999. Immunopathogenesis of Dengue hemorrhagic fever. Virology 257:1-6. [DOI] [PubMed] [Google Scholar]
- 51.Salazar-Mather, T. P., T. A. Hamilton, and C. A. Biron. 2000. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J. Clin. Investig. 105:985-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salazar-Mather, T. P., J. S. Orange, and C. A. Biron. 1998. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1α (MIP-1α)-dependent pathways. J. Exp. Med. 187:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature 347:669-671. [DOI] [PubMed] [Google Scholar]
- 55.Spain-Santana, T. A., S. Marglin, F. A. Ennis, and A. L. Rothman. 2001. MIP-1 alpha and MIP-1 beta induction by dengue virus. J. Med. Virol. 65:324-330. [DOI] [PubMed] [Google Scholar]
- 56.Stockl, J., H. Vetr, O. Majdic, G. Zlabinger, E. Kuechler, and W. Knapp. 1999. Human major group rhinoviruses downmodulate the accessory function of monocytes by inducing IL-10. J. Clin. Investig. 104:957-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Streatfield, R., G. Bielby, and D. Sinclair. 1993. A primary dengue 2 epidemic with spontaneous haemorrhagic manifestations. Lancet 342:560-561. [DOI] [PubMed] [Google Scholar]
- 58.Taoufik, Y., O. Lantz, C. Wallon, A. Charles, E. Dussaix, and J. F. Delfraissy. 1997. Human immunodeficiency virus gp120 inhibits interleukin-12 secretion by human monocytes: an indirect interleukin-10-mediated effect. Blood 89:2842-2848. [PubMed] [Google Scholar]
- 59.Temonen, M., H. Lankinen, O. Vapalahti, T. Ronni, I. Julkunen, and A. Vaheri. 1995. Effect of interferon-alpha and cell differentiation on Puumala virus infection in human monocyte/macrophages. Virology 206:8-15. [DOI] [PubMed] [Google Scholar]
- 60.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 70:83-243. [DOI] [PubMed] [Google Scholar]
- 61.Tuttle, D. L., J. K. Harrison, C. Anders, J. W. Sleasman, and M. M. Goodenow. 1998. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J. Virol. 72:4962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang, S. Y., H. Castro-Malaspina, L. Lu, and M. A. Moore. 1985. Biological characterization of a granulomonopoietic enhancing activity derived from cultured human lipid-containing macrophages. Blood 65:1181-1190. [PubMed] [Google Scholar]
- 63.Wang, S. Y., H. Castro-Malaspina, and M. A. Moore. 1985. Long-term culture of human bone marrow macrophages: macrophage development is associated with the production of granulomonopoietic enhancing activity (GM-EA). J. Immunol. 135:1186-1193. [PubMed] [Google Scholar]
- 64.Wang, S. Y., C. K. Ho, L. Y. Chen, R. C. Wang, M. H. Huang, H. Castro-Malaspina, and M. A. Moore. 1988. Down regulation of myelopoiesis by mediators inhibiting the production of macrophage-derived granulomonopoietic enhancing activity (GM-EA). Blood 72:2001-2006. [PubMed] [Google Scholar]
- 65.Wang, S. Y., K. L. Mak, L. Y. Chen, M. P. Chou, and C. K. Ho. 1992. Heterogeneity of human blood monocyte: two subpopulations with different sizes, phenotypes and functions. Immunology 77:298-303. [PMC free article] [PubMed] [Google Scholar]
- 66.Watts, D. M., K. R. Porter, P. Putvatana, B. Vasquez, C. Calampa, C. G. Hayes, and S. B. Halstead. 1999. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 354:1431-1434. [DOI] [PubMed] [Google Scholar]
- 67.Wu, S. J., G. Grouard-Vogel, W. Sun, J. R. Mascola, E. Brachtel, R. Putvatana, M. K. Louder, L. Filgueira, M. A. Marovich, H. K. Wong, A. Blauvelt, G. S. Murphy, M. L. Robb, B. L. Innes, D. L. Birx, C. G. Hayes, and S. S. Frankel. 2000. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 6:816-820. [DOI] [PubMed] [Google Scholar]