Abstract
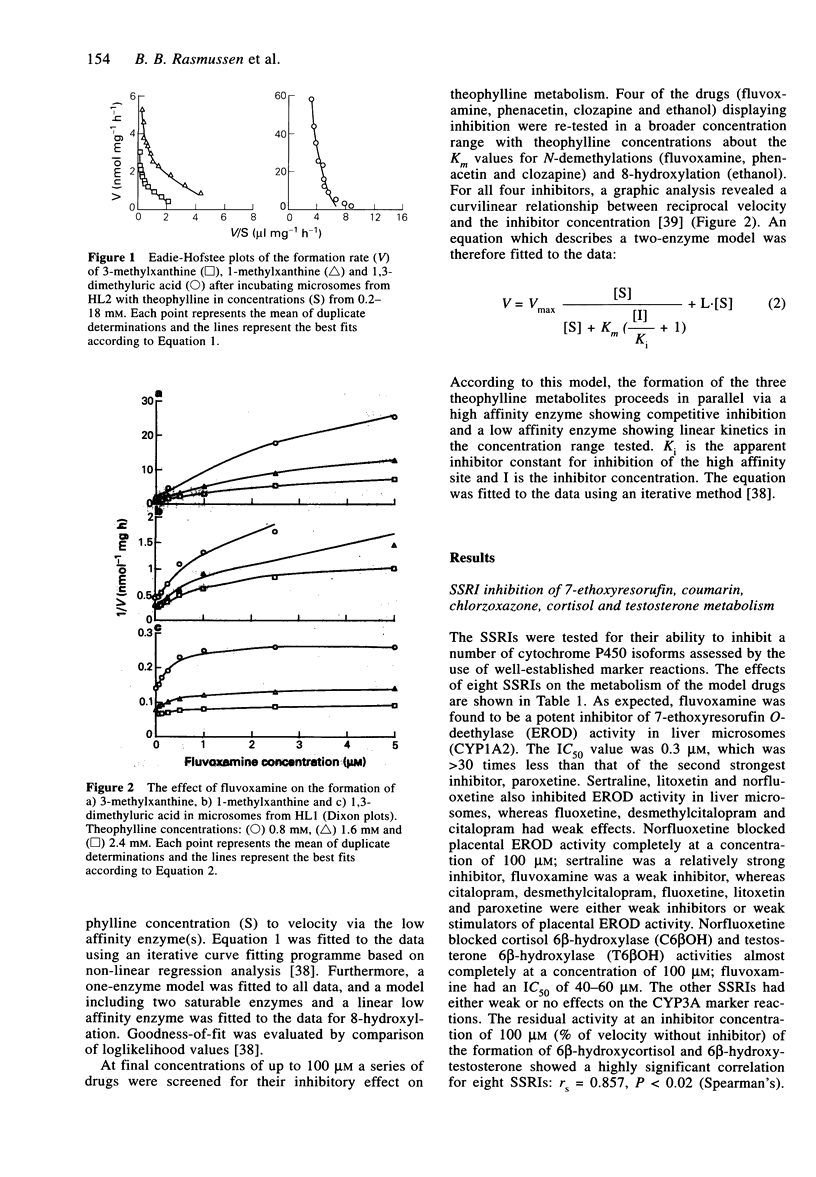
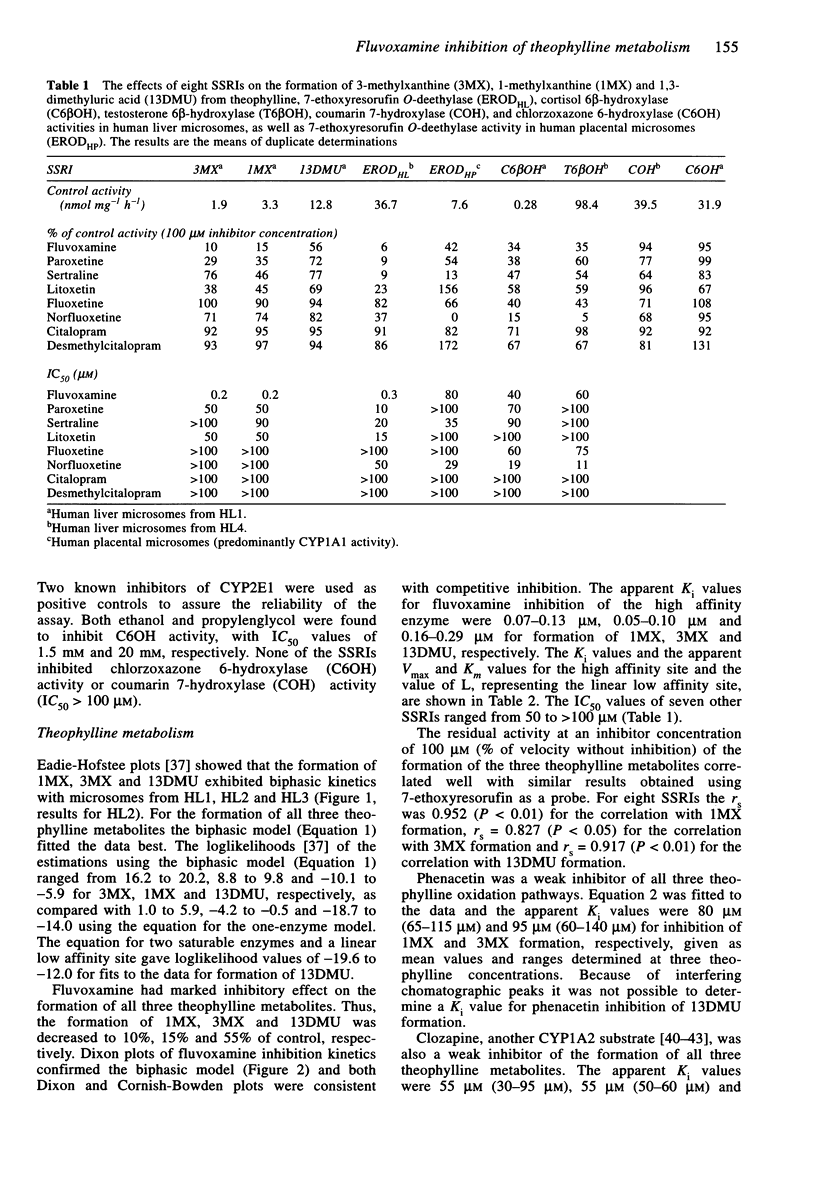
1. Fluvoxamine and seven other selective serotonin reuptake inhibitors (SRRI) were tested for their ability to inhibit a number of human cytochrome P450 isoforms (CYPs). 2. None of the drugs showed potent inhibition of CYP2A6 (coumarin 7-hydroxylase) or CYP2E1 (chlorzoxazone 6-hydroxylase), while norfluoxetine was the only potent inhibitor of CYP3A having IC50 values of 11 microM and 19 microM for testosterone 6 beta-hydroxylase and cortisol 6 beta-hydroxylase, respectively. 3. Norfluoxetine, sertraline and fluvoxamine inhibited CYP1A1 (7-ethoxyresorufin O-deethylase) in microsomes from human placenta (IC50 values 29 microM, 35 microM and 80 microM, respectively). Fluvoxamine was a potent inhibitor of CYP1A2-mediated 7-ethoxyresorufin O-deethylase activity (IC50 = 0.3 microM) in human liver. 4. In microsomes from three human livers fluvoxamine potently inhibited all pathways of theophylline biotransformation, the apparent inhibitor constant, Ki, was 0.07-0.13 microM, 0.05-0.10 microM and 0.16-0.29 microM for inhibition of 1-methylxanthine, 3-methylxanthine and 1,3-dimethyluric acid formation, respectively. Seven other SSRIs showed either weak or no inhibition of theophylline metabolism. 5. Ethanol inhibited the formation of 1,3-dimethyluric acid with K(i) value of 300 microM, a value which is consistent with inhibition of CYP2E1. Ethanol and fluvoxamine both inhibited 8-hydroxylation by about 45% and, in combination, the compounds decreased the formation of 1,3-dimethyluric acid by 90%, indicating that CYP1A2 and CYP2E1 are equally important isoforms for the 8-hydroxylation of theophylline. 6. It is concluded that pharmacokinetic interaction between fluvoxamine and theophylline is due to potent inhibition of CYP1A2.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitio A. A simple and sensitive assay of 7-ethoxycoumarin deethylation. Anal Biochem. 1978 Apr;85(2):488–491. doi: 10.1016/0003-2697(78)90245-2. [DOI] [PubMed] [Google Scholar]
- Benfield P., Ward A. Fluvoxamine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs. 1986 Oct;32(4):313–334. doi: 10.2165/00003495-198632040-00002. [DOI] [PubMed] [Google Scholar]
- Birkett D. J., Miners J. O., Attwood J. Secondary metabolism of theophylline biotransformation products in man--route of formation of 1-methyluric acid. Br J Clin Pharmacol. 1983 Jan;15(1):117–119. doi: 10.1111/j.1365-2125.1983.tb01475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boobis A. R., Lynch A. M., Murray S., de la Torre R., Solans A., Farré M., Segura J., Gooderham N. J., Davies D. S. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994 Jan 1;54(1):89–94. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Skjelbo E., Rasmussen B. B., Poulsen H. E., Loft S. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol. 1993 Mar 24;45(6):1211–1214. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- Burke M. D., Prough R. A., Mayer R. T. Characteristics of a microsomal cytochrome P-448-mediated reaction. Ethoxyresorufin O-de-ethylation. Drug Metab Dispos. 1977 Jan-Feb;5(1):1–8. [PubMed] [Google Scholar]
- Butler M. A., Iwasaki M., Guengerich F. P., Kadlubar F. F. Human cytochrome P-450PA (P-450IA2), the phenacetin O-deethylase, is primarily responsible for the hepatic 3-demethylation of caffeine and N-oxidation of carcinogenic arylamines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7696–7700. doi: 10.1073/pnas.86.20.7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. E., Grant D. M., Inaba T., Kalow W. Biotransformation of caffeine, paraxanthine, theophylline, and theobromine by polycyclic aromatic hydrocarbon-inducible cytochrome(s) P-450 in human liver microsomes. Drug Metab Dispos. 1987 Mar-Apr;15(2):237–249. [PubMed] [Google Scholar]
- Crewe H. K., Lennard M. S., Tucker G. T., Woods F. R., Haddock R. E. The effect of selective serotonin re-uptake inhibitors on cytochrome P4502D6 (CYP2D6) activity in human liver microsomes. Br J Clin Pharmacol. 1992 Sep;34(3):262–265. doi: 10.1111/j.1365-2125.1992.tb04134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist R., Bertilsson L., Birkett D. J., Eichelbaum M., Säwe J., Sjöqvist F. Theophylline metabolism in relation to antipyrine, debrisoquine, and sparteine metabolism. Clin Pharmacol Ther. 1984 Jun;35(6):815–821. doi: 10.1038/clpt.1984.118. [DOI] [PubMed] [Google Scholar]
- Diot P., Jonville A. P., Gerard F., Bonnelle M., Autret E., Breteau M., Lemarie E., Lavandier M. Possible interaction entre théophylline et fluvoxamine. Therapie. 1991 Mar-Apr;46(2):170–171. [PubMed] [Google Scholar]
- Gascon M. P., Dayer P. In vitro forecasting of drugs which may interfere with the biotransformation of midazolam. Eur J Clin Pharmacol. 1991;41(6):573–578. doi: 10.1007/BF00314987. [DOI] [PubMed] [Google Scholar]
- Ged C., Rouillon J. M., Pichard L., Combalbert J., Bressot N., Bories P., Michel H., Beaune P., Maurel P. The increase in urinary excretion of 6 beta-hydroxycortisol as a marker of human hepatic cytochrome P450IIIA induction. Br J Clin Pharmacol. 1989 Oct;28(4):373–387. doi: 10.1111/j.1365-2125.1989.tb03516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grygiel J. J., Birkett D. J. Cigarette smoking and theophylline clearance and metabolism. Clin Pharmacol Ther. 1981 Oct;30(4):491–496. doi: 10.1038/clpt.1981.193. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Hakkola J., Mäenpä J., Mayer R. T., Park S. S., Gelboin H. V., Pelkonen O. 7-Alkoxyquinoline O-dealkylation by microsomes from human liver and placenta. Br J Clin Pharmacol. 1992 Nov;34(5):415–420. doi: 10.1111/j.1365-2125.1992.tb05650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. N., Jusko W. J., Yurchak A. M. Effect of smoking on theophylline disposition. Clin Pharmacol Ther. 1976 May;19(5 Pt 1):546–551. doi: 10.1002/cpt1976195part1546. [DOI] [PubMed] [Google Scholar]
- Jerling M., Lindström L., Bondesson U., Bertilsson L. Fluvoxamine inhibition and carbamazepine induction of the metabolism of clozapine: evidence from a therapeutic drug monitoring service. Ther Drug Monit. 1994 Aug;16(4):368–374. doi: 10.1097/00007691-199408000-00006. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lemoine A., Gautier J. C., Azoulay D., Kiffel L., Belloc C., Guengerich F. P., Maurel P., Beaune P., Leroux J. P. Major pathway of imipramine metabolism is catalyzed by cytochromes P-450 1A2 and P-450 3A4 in human liver. Mol Pharmacol. 1993 May;43(5):827–832. [PubMed] [Google Scholar]
- Lykkesfeldt J., Loft S., Poulsen H. E. Simultaneous determination of urinary free cortisol and 6 beta-hydroxycortisol by high-performance liquid chromatography to measure human CYP3A activity. J Chromatogr B Biomed Appl. 1994 Oct 3;660(1):23–29. doi: 10.1016/0378-4347(94)00265-7. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Mueller H. K., Dick B., Meyer U. A. Hepatic monooxygenase activities in subjects with a genetic defect in drug oxidation. Gastroenterology. 1983 Sep;85(3):682–692. [PubMed] [Google Scholar]
- Mäenpä J., Pelkonen O., Cresteil T., Rane A. The role of cytochrome P450 3A (CYP3A) isoform(s) in oxidative metabolism of testosterone and benzphetamine in human adult and fetal liver. J Steroid Biochem Mol Biol. 1993 Jan;44(1):61–67. doi: 10.1016/0960-0760(93)90152-m. [DOI] [PubMed] [Google Scholar]
- Mäenpä J., Syngelmä T., Honkakoski P., Lang M. A., Pelkonen O. Comparative studies on coumarin and testosterone metabolism in mouse and human livers. Differential inhibitions by the anti-P450Coh antibody and metyrapone. Biochem Pharmacol. 1991 Aug 22;42(6):1229–1235. doi: 10.1016/0006-2952(91)90258-7. [DOI] [PubMed] [Google Scholar]
- Ogilvie R. I. Clinical pharmacokinetics of theophylline. Clin Pharmacokinet. 1978 Jul-Aug;3(4):267–293. doi: 10.2165/00003088-197803040-00002. [DOI] [PubMed] [Google Scholar]
- Otton S. V., Wu D., Joffe R. T., Cheung S. W., Sellers E. M. Inhibition by fluoxetine of cytochrome P450 2D6 activity. Clin Pharmacol Ther. 1993 Apr;53(4):401–409. doi: 10.1038/clpt.1993.43. [DOI] [PubMed] [Google Scholar]
- Pasanen M., Pelkonen O. The expression and environmental regulation of P450 enzymes in human placenta. Crit Rev Toxicol. 1994;24(3):211–229. doi: 10.3109/10408449409021606. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Pasanen M., Kuha H., Gachalyi B., Kairaluoma M., Sotaniemi E. A., Park S. S., Friedman F. K., Gelboin H. V. The effect of cigarette smoking on 7-ethoxyresorufin O-deethylase and other monooxygenase activities in human liver: analyses with monoclonal antibodies. Br J Clin Pharmacol. 1986 Aug;22(2):125–134. doi: 10.1111/j.1365-2125.1986.tb05239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R., Tu Y. Y., Yang C. S. The induction and competitive inhibition of a high affinity microsomal nitrosodimethylamine demethylase by ethanol. Carcinogenesis. 1982;3(12):1457–1461. doi: 10.1093/carcin/3.12.1457. [DOI] [PubMed] [Google Scholar]
- Peter R., Böcker R., Beaune P. H., Iwasaki M., Guengerich F. P., Yang C. S. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990 Nov-Dec;3(6):566–573. doi: 10.1021/tx00018a012. [DOI] [PubMed] [Google Scholar]
- Rasmussen B. B., Nielsen K. K., Brøsen K. Determination of theophylline metabolites in human liver microsomes by high-performance liquid chromatography. Anal Biochem. 1994 Oct;222(1):9–13. doi: 10.1006/abio.1994.1446. [DOI] [PubMed] [Google Scholar]
- Raunio H., Syngelmä T., Pasanen M., Juvonen R., Honkakoski P., Kairaluoma M. A., Sotaniemi E., Lang M. A., Pelkonen O. Immunochemical and catalytical studies on hepatic coumarin 7-hydroxylase in man, rat, and mouse. Biochem Pharmacol. 1988 Oct 15;37(20):3889–3895. doi: 10.1016/0006-2952(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Robson R. A., Miners J. O., Matthews A. P., Stupans I., Meller D., McManus M. E., Birkett D. J. Characterisation of theophylline metabolism by human liver microsomes. Inhibition and immunochemical studies. Biochem Pharmacol. 1988 May 1;37(9):1651–1659. doi: 10.1016/0006-2952(88)90423-6. [DOI] [PubMed] [Google Scholar]
- Sarkar M. A., Hunt C., Guzelian P. S., Karnes H. T. Characterization of human liver cytochromes P-450 involved in theophylline metabolism. Drug Metab Dispos. 1992 Jan-Feb;20(1):31–37. [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Edwards R. J., Davies D. S. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1988 Oct;26(4):363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Murray B. P., Murray S., Segura J., de la Torre R., Davies D. S. Furafylline is a potent and selective inhibitor of cytochrome P450IA2 in man. Br J Clin Pharmacol. 1990 Jun;29(6):651–663. doi: 10.1111/j.1365-2125.1990.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelbo E., Brøsen K. Inhibitors of imipramine metabolism by human liver microsomes. Br J Clin Pharmacol. 1992 Sep;34(3):256–261. doi: 10.1111/j.1365-2125.1992.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber A. D. Toxic interaction between fluvoxamine and sustained release theophylline in an 11-year-old boy. Drug Saf. 1991 Nov-Dec;6(6):460–462. doi: 10.2165/00002018-199106060-00006. [DOI] [PubMed] [Google Scholar]
- Spina E., Campo G. M., Avenoso A., Pollicino M. A., Caputi A. P. Interaction between fluvoxamine and imipramine/desipramine in four patients. Ther Drug Monit. 1992 Jun;14(3):194–196. doi: 10.1097/00007691-199206000-00004. [DOI] [PubMed] [Google Scholar]
- Spina E., Pollicino A. M., Avenoso A., Campo G. M., Perucca E., Caputi A. P. Effect of fluvoxamine on the pharmacokinetics of imipramine and desipramine in healthy subjects. Ther Drug Monit. 1993 Jun;15(3):243–246. doi: 10.1097/00007691-199306000-00011. [DOI] [PubMed] [Google Scholar]
- Waxman D. J., Ko A., Walsh C. Regioselectivity and stereoselectivity of androgen hydroxylations catalyzed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J Biol Chem. 1983 Oct 10;258(19):11937–11947. [PubMed] [Google Scholar]
- Waxman D. J., Lapenson D. P., Aoyama T., Gelboin H. V., Gonzalez F. J., Korzekwa K. Steroid hormone hydroxylase specificities of eleven cDNA-expressed human cytochrome P450s. Arch Biochem Biophys. 1991 Oct;290(1):160–166. doi: 10.1016/0003-9861(91)90602-f. [DOI] [PubMed] [Google Scholar]
- Yamano S., Tatsuno J., Gonzalez F. J. The CYP2A3 gene product catalyzes coumarin 7-hydroxylation in human liver microsomes. Biochemistry. 1990 Feb 6;29(5):1322–1329. doi: 10.1021/bi00457a031. [DOI] [PubMed] [Google Scholar]



