Abstract
Six structures of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase (RT) containing combinations of resistance mutations for zidovudine (AZT) (M41L and T215Y) or lamivudine (M184V) have been determined as inhibitor complexes. Minimal conformational changes in the polymerase or nonnucleoside RT inhibitor sites compared to the mutant RTMC (D67N, K70R, T215F, and K219N) are observed, indicating that such changes may occur only with certain combinations of mutations. Model building M41L and T215Y into HIV-1 RT-DNA and docking in ATP that is utilized in the pyrophosphorolysis reaction for AZT resistance indicates that some conformational rearrangement appears necessary in RT for ATP to interact simultaneously with the M41L and T215Y mutations.
Codon 215 mutations in combination with M41L in reverse transcriptase (RT) confer high-level zidovudine (AZT) resistance on human immunodeficiency virus (HIV) (9, 11). M184V gives resistance to lamivudine (3TC) (20, 23) and restores AZT sensitivity to codon 41 and 215 mutations (12). X-ray crystallographic results indicate that residues 41 and 215 in HIV-1 RT are distal to the deoxynucleoside triphosphate binding site (6, 7, 10, 16), and ideas as to how AZT resistance is induced include template rearrangement (3) and conformational changes propagated from residues 215 and 219 for the mutant RTMC (D67N, K70R, T215F, and K219N) (17). Biochemical studies have shown a mechanism of AZT resistance involving AZTMP removal from the blocked primer strand in a pyrophosphate- or ATP-dependent pyrophosphorolysis reaction enhanced by RTMC and other mutant RTs (1, 2, 13, 14). Model building studies suggest that T215Y might promote ATP binding, the presumed physiological acceptor for the pyrophosphorolysis reaction (2). To investigate further the effects of AZT and 3TC drug resistance mutations on the conformational states of HIV-1 RT, structures of a series of six AZT- and/or 3TC-resistant RTs mainly containing combinations of M41L, T215Y, and M184V mutations were determined in the absence of oligonucleotide or deoxynucleoside triphosphate substrates to assess whether underlying baseline structural rearrangements are induced by these mutations in a way analogous to that previously observed for RTMC.
Site-directed mutants were introduced into the HIV-1 HXB2-D RT coding region by using ExSite or QuikChange kits (Stratagene). Expression, purification, and crystallization of RT (as complexes with nevirapine or the nevirapine analogue 1051U91) were performed as described previously (5, 22, 24). Six RT mutants were purified and crystallized, and structures were determined for five of these (Table 1), whereas the sixth mutant, RT+M41L, gave disordered crystals unsuitable for data collection. X-ray data were collected at synchrotron sources (Table 1), and DENZO and SCALEPACK were used for data processing (15). The orientation and translation of HIV-1 RT in the unit cell were determined by using rigid-body refinement with XPLOR, and refinement was done with XPLOR or CNS (4) as described previously (18). For data sets too small for full refinement, tight positional restraints were applied. Table 1 gives statistics on X-ray data quality and refinement statistics. Coordinates and structure factors have been deposited in the Protein Data Bank (PDB) for immediate release (see Table 1 for PDB codes). O (8) was used for mutating side chains in the RT-DNA complex (PDB code 1rtd) (6) and docking in ATP.
TABLE 1.
Statistics for crystallographic structure determinationsa
| Parameter | Data set (mutant name and mutation[s])
|
|||||
|---|---|---|---|---|---|---|
| RTMF T215Y | RTMF (1051U91) T215Y | RT+M184V M184V | RTMN M41L, T215Y | RTMN+M184V M41L, 215Y, M184V | RTMQ+M184V M41L, D67N, K70R, M184V, T215Y | |
| Data collection details | ||||||
| Data collection site | ESRF ID2 | ESRF ID14-EH3 | ESRF ID2 | SRS PX7.2 | KEK BL-6A | SRS PX9.6 |
| Detector | MAR345 | MAR CCD | MAR345 | MAR 30 cm | Fuji BAS III | ADSC CCD |
| Temperature (K) | 100 | 100 | 100 | 100 | 290 | 100 |
| Wavelength (Å) | 0.9903 | 0.9310 | 0.9903 | 1.488 | 1.000 | 0.870 |
| Unit cell dimensions (a, b, c in Å) | 139.7, 115.0, 65.6 | 137.3, 115.2, 64.9 | 140.0, 115.2, 65.3 | 139.7, 111.3, 74.5 | 140.3, 111.2, 74.4 | 137.9, 109.5, 74.1 |
| Resolution range (Å) | 30-2.80 | 30-3.00 | 30-2.62 | 30-2.80 | 30-3.30 | 30-2.80 |
| Observations (no.) | 91,111 | 91,720 | 134,485 | 106,768 | 62,933 | 117,013 |
| Unique reflections (no.) | 25,400 | 21,006 | 28,631 | 27,248 | 17,485 | 28,293 |
| Completeness (%) | 93.2 | 98.6 | 86.8 | 93.9 | 96.0 | 97.5 |
| Avg I/σ(I) | 13.8 | 8.3 | 17.7 | 14.5 | 6.9 | 19.6 |
| Rmergeb | 0.061 | 0.111 | 0.075 | 0.066 | 0.137 | 0.054 |
| Outer resolution shell | ||||||
| Resolution range (Å) | 2.90-2.80 | 3.11-3.00 | 2.71-2.62 | 2.90-2.80 | 3.42-3.30 | 2.90-2.80 |
| Unique reflections (no.) | 2,107 | 1,996 | 2,326 | 2,197 | 1,575 | 2,520 |
| Completeness (%) | 78.5 | 95.3 | 71.6 | 77.0 | 88.2 | 87.6 |
| Average I/σ(I) | 1.6 | 1.0 | 2.0 | 1.6 | 0.94 | 2.4 |
| Refinement statistics | ||||||
| Resolution range (Å) | 30-2.80 | 30-3.00 | 30-2.62 | 30-2.80 | 30-3.30e | 30-2.80 |
| No. of reflections (working/test) | 23,850/1,257 | 19,965/1,013 | 26,913/1,437 | 25,899/1,329 | 16,586/843 | 26,526/1,345 |
| R factorc (Rwork/Rfree) | 0.220/0.302 | 0.206/0.270 | 0.214/0.280 | 0.214/0.302 | 0.285/0.322 | 0.237/0.295 |
| R factorc (all data) | 0.209 | 0.189 | 0.207 | 0.202 | 0.220 | |
| No. of atoms (protein/inhibitor/water) | 7,517/20/− | 7,639/22/− | 7,556/20/18 | 7,767/20/19 | 7,719/20/− | |
| Rmsf bond length deviation (Å) | 0.0116 | 0.0117 | 0.0113 | 0.0113 | 0.0110 | |
| Rms bond angle deviation (°) | 1.71 | 1.61 | 1.69 | 1.69 | 1.63 | |
| Mean B factor (Å2)d | 80/87/60/− | 58/66/48/− | 73/81/50/54 | 67/73/48/44 | 73/79/51/− | |
| PBD code | 1LW0 | 1LW2 | 1LWC | 1LWE | — | 1LWF |
All mutants were crystallized as nevirapine complexes except as indicated (1051U91). Abbreviations: ESRF, European Synchrotron Radiation Facility, Grenoble, France; SRS, Synchrotron Radiation Source, Daresbury, United Kingdom; KEK, National Laboratory for High Energy Physics, Tsukuba, Japan; CCD, charge-coupled device. −, no value.
Rmerge = ∑|I − 〈I〉 |/∑ 〈I〉.
R factor = ∑|Fo − Fc |/∑Fo.
Mean B factor for main chain, side chain, inhibitor, and water atoms, respectively.
Rigid-body and grouped B-factor refinement only.
Rms, root mean square.
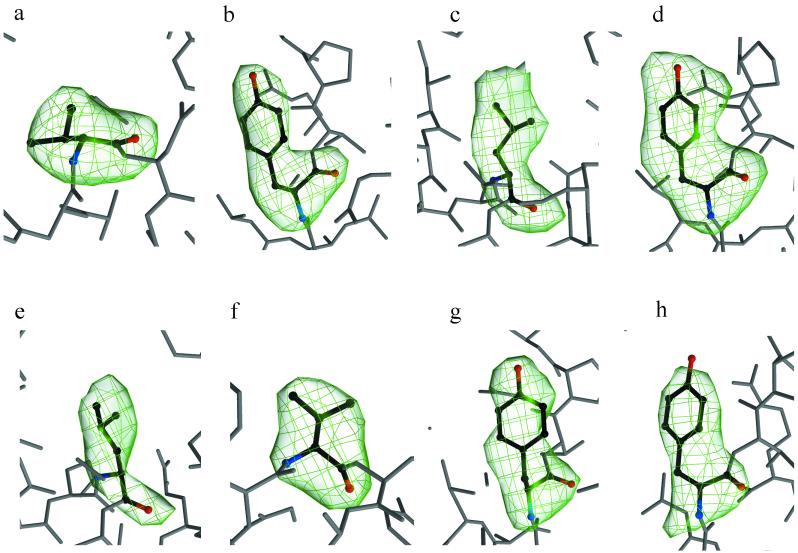
Omit maps show electron density for the mutated side chains in p66 (Fig. 1). Y215 is clearly defined (Fig. 1b, d, g, and h), while for L41 the density is less clear within p66 (Fig. 1c and e) than in p51; nevertheless, its conformation could be determined. In the RTMQ+M184V structure the 67 and 70 side chains are disordered.
FIG. 1.
Simulated annealing omit electron density maps showing the drug resistance mutations of M184V in RT+M184V (a); T215Y in RTMF-nevirapine (b); M41L (c) and T215Y (d) in RTMN; M41L (e), M184V (f), and T215Y (g) in RTMQ+M184V; and T215Y in RTMF-1051U91 (h).
Electron density for the M184V mutation is clear in all cases (Fig. 1a and f) compared to the previously reported RT+M184I structure which had the mutated residue disordered in the unliganded structure but defined in the binary complex with DNA (19).
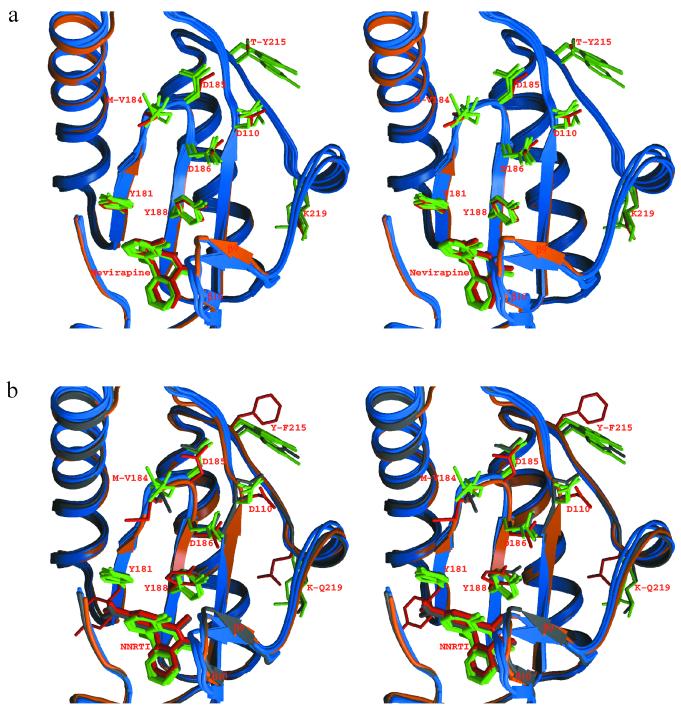
Comparison of the structures of this present set of mutant RTs with wild-type RT-nevirapine complexes shows only minor conformational changes in the protein (Fig. 2a). Structural differences at the β9-β10 loop region appear related to the various crystal forms, and such effects can be separated from differences in inhibitor binding or drug resistance mutations. These results are distinct from the previously determined structure of an AZT-resistant RT containing a different set of mutations (RTMC: D67N, K70R, T215F, and K219N), where significant movement of the polymerase active site and the Y181 side chain is observed (17). The RTMF-1051U91 structure is essentially identical to that with nevirapine, indicating that the conformational variation compared to RTMC could not be ascribed to these different inhibitors (Fig. 2b). There are differences between the particular AZT resistance mutations for RTMC and those for RTMF or RTMN. RTMC has, in addition to the change at codon 215, mutations at 67, 70, and 219, and none of the latter are present in RTMF or RTMN. It was determined from the earlier structural studies that the observed conformational changes that propagated toward the active site and the nonnucleoside RT inhibitor (NNRTI)-binding site apparently originated from residues 215 and 219 (17), and it is possible that the 219 mutation in combination with T215F (in place of T215Y in RTMN) could give rise to the conformational rearrangement in the RTMC active and NNRTI sites. RTMN is from a mutant virus that shows high-level AZT resistance, and yet the polymerase active site has essentially the same conformation as does wild-type RT, hence indicating a lack of correlation between resistance phenotype and the active site conformational state of substrate-free RT. RTs containing the M184V mutation either in the absence or in the presence of AZT resistance mutations have similar conformational states. The reversal effect of M184V on AZT resistance may in any case be mediated by more direct effects, since residue 184 is positioned close to the termination site on the blocked primer. However, drug resistance mutation-dependent conformational changes might occur in higher-order complexes involving bound substrates or other ligands.
FIG. 2.
Comparison of the polymerase active-site regions. (a) RT+M184V, RTMQ+M184V, RTMN, and RTMF-nevirapine are shown overlapped onto the wild-type RT-nevirapine complex. The main chain, side chains, and the inhibitor of wild-type RT are shown in orange and red, and those of mutant RTs are shown in blue and green. (b) RTMF-nevirapine, RTMF-1051U91, RTMN, and RTMQ+M184V are superimposed onto RTMC. The main chain, side chains, and the inhibitor of RTMC are colored in orange and red, those of RTMF-1051U91 are all in gray, and those of other mutant RTs are in blue and green, respectively.
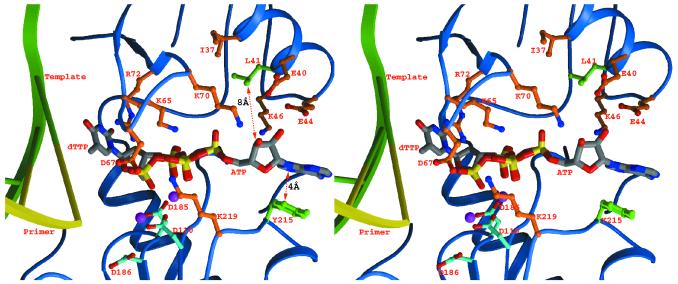
The side chain conformations of the mutated 41 and 215 residues from the structures determined here were modeled into the ternary complex of RT-template-primer-dTTP (6) followed by the docking of ATP, based on the mode predicted from the nature of the pyrophosphorolysis reaction (2, 21). This showed that ATP cannot simultaneously contact the mutated side chains of residues 215 and 41 (Fig. 3). Contacts with the Y215 side chain could be made via parallel aromatic ring stacking of the adenine base of ATP, forming a number of van der Waals contacts as has been noted previously (2, 21). Structural mechanisms whereby the mutation of M41L could lead to increased ATP binding are less clear. M41 is located some distance from Y215 and is somewhat buried, making direct interaction with ATP unlikely. The M41L mutation introduces a branched side chain that potentially might disrupt this buried region; however, structures of RTMN and RTMN+M184V reveal that this change is accommodated without significant rearrangement of the protein. It thus seems more likely that a conformational rearrangement occurs either within the catalytic complex or on binding of ATP rather than that there are two distinct ATP binding positions, each of which is strengthened by the mutations at either residue 41 or residue 215. The former model would be consistent with the synergistic effect of the combination of M41L and T215Y on AZT drug resistance. The putative further conformational change could lead to an indirect effect on ATP binding via intervening side chains, such as that formed by contact between residues 116 and 41, or main chain elements rather than repositioning the mutated 41 and 215 side chains to simultaneously interact with ATP. Further work is required to test such possibilities, in particular determination of a range of HIV-1 RT mutant-DNA structures including complexes with ATP.
FIG. 3.
Diagram showing the M41L and T215Y mutations (colored green) transferred into the HIV-1 RT double-stranded DNA-dTTP complex (6). An ATP molecule (atom-colored thick bonds with carbon atoms in gray) capable of the pyrophosphorolysis reaction has also been modeled into the protein by overlapping the β and γ phosphates of the bound dTTP. The protein side chains in the vicinity are shown in orange, and the three polymerase active-site aspartates are shown in cyan. The two magenta spheres represent the bound magnesiums. Distances for the putative interaction between ATP and the side chain of Y215 and the distance of the closest approach of L41 to ATP are marked.
Acknowledgments
We thank the staff of synchrotron facilities at SRS, Daresbury, United Kingdom; ESRF, Grenoble, France; and the Photon Factory, Tsukuba, Japan.
D.I.S. is a member of the TARA project. The UK MRC provided long-term funding of the RT work with grants to D.K.S. and D.I.S. D.K.S. acknowledges the support of the EU via grant QLKT-2000-00291.
REFERENCES
- 1.Arion, D., N. Kaushik, S. McCormick, G. Borkow, and M. A. Parniak. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry 37:15908-15917. [DOI] [PubMed] [Google Scholar]
- 2.Boyer, P. L., S. G. Sarafianos, E. Arnold, and S. H. Hughes. 2001. Selective excision of AZTMP by drug-resistant human immunodeficiency virus reverse transcriptase. J. Virol. 75:4832-4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyer, P. L., C. Tantillo, A. Jacobo-Molina, R. G. Nanni, J. Ding, E. Arnold, and S. H. Hughes. 1994. Sensitivity of wild-type human immunodeficiency virus type 1 reverse transcriptase to dideoxynucleotides depends on template length; the sensitivity of drug-resistant mutants does not. Proc. Natl. Acad. Sci. USA 91:4882-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunger, A. T., P. D. Adams, G. M. Clore, W. L. Delano, P. Gros, K. R. W. Grosse, J. S. Jiang, J. Kuszewski, M. Nilges, N. S. Pannu, R. J. Read, L. M. Rice, T. Simonson, and G. L. Warren. 1998. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr. D54:905-921. [DOI] [PubMed] [Google Scholar]
- 5.Esnouf, R. M., J. Ren, E. F. Garman, D. O. N. Somers, C. K. Ross, E. Y. Jones, D. K. Stammers, and D. I. Stuart. 1998. Continuous and discontinuous changes in the unit cell of HIV-1 reverse transcriptase crystals on dehydration. Acta Crystallogr. D54:938-954. [DOI] [PubMed] [Google Scholar]
- 6.Huang, H., R. Chopra, G. L. Verdine, and S. C. Harrison. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669-1675. [DOI] [PubMed] [Google Scholar]
- 7.Jacobo-Molina, A., J. P. Ding, R. G. Nanni, A. D. J. Clark, X. Lu, C. Tantillo, R. L. Williams, G. Kamer, A. L. Ferris, P. Clark, A. Hizi, S. H. Hughes, and E. Arnold. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 90:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, T. A., J. Y. Zou, S. W. Cowan, and M. Kjeldgaard. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A47:110-119. [DOI] [PubMed] [Google Scholar]
- 9.Kellam, P., C. A. B. Boucher, J. M. G. H. Tijnagel, and B. A. Larder. 1994. Zidovudine treatment results in the selection of human immunodeficiency virus type 1 variants whose genotypes confer increasing levels of drug resistance. J. Gen. Virol. 75:341-351. [DOI] [PubMed] [Google Scholar]
- 10.Kohlstaedt, L. A., J. Wang, J. M. Friedman, P. A. Rice, and T. A. Steitz. 1992. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science 256:1783-1790. [DOI] [PubMed] [Google Scholar]
- 11.Larder, B. A., and S. D. Kemp. 1989. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science 246:1155-1158. [DOI] [PubMed] [Google Scholar]
- 12.Larder, B. A., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 13.Lennerstrand, J., K. Hertogs, D. K. Stammers, and B. A. Larder. 2001. Correlation between viral resistance to zidovudine and resistance at the reverse transcriptase level for a panel of human immunodeficiency virus type 1 mutants. J. Virol. 75:7202-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer, P. R., S. E. Matsuura, A. M. Mian, A. G. So, and W. A. Scott. 1999. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell 4:35-43. [DOI] [PubMed] [Google Scholar]
- 15.Otwinowski, Z., and W. Minor. 1996. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-326. [DOI] [PubMed] [Google Scholar]
- 16.Ren, J., R. Esnouf, E. Garman, D. Somers, C. Ross, I. Kirby, J. Keeling, G. Darby, Y. Jones, D. Stuart, and D. Stammers. 1995. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat. Struct. Biol. 2:293-302. [DOI] [PubMed] [Google Scholar]
- 17.Ren, J., R. M. Esnouf, A. L. Hopkins, E. Y. Jones, I. Kirby, J. Keeling, C. K. Ross, B. A. Larder, D. I. Stuart, and D. K. Stammers. 1998. 3′-Azido-3′-deoxythymidine drug resistance mutations in HIV-1 reverse transcriptase can induce-long range conformational changes. Proc. Natl. Acad. Sci. USA 95:9518-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren, J., R. M. Esnouf, A. L. Hopkins, J. Warren, J. Balzarini, D. I. Stuart, and D. K. Stammers. 1998. Crystal structures of HIV-1 reverse transcriptase in complex with carboxanilide derivatives. Biochemistry 37:14394-14403. [DOI] [PubMed] [Google Scholar]
- 19.Sarafianos, S. G., K. Das, A. D. Clark, Jr., J. Ding, P. L. Boyer, S. H. Hughes, and E. Arnold. 1999. Lamivudine (3TC) resistance in HIV-1 reverse transcriptase involves steric hindrance with beta-branched amino acids. Proc. Natl. Acad. Sci. USA 96:10027-10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schinazi, R. F., R. M. Lloyd, Jr., M. H. Nguyen, D. L. Cannon, A. McMillan, N. Ilksoy, C. K. Chu, D. C. Liotta, H. Z. Bazmi, and J. W. Mellors. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob. Agents Chemother. 37:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sluis-Cremer, N., D. Arion, and M. A. Parniak. 2000. Molecular mechanisms of HIV-1 resistance to nucleoside reverse transcriptase inhibitors (NRTIs). Cell Mol. Life Sci. 57:1408-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stammers, D. K., D. O. N. Somers, C. K. Ross, I. Kirby, P. H. Ray, J. E. Wilson, M. Norman, J. S. Ren, R. M. Esnouf, E. F. Garman, E. Y. Jones, and D. I. Stuart. 1994. Crystals of HIV-1 reverse transcriptase diffracting to 2.2 Å resolution. J. Mol. Biol. 242:586-588. [DOI] [PubMed] [Google Scholar]
- 23.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson, J. E., L. L. Wright, J. L. Martin, S. E. Haire, P. H. Ray, G. R. Painter, and P. A. Furman. 1996. Recombinant human immunodeficiency virus type 1 reverse transcriptase is heterogeneous. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:20-30. [DOI] [PubMed] [Google Scholar]