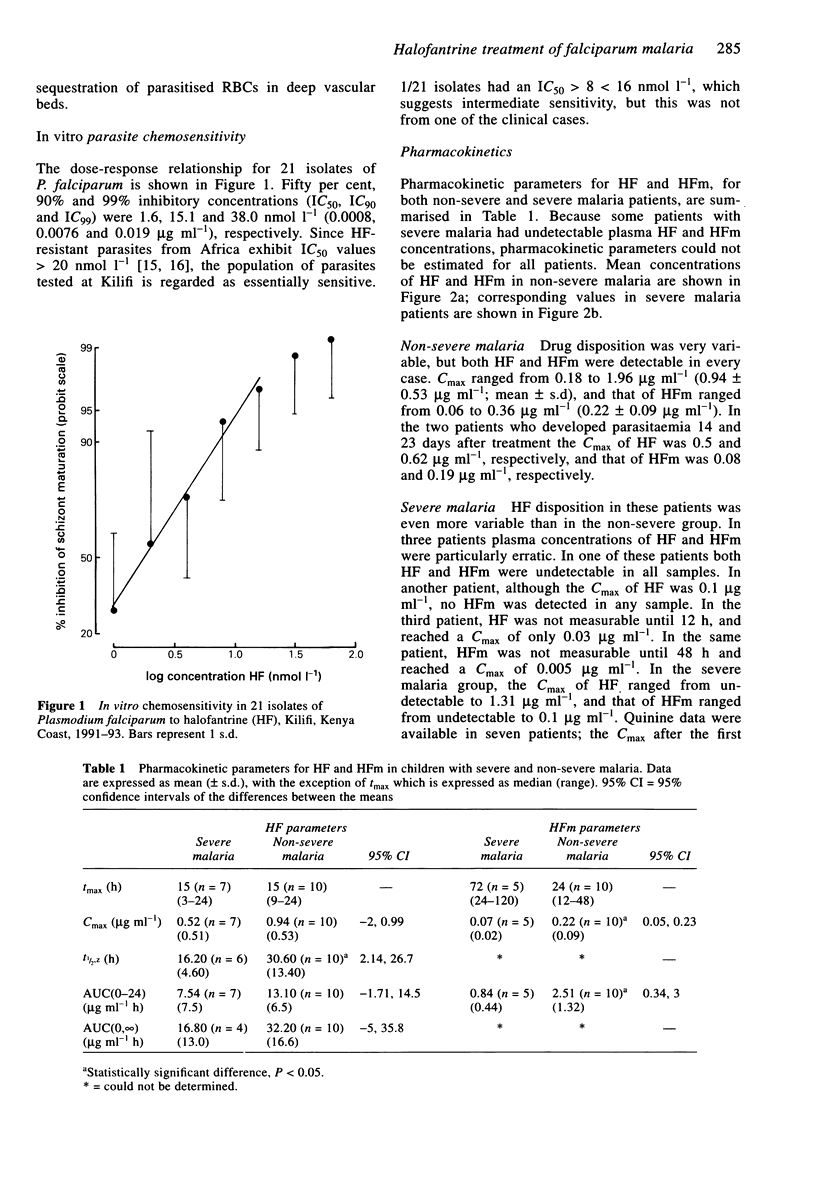
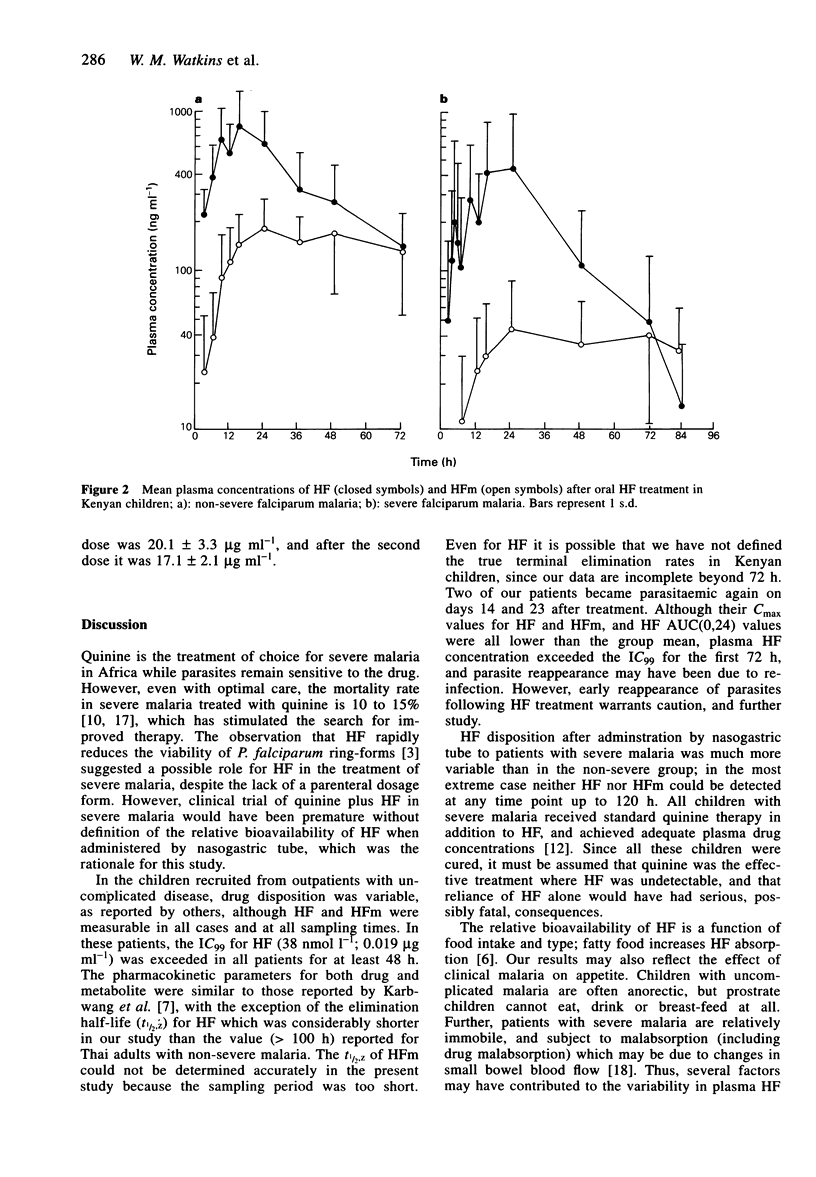
Abstract
1. Kenyan children with uncomplicated malaria given oral halofantrine (HF; non-micronised suspension; 8 mg base kg-1 body weight 6 hourly for three doses) showed wide variation in the disposition of HF and desbutylhalofantrine (HFm). 2. Eight Kenyan children with severe (prostrate) falciparum malaria who were receiving intravenous quinine, were given the same HF regimen by nasogastric tube. One patient had undetectable HF and two had undetectable HFm at all times after drug administration. 3. The mean AUC(0,24 h) of HF in prostrate children was half (7.54 compared with 13.10 micrograms ml-1 h) (P = 0.06), and that for HFm one-third (0.84 compared with 2.51 micrograms ml-1 h) (P < 0.05) of the value in children with uncomplicated malaria. 4. Oral HF may be appropriate for some cases of uncomplicated falciparum malaria in Africa, but in patients with severe malaria, the bioavailability of HF and HFm may be inadequate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brasseur P., Bitsindou P., Moyou R. S., Eggelte T. A., Samba G., Penchenier L., Druilhe P. Fast emergence of Plasmodium falciparum resistance to halofantrine. Lancet. 1993 Apr 3;341(8849):901–902. doi: 10.1016/0140-6736(93)93115-h. [DOI] [PubMed] [Google Scholar]
- Chanthavanich P., Looareesuwan S., White N. J., Warrell D. A., Warrell M. J., DiGiovanni J. H., von Bredow J. Intragastric mefloquine is absorbed rapidly in patients with cerebral malaria. Am J Trop Med Hyg. 1985 Nov;34(6):1028–1036. doi: 10.4269/ajtmh.1985.34.1028. [DOI] [PubMed] [Google Scholar]
- Karbwang J., Milton K. A., Na Bangchang K., Ward S. A., Edwards G., Bunnag D. Pharmacokinetics of halofantrine in Thai patients with acute uncomplicated falciparum malaria. Br J Clin Pharmacol. 1991 Apr;31(4):484–487. doi: 10.1111/j.1365-2125.1991.tb05567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbwang J., Na Bangchang K., Bunnag D., Harinasuta T., Laothavorn P. Cardiac effect of halofantrine. Lancet. 1993 Aug 21;342(8869):501–501. doi: 10.1016/0140-6736(93)91631-u. [DOI] [PubMed] [Google Scholar]
- Mberu E. K., Muhia D. K., Watkins W. M. Measurement of halofantrine and its major metabolite desbutylhalofantrine in plasma and blood by high-performance liquid chromatography: a new methodology. J Chromatogr. 1992 Oct 2;581(1):156–160. doi: 10.1016/0378-4347(92)80461-x. [DOI] [PubMed] [Google Scholar]
- Mberu E. K., Ward S. A., Winstanley P. A., Watkins W. M. Measurement of quinine in filter paper-absorbed blood by high-performance liquid chromatography. J Chromatogr. 1991 Sep 18;570(1):180–184. doi: 10.1016/0378-4347(91)80213-v. [DOI] [PubMed] [Google Scholar]
- Milton K. A., Edwards G., Ward S. A., Orme M. L., Breckenridge A. M. Pharmacokinetics of halofantrine in man: effects of food and dose size. Br J Clin Pharmacol. 1989 Jul;28(1):71–77. doi: 10.1111/j.1365-2125.1989.tb03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F., ter Kuile F. O., Luxemburger C., Woodrow C., Kyle D. E., Chongsuphajaisiddhi T., White N. J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993 Apr 24;341(8852):1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Newton C. R., Winstanley P. A., Watkins W. M., Peshu N. M., Were J. B., Marsh K., Warrell D. A. Quinine treatment of severe falciparum malaria in African children: a randomized comparison of three regimens. Am J Trop Med Hyg. 1991 Dec;45(6):702–713. doi: 10.4269/ajtmh.1991.45.702. [DOI] [PubMed] [Google Scholar]
- Phillips R. E., Warrell D. A. The pathophysiology of severe falciparum malaria. Parasitol Today. 1986 Oct;2(10):271–282. doi: 10.1016/0169-4758(86)90136-5. [DOI] [PubMed] [Google Scholar]
- Ringwald P., LeBras J., Voyer C., Coulaud J. P. Reduced in vitro susceptibility to halofantrine of Plasmodium falciparum in West Africa. Lancet. 1990 Feb 17;335(8686):421–422. doi: 10.1016/0140-6736(90)90264-6. [DOI] [PubMed] [Google Scholar]
- Watkins W. M., Oloo J. A., Lury J. D., Mosoba M., Kariuki D., Mjomba M., Koech D. K., Gilles H. M. Efficacy of multiple-dose halofantrine in treatment of chloroquine-resistant falciparum malaria in children in Kenya. Lancet. 1988 Jul 30;2(8605):247–250. doi: 10.1016/s0140-6736(88)92538-x. [DOI] [PubMed] [Google Scholar]
- Watkins W. M., Woodrow C., Marsh K. Falciparum malaria: differential effects of antimalarial drugs on ex vivo parasite viability during the critical early phase of therapy. Am J Trop Med Hyg. 1993 Jul;49(1):106–112. doi: 10.4269/ajtmh.1993.49.106. [DOI] [PubMed] [Google Scholar]
- White N. J., Miller K. D., Churchill F. C., Berry C., Brown J., Williams S. B., Greenwood B. M. Chloroquine treatment of severe malaria in children. Pharmacokinetics, toxicity, and new dosage recommendations. N Engl J Med. 1988 Dec 8;319(23):1493–1500. doi: 10.1056/NEJM198812083192301. [DOI] [PubMed] [Google Scholar]
- ter Kuile F. O., Dolan G., Nosten F., Edstein M. D., Luxemburger C., Phaipun L., Chongsuphajaisiddhi T., Webster H. K., White N. J. Halofantrine versus mefloquine in treatment of multidrug-resistant falciparum malaria. Lancet. 1993 Apr 24;341(8852):1044–1049. doi: 10.1016/0140-6736(93)92409-m. [DOI] [PubMed] [Google Scholar]


