Abstract
The papillomavirus minor capsid protein, L2, has been shown to exhibit immunogenicity, whereby a variety of B-cell epitopes, predominantly in the amino terminus of L2, have been deduced. However, immunity to L2 in vivo has not been examined extensively. Notably, a common neutralization epitope for human papillomavirus (HPV) types 6 and 16 was mapped to amino acids (aa) 108 to 120. The objectives of this study were to derive antisera from rabbits using the corresponding sequences from rabbit viruses and to assess the ability of these peptides to protect against infection. Synthetic peptides consisting of two overlapping sequences each in the region of aa 94 to 122 of the rabbit oral (ROPV) and cottontail rabbit (CRPV) papillomaviruses were used to immunize rabbits. Rabbits were then infected with both ROPV and CRPV and monitored for the development of oral and cutaneous papillomas, respectively. Serum derived from rabbits immunized with either of the two peptides was shown to (i) react to purified L2 from the cognate virus, (ii) specifically recognize L2 within virus-infected cells, and (iii) neutralize virus in vitro. Following viral challenge, cutaneous papilloma growth was completely absent in rabbits immunized with either CRPV peptide. Likewise, ROPV peptide-immunized rabbits were protected from oral papillomatosis. Challenge of CRPV peptide-immune rabbits with the viral genome resulted in efficient papilloma growth, suggesting a neutralizing antibody-mediated mechanism of protection. These results afford in vivo evidence for the immunogenicity provided by a distinct region of L2 and further support previous evidence for the ability of this region to elicit antiviral immunity.
Papillomaviruses are the etiologic agents of a variety of diseases involving hyperproliferative lesions of cutaneous or mucosal epithelium. Many different virus types exist in nature, spanning the animal kingdom and including the over 100 types found to infect humans (http://hpv-web.lanl.gov/). A subset of human papillomaviruses that infect the genital tract can produce invasive carcinoma and are associated with >90% of cervical cancers (37).
Papillomavirus genomes encode structural proteins L1 and L2, which comprise the viral capsid. L1 is the more abundant protein within the viral capsid. When expressed in vitro, L1 molecules self-assemble into virus-like particles (VLPs), which structurally resemble native virions (7, 18, 20). As L1 is highly immunogenic, a variety of antigenic determinants for this protein have been characterized. Regions of L1 that elicit antibodies capable of neutralization have been localized to hypervariable loops on the capsid surface (4, 26-29, 38). The degree to which these epitopes vary among viral types is significant enough that immunologic cross-reactivity is limited to only the most closely related types (8, 12, 32, 33). L2 proteins are a minor structural element within the viral capsid but appear to have a role in viral genome encapsidation (40-42) and recruitment of L1 and early transcription/replication regulatory protein E2 to promonocytic leukemia protein oncogenic domains (11). The physical orientation of L2 molecules within the viral capsid and their explicit surface determinants remain elusive; however, several B-cell epitopes of L2 have been mapped by the use of monoclonal antibodies. These studies have shown a propensity for the amino terminal 170 amino acids (aa) to elicit neutralizing antibodies (2, 15, 23, 36).
A number of studies with animals have shown that both the L1 and L2 proteins are capable of inducing humoral responses sufficient for virus neutralization and subsequent protection from viral challenge. Vaccination with L1 VLPs induces high-titer neutralizing antibodies (24, 25, 30); the nondenatured (21) product administered systemically can provide humorally mediated virus neutralization at both cutaneous and mucosal infection sites (1, 5, 19, 35). However, the extent to which this protection may apply to natural human infection is complicated by the presence of a wide array of viral types containing distinctly different antigenic determinants. As determined efficacious, several VLP-based vaccines are currently being tested (13, 34) with notable success; yet, the challenge of producing broad-based protection to human papillomavirus (HPV) infection remains.
L2 has also been shown to evoke protective immunity. Immunization of rabbits with whole L2 (22) or the C-terminal half (9) of cottontail rabbit papillomavirus (CRPV) L2 produces neutralizing antibodies and protection, albeit considerably less than immunization with L1. In the bovine system, both the N-terminal and C-terminal thirds of the bovine papillomavirus type 4 L2 protein were shown to elicit strong antibody responses; however, complete protection was achieved only by vaccination with the N-terminal portion (3). This neutralization epitope was later mapped to aa 131 to 151 (2). Furthermore, although L2 epitopes appear to be subdominant in comparison to those of L1, L2 holds the potential for cross-neutralization (16, 31). A monoclonal antibody capable of neutralizing both HPV 6 and HPV 16 pseudovirions has been derived from immunization of mice with a specific peptide derived from a region spanning aa 108 to 120 of HPV 16 L2 (16). This peptide has been used as an immunogen to produce systemic and mucosal antibodies capable of neutralizing HPV 16 pseudovirions (14).
The rabbit model system provides a medium to study protective immunity to both cutaneous and mucosotropic papillomaviruses. To examine the in vivo protective potential of this peptide as an antigen, we constructed peptides from the aforementioned L2 region of the rabbit cutaneous (CRPV) and oral (10) viruses. Serum derived from immunized rabbits was highly reactive to L2 in immunoblots and within infected tissue. CRPV peptide-immunized rabbit serum was also capable of virus neutralization in vitro and completely protected rabbits from infection with the cognate virus. Rabbits were not protected from CRPV DNA challenge, implying that prophylaxis is mediated predominantly by neutralizing antibodies. Oral papillomas were absent or substantially diminished in rabbit oral papillomavirus (ROPV) peptide-immunized rabbits compared to controls. Despite serum reactivity to the heterologous peptides, little cross-protection was observed.
MATERIALS AND METHODS
Peptides and rabbit immunizations.
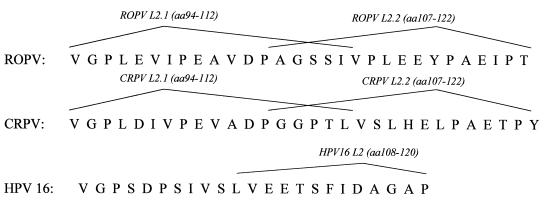
Synthetic peptides (Genemed Synthesis Inc., San Francisco, Calif.) were constructed as shown in Fig. 1. Each peptide was conjugated to keyhole limpet hemocyanin (KLH) for immunization or to bovine serum albumin for enzyme-linked immunosorbent assays (ELISA). Peptides were conjugated to proteins via the sulfosuccinimidyl 4-[N-maleimidomethyl]-cyclohexane-1-carboxylate linker (Pierce, Rockford, Ill.).
FIG. 1.
Sequences of L2-derived peptides used for vaccination
Twenty-three New Zealand White rabbits were divided into five groups; four rabbits per group received one of the following peptides: CRPV L2.1, CRPV L2.2, ROPV L2.1, and ROPV L2.2. Three control rabbits received the HPV 16 L2 peptide. Primary immunizations consisted of 500 μg of peptide-KLH emulsified in Freund's complete adjuvant and delivered intradermally. Rabbits were boosted twice, at 3- to 4-week intervals, with 500 μg of peptide in Freund's incomplete adjuvant delivered subcutaneously. Blood was collected through the ear vein after the second boost, allowed to clot, and centrifuged (10 min, 3,000 × g) to obtain serum.
Viral and viral DNA challenge.
All procedures were approved by the institutional review board at The Pennsylvania State University College of Medicine. Each experimental animal was challenged with both ROPV and CRPV 4 to 12 weeks after the last immunization. For CRPV infection, rabbits received viral doses at 10−2 and 10−3 dilution of crude viral stock (CRPVHershey strain). Virus was applied, under anesthesia, to scarified skin at two sites per dilution. For ROPV infection, 5 μl of crude stock (local isolate [10]) was applied to 15 needle puncture sites on the right underside of tongues. Cutaneous papilloma growth was monitored with weekly measurements for 10 weeks. Oral papillomas were counted and photographed at weeks 3 and 4 postinfection. ROPV-induced papillomas are at maximum growth at this time and naturally regress by 6 to 8 weeks (6).
After >12 weeks of observation, cutaneous papillomas did not appear on any rabbits immunized with either CRPV peptide. To determine if the protection was mediated by the antibody response to the L2 protein, four experimental rabbits (two immunized with CRPV L2.1 and two immunized with CRPV L2.2) and two nonimmune inbred controls were challenged with infectious CRPV DNA. Eight micrograms of the pUC19 vector containing the CRPV genome was applied to scarified skin at three sites each. One rabbit from each experimental group and both controls also received a genome deficient for the expression of L2 (ATG mutant) at three sites each. Papilloma growth was monitored for 9 weeks.
Serological analyses. (i) Immunoblots.
Histidine-tagged ROPV and CRPV L2s were produced by the recombinant baculovirus expression system and HPV 16 L2-His6 was produced in bacteria. Briefly, the L2 genes were amplified from genomic DNA and cloned into pBlueBacHis (CPRV and ROPV) and pTrcHis (HPV 16). The production of recombinant baculoviruses and expression of proteins were carried out according to the manufacturer's protocols (InVitrogen Corp., Carpinteria, Calif.). Nickel column-purified His6-tagged L2 proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% polyacrylamide) and transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat milk and incubated overnight with a 1:250 dilution of immune rabbit serum. Blots were probed with an alkaline phosphatase-conjugated swine anti-rabbit secondary antibody (DAKO Corp., Carpinteria, Calif.) at a 1:2,000 dilution and were developed with BCIP (5-bromo-4-chloro-3-indolylphosphate)-nitroblue tetrazolium substrate. Positive controls for purified L2 proteins were probed with an anti-His6G mouse monoclonal antibody (InVitrogen Corp.), followed by rabbit anti-mouse immunoglobulin G (IgG)/IgM (Pierce) at 1:2,000.
(ii) ELISA.
Synthetic L2 peptides conjugated to bovine serum albumin were used as the antigen to determine the peptide specificity of rabbit polyclonal sera. Peptides (0.5 μg/well) were bound to 96-well plates overnight in 0.1 M NaHCO3 buffer, pH 9.0, washed, and blocked for 1 h with 5% nonfat milk. Rabbit serum was diluted 1:100 in blocking buffer and incubated for 1 h, followed by incubation with swine anti-rabbit alkaline phosphatase-conjugated antibody (DAKO; 1:1,000) and detection with 1 mg/ml of p-nitrophenyl phosphate substrate. Wells probed with preimmune sera were used as blanks. Absorbance at 405 nm (A405) was measured with a microplate reader 25 min after the addition of substrate.
(iii) Immunohistochemistry.
CRPV- and ROPV-infected rabbit tissues grown in the mouse xenograft system (10) (CRPV, subcutaneous; ROPV, subrenal) were fixed in formalin, embedded in paraffin, and sectioned for histology. Rabbit polyclonal serum was diluted 1:200 and used to probe tissue sections containing virus-infected cells. Serum reactivity was resolved by using Histostain (Zymed Laboratories, San Francisco, Calif.) with goat anti-rabbit streptavidin peroxidase, followed by aminoethylcarbazol. Positive staining appears dark red. As a control for capsid protein, tissues were probed with either rabbit polyclonal HPV (Signet Pathology Systems, Dedham, Mass.) at 1:50 dilution for CRPV or mouse anti-L2 monoclonal antibody RL2.5D11 (M. Embers, unpublished data) at 1:100 dilution for ROPV.
(iv) Virus neutralization assays.
In vitro virus neutralization by immune rabbit serum was assayed by reverse transcriptase-PCR (RT-PCR) designed to amplify CRPV early spliced transcript E1^E4. RK13 (American Type Culture Collection, Manassas, Va.) rabbit kidney cells were seeded at 4 × 105 cells/well onto six-well culture plates. A 1:100 dilution of viral stock was incubated with or without serum in a 20-μl total volume for 1 h at 37°C. The mixture was added to cells in 1 ml of Eagle's medium, and incubation for 24 h was performed. On day 2, 2 ml of medium was added to cells. At 3 days postinfection, cells were lysed and RNA was extracted with Trizol (InVitrogen) reagent. cDNA was obtained by using outside downstream primer 5′-GTGCCCCCCTTTCAAGCAAAT-3′. Two rounds of PCR using nested primers (outside upstream, 5′-CCAGAAGCCATAAGAACCTTGAAT-3′; inside upstream, 5′-CCCGGAGTGTTGTAACTGAAAA-3′; inside downstream, 5′-AAGCTCGCGAAGCCGTCTATT-3′) were performed to amplify the specific transcript of 329 bp. Sequencing the 329-bp fragment confirmed CRPV origin and the spliced viral product. A 717-bp β actin transcript was amplified as a control.
RESULTS
Serum reactivity to peptide immunogens.
Immune and preimmune sera from each individual rabbit were tested against all peptide immunogens by ELISA. Preimmune serum was nonreactive in all cases (data not shown). The results are shown in Table 1. As expected, the serum antibody was strongly responsive to the peptide used for immunization. Cross-reactivity to the heterologous peptide was present and slightly weaker for both CRPV L2- and ROPV L2-immunized rabbits. Comparison of the relative intensities of cross-reactivity showed some unidirectional response. For example, ROPV L2.2 immune sera were highly cross-reactive to the CPRV L2.2 peptide, yet the converse (CRPV L2.2 immune serum reactivity to the ROPV L2.2 peptide) response was significantly weaker. Very little cross-reactivity between adjacent peptides was evident, implying that the overlapping region does not contribute significantly to this antibody response. Serum dilutions of 10−3 and lower showed positive reactivity (optical density > 0.100) under conditions identical to those presented here (data not shown).
TABLE 1.
ELISA reactivity of immune serum against L2-derived peptides
| Rabbit group | Reactivitya (OD405)b against:
|
||||
|---|---|---|---|---|---|
| ROPV L2.1 | ROPV L2.2 | CRPV L2.1 | CRPV L2.2 | HPV 16 L2 | |
| R1 | 0.778, 0.802, 0.703, 0.691 | 0.040, 0, 0.023, 0.046 | 0.341, 0.219, 0.167, 0.353 | 0.034, 0.004, 0.003, 0.013 | 0.075, 0.017, 0.015, 0.004 |
| R2 | 0.047, 0.075, 0.101, 0.114 | 0.846, 0.631, 0.539, 0.660 | 0.014, 0.013, 0.074, 0.008 | 0.746, 0.493, 0.492, 0.481 | 0.236, 0.083, 0.087, 0.029 |
| C1 | 0.430, 0.457, 0.257, 0.574 | 0.122, 0.104, 0.150, 0.178 | 0.848, 0.875, 0.764, 0.960 | 0.023, 0.039, 0.015, 0.071 | 0.021, 0.028, 0.042, 0.115 |
| C2 | 0.086, 0.089, 0.040, 0.055 | 0.143, 0.398, 0.026, 0.268 | 0.154, 0.299, 0.201, 0.401 | 0.579, 0.580, 0.409, 0.793 | 0.014, 0.032, 0.021, 0.016 |
| 16 | 0.055, 0.379, 0 | 0.072, 0.198, 0 | 0, 0.184, 0 | 0, 0.058, 0 | 0.995, 0.990, 0.710 |
Values for three or four rabbits are shown.
OD405, optical density at 405 nm.
With regard to whole L2 proteins, assays testing immune serum were indicative of robust reactivity to the cognate L2 proteins, but cross-reactivity was largely absent. Immunoblots using rabbit sera to probe for recognition of L2 antigens are shown in Fig. 2. These assays indicated that, in some rabbits, a low level of cross-reactivity to the heterologous protein appeared but that the antibody response is predominantly specific. These findings are further supported by the analysis of serum responses to virally infected tissues using immunohistochemistry. Figure 3 shows detection of L2 in the nuclei of rabbit tissue epithelial cells infected with each rabbit papillomavirus. Positive immunoperoxidase staining appears dark red within nuclei of cells found in the intermediate layer of the stratified squamous epithelium. Keratinized cells contributed some staining in the highly differentiated, flattened surface layer. Serum derived from rabbits immunized with both L2.1 and L2.2 peptides contained significant antibody responsiveness to viral protein within tissue, as a 1:200 dilution gave results comparable to those for the anti-L1 polyclonal antibody and ROPV L2-specific monoclonal antibody used as positive controls. Positive staining was undetectable when serum from HPV 16 L2 peptide-immunized rabbits was used. Furthermore, no cross-reactivity was apparent when tissues were probed with serum derived from immunization with peptides from the alternative rabbit virus.
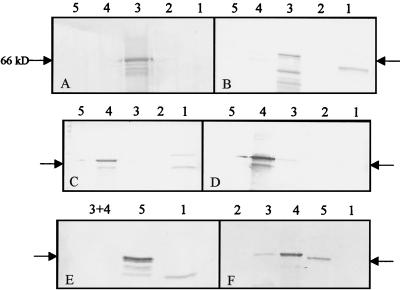
FIG. 2.
Immunoblots of serum reactivity for purified L2 proteins. Immune rabbit serum at a 1:250 dilution was used to probe blots of His6-tagged, nickel-purified fusion proteins of insect cell-derived ROPV L2 (lanes 3), bacterially derived CRPV L2 (lanes 4), and HPV 16 L2 (lanes 5); included are bacterial cell lysates (lanes 1) and insect cell lysates (lanes 2). Sera from rabbits immunized with ROPV L2.1 (A), ROPV L2.2 (B), CRPV L2.1 (C), CRPV L2.2 (D), and HPV 16 L2 (E) are shown. An anti-His6 antibody was used as a control (F). Arrows, 66-kDa marker. L2 is ∼70 to 75 kDa.
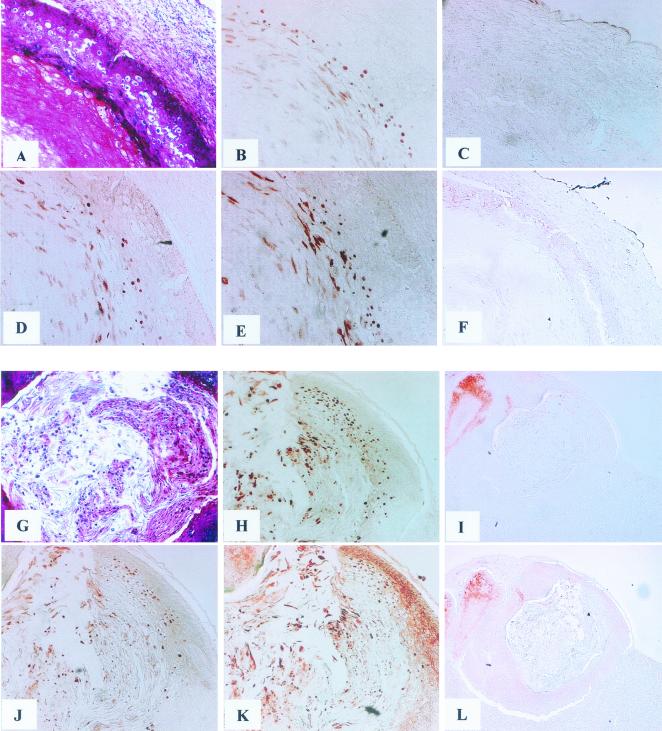
FIG. 3.
Immunohistochemical staining of rabbit tissue infected with CRPV (A to F) and ROPV (G to L). A positive antibody response is indicated by dark red nuclear staining in virus-infected cells. Hematoxylin and eosin staining reveals tissue morphology (A and G). Sera from immunization with HPV 16 (C and I), CRPV L2.1 (D), CRPV L2.2 (E and L), ROPV L2.1 (F and J), and ROPV L2.2 (K) are shown. The rabbit polyclonal HPV L1 control antibody recognizes CRPV (B). ROPV L2-specific control monoclonal antibody RL2.5D11 is also shown (H). Magnifications: ×40 (C, F, I, and L) and ×100 (A, B, D, E, G, H, J, and K).
Virus neutralization and the protective response.
The aforementioned assays to evaluate the antibody responses of peptide-immunized rabbits to L2 protein utilized denatured antigens. Rabbit serum imparted significant nonspecific reactivity to VLP antigen and virus extract contaminants; thus, we were unable to test antibody responses to intact antigen in ELISA. Rather, the ability of peptide-immune rabbit serum to neutralize virus directly was evaluated by RT-PCR for the detection of CRPV E1^E4 spliced transcripts. Virus was incubated with various dilutions of serum and then used to infect RK13 cells. Although in vitro infection of monolayer cells was abortive, this early transcript was produced. Reverse transcription followed by two rounds of PCR using nested primers yielded a 329-bp product. Both CRPV L2.1 and CRPV L2.2 serum was found to neutralize the infection at serum dilutions between 1:5 and 1:10 (Fig. 4), whereas ROPV L2-immune, HPV 16 L2-immune, and preimmune sera did not. The results from two separate rabbits immunized with each CRPV L2 peptide are shown. The virus titer used for infection was a 1,000-fold-lower dilution of stock than that which yields detectable transcripts (data not shown) and thus corresponds to a significant quantity.
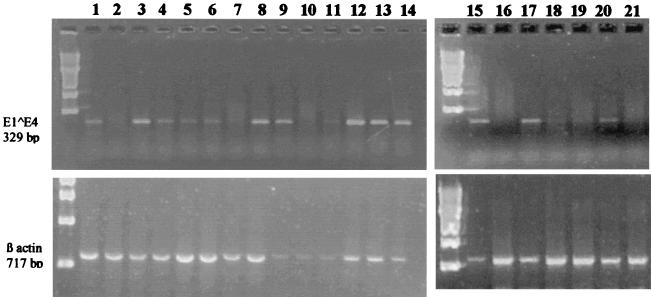
FIG. 4.
RT-PCR analysis of CRPV neutralization by rabbit CRPV L2 peptide immune serum. (Top) Amplimers of the E1^E4 transcript. Lanes 1 and 15, virus only; lanes 2 and 16, uninfected cells; lanes 3 to 8 and 17 to 19, CRPV L2.2 serum diluted 1:80 (lane 3), 1:40 (lane 4), 1:20 (lanes 5 and 18), 1:10 (lanes 6 and 19), and 1:5 (lane 7) and undiluted preimmune serum (lanes 8 and 17); lanes 9 to 11 and 20 and 21, CRPV L2.1 serum diluted 1:20 (lanes 9 and 21) and 1:10 (lane 10) and undiluted preimmune serum (lanes 11 and 20); lane 12, 1:5 dilution of ROPV L2.1 serum; lane 13, ROPV L2.2 serum; lane 14, HPV 16 serum. (Bottom) β actin controls for each lane.
Upon challenge with virus, rabbits immunized with the corresponding peptide were completely protected from CRPV infection and were significantly more resistant to ROPV-induced oral papillomatosis. As Fig. 5 indicates, cutaneous papillomas were completely absent from rabbits immunized with either CRPV L2 peptide. With the exception of one rabbit, ROPV L2 peptide-immune rabbits were not resistant to CRPV challenge, as papillomas grew progressively. The rabbit that appeared resistant showed no cross-reactivity in serum response assays, possibly implying nonproductive infection. Papillomas also grew readily on HPV 16 L2 peptide-immunized controls as expected. Following ROPV infection, papillomas of >1 mm grew at over 75% of challenge sites in HPV 16 and CRPV L2 peptide-immune rabbits. In contrast, both the sizes and numbers of oral papillomas were substantially reduced, or papillomas were completely absent, in ROPV peptide-immune rabbits (Table 2), indicating that these rabbits were less susceptible to ROPV infection. The appearance of cutaneous and oral papillomas is shown in Fig. 6.
FIG. 5.
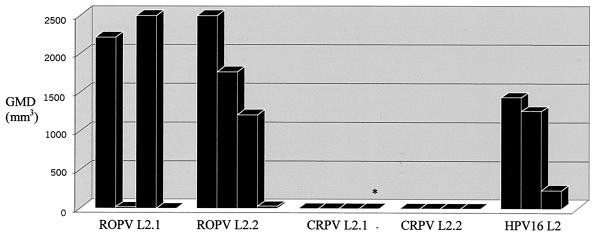
Cutaneous papilloma sizes on peptide-immunized rabbits following infection with CRPV. Sizes are the average geometric mean diameters (GMD) of papillomas at sites (two sites per rabbit) infected with a 10−2 dilution of viral stock 11 weeks postinfection. Each bar corresponds to one animal. ∗, rabbit died on day 28.
TABLE 2.
Numbers of oral papillomas (total and sorted by size) in peptide-immunized rabbits following infection with ROPVa
| Peptide | No. of papillomas
|
||
|---|---|---|---|
| Total | >1 mm | <1 mm | |
| ROPV L2.1 | 1 | 0 | 1 |
| 2 | 0 | 2 | |
| 1 | 0 | 1 | |
| 0 | 0 | 0 | |
| ROPV L2.2 | 1 | 0 | 1 |
| 0 | 0 | 0 | |
| 5 | 0 | 5 | |
| 0 | 0 | 0 | |
| CRPV L2.1 | 15 | 13 | 2 |
| 12 | 10 | 2 | |
| 15 | 15 | 0 | |
| 13 | 13 | 0 | |
| CRPV L2.2 | 14 | 12 | 2 |
| 14 | 14 | 0 | |
| 14 | 14 | 0 | |
| 14 | 14 | 0 | |
| HPV 16 L2 | 11 | 9 | 2 |
| 9 | 5 | 4 | |
| 13 | 13 | 0 | |
Values are for individual rabbits from day 28, at the height of papilloma growth.
FIG. 6.
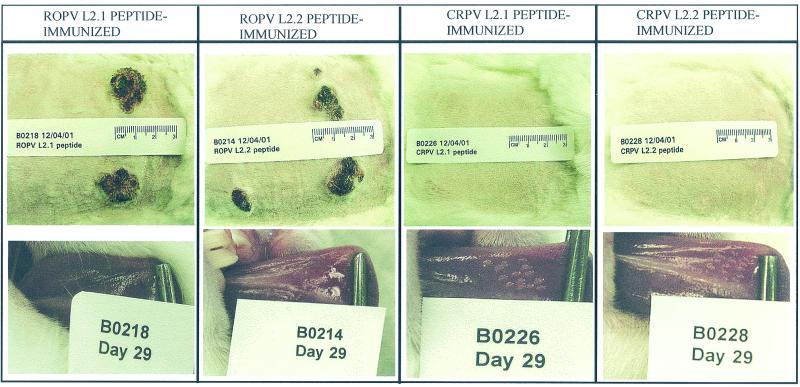
Physical appearance of cutaneous and oral papillomas on vaccinated rabbits. (Top) CRPV infections at week 10; (bottom) ROPV infections on day 28.
To determine whether the immunization peptides may have induced cell-mediated immunity as part of the protective response, rabbits were challenged with infectious CRPV DNA and monitored for papilloma growth. Two of the four rabbits from each CRPV peptide-immunized group received the CPRV genome at three sites on the back behind the original challenge sites. One from each group also received a CRPV L2-deficient genome. Papillomas grew on all rabbits (Table 3), despite the absence of viral protein in the agent used for infection. Control rabbits from an inbred strain grew larger papillomas; this is likely a result of genetic variance rather than comparative immunity in the experimental New Zealand White rabbits. A low level of cell-mediated immunity or bystander effect from adjuvant, KLH, or previous challenge, however, may have contributed to the slower papilloma growth in these animals. The results of this experiment provide strong evidence that protective immunity provided by immunization with the L2 peptides is largely due to antibody-mediated neutralization of virus.
TABLE 3.
Geometric mean diameters of papillomas on rabbits challenged with infectious CRPV DNAa
| Peptide | CRPV genome
|
CRPV L2− genomeb
|
||
|---|---|---|---|---|
| No. of papillomas/no. of challenge sites | GMD (cm3) | No. of papillomas/no. of challenge sites | GMD (cm3) | |
| CRPV L2.1 1 | 3/3 | 10.2 | 3/3 | 8.3 |
| CRPV L2.1 2 | 3/3 | 10.2 | NDc | ND |
| CRPV L2.2 1 | 2/3 | 7.3 | 2/3 | 6.0 |
| CRPV L2.2 2 | 3/3 | 5.1 | ND | ND |
| Control 1 | 3/3 | 18.1 | 3/3 | 17.3 |
| Control 2 | 3/3 | 16.5 | 3/3 | 20.6 |
Geometric mean diameters (GMD) are means for papillomas on three sites per treatment at 9 weeks postinfection.
CRPV L2− genome, genome rendered deficient for the expression of L2 via mutation in the start (ATG) codon.
ND, not determined.
A smaller pilot experiment using one rabbit for immunization with each of the L2 peptides described above yielded similar results, i.e., protection against ROPV and complete protection against CRPV infection (data not shown).
DISCUSSION
In this study, we have shown that a specific B-cell epitope within L2, when administered as a peptide vaccine, provides protection from cutaneous and mucosal papillomavirus infection in rabbits. The level of protection afforded by these peptides is comparable to that seen with L1 VLP vaccination, where cutaneous papillomas were completely absent after challenge with a 10−2 dilution of viral stock (5). Furthermore, rabbits were significantly less susceptible to challenge with high-titer ROPV stock when immunized with the L2 peptides.
In a previous pilot experiment, the postinfection serum antibody did not appear to recognize the highly immunogenic major capsid protein, L1, as evidenced by a lack of reactivity to L1 VLP. This finding, along with the results of the DNA challenge experiment, provides sufficient evidence to indicate that the protective response from immunization with these L2 peptides is due predominantly, if not completely, to the neutralization of viral infection by peptide-specific antibody targeted to the L2 protein.
The ability of short peptides containing specific B-cell epitopes to provide neutralizing antibodies to viruses is rare. Typically, B-cell epitopes are discontinuous, with antibody recognition sites resulting from folding of the target protein into secondary or tertiary structures. Furthermore, effective humoral responses often require the inclusion of T-cell epitopes in a multiple-antigen peptide vaccine (reviewed in reference 39). Due to the ability of linear epitopes to invoke neutralizing antibodies, we speculate that those used in this experiment must not conformationally diverge significantly from the peptide structure as the protein is folded and incorporated into a capsid. The region of papillomavirus L2 used in this study shows hydrophilic-to-neutral polarity in hydrophobicity plots. Protein structure prediction software indicates that this epitope, with >80% probability, would form a strand-coil-strand motif, with the coil lying in the overlapping region of the peptides used for this experiment. Perhaps this portion of L2 loops out at the capsid surface, and its position, along with weak hydrophilic interactions, allows for the epitope structure to remain rigid. This sequence may also be involved in an interaction at the cell surface; the HPV 16 L2 peptide counterpart, when fused with green fluorescent protein, was shown to bind the surfaces of a variety of cell types (17).
Given the previous findings for mice with the HPV 16 and 6/11 L2 peptide counterparts, we speculate that peptides incorporating a larger segment of this region, or a combination of the L2.1 and L2.2 peptides used here, may be capable of eliciting cross-reactive responses in rabbits. However, this is difficult to predict, as discrepancies may result from differences between rabbits and mice in the mechanisms they utilize to generate the diverse B-cell receptor repertoire. In accordance with our findings on the ability of these B-cell epitope peptides to elicit protective neutralizing antibodies, we conclude that this region of L2 could provide a feasible and effective component of a multiple-antigen peptide vaccine for prevention of papillomavirus infections.
Acknowledgments
This study was supported by Public Health Service grant R01CA47622 from the National Cancer Institute, the National Institutes of Health, and by the Jake Gittlen Memorial Golf Tournament.
We thank Syndi Reed for optimization of the RT-PCR assay and Ricai Han for the CRPV L2-expressing baculovirus.
REFERENCES
- 1.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmarquet, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campo, M. S., B. W. O'Neil, G. J. Grindlay, F. Curtis, G. Knowles, and L. Chandrachud. 1997. A peptide encoding a B-cell epitope from the N-terminus of the capsid protein L2 of bovine papillomavirus-4 prevents disease. Virology 234:261-266. [DOI] [PubMed] [Google Scholar]
- 3.Chandrachud, L. M., G. J. Grindlay, G. M. McGarvie, B. W. O'Neil, E. R. Wagner, W. F. Jarrett, and M. S. Campo. 1995. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology 211:204-208. [DOI] [PubMed] [Google Scholar]
- 4.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, N. D., C. A. Reed, N. M. Cladel, R. Han, and J. W. Kreider. 1996. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J. Virol. 70:960-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen, N. D., N. M. Cladel, C. A. Reed, and R. Han. 2000. Rabbit oral papillomavirus complete genome sequence and immunity following genital infection. Virology 269:451-461. [DOI] [PubMed] [Google Scholar]
- 7.Christensen, N. D., R. Hopfl, S. L. DiAngelo, N. M. Cladel, S. D. Patrick, P. A. Welsh, L. R. Budgeon, C. A. Reed, and J. W. Kreider. 1994. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J. Gen. Virol. 75:2271-2276. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, N. D., R. Kirnbauer, J. T. Schiller, S. J. Ghim, R. Schlegel, A. B. Jenson, and J. W. Kreider. 1994. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology 205:329-335. [DOI] [PubMed] [Google Scholar]
- 9.Christensen, N. D., J. W. Kreider, N. C. Kan, and S. L. DiAngelo. 1991. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology 181:572-579. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, N. D., N. M. Cladel, C. A. Reed, L. R. Budgeon, P. A. Welsh, S. D. Patrick, and J. W. Kreider. 1996. Laboratory production of infectious stocks of rabbit oral papillomavirus. J. Gen. Virol. 77:1793-1798. [DOI] [PubMed] [Google Scholar]
- 11.Day, P. M., R. B. Roden, D. R. Lowy, and J. T. Schiller. 1998. The papillomavirus minor capsid protein, L2, induces localization of the major capsid protein, L1, and the viral transcription/replication protein, E2, to PML oncogenic domains. J. Virol. 72:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 13.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 14.Kawana, K., Y. Kawana, H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 2001. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit (sic) systemic and mucosal antibodies. Vaccine 19:1496-1502. [DOI] [PubMed] [Google Scholar]
- 15.Kawana, K., K. Matsumoto, H. Yoshikawa, Y. Taketani, T. Kawana, K. Yoshiike, and T. Kanda. 1998. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology 245:353-359. [DOI] [PubMed] [Google Scholar]
- 16.Kawana, K., H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 1999. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J. Virol. 73:6188-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawana, Y., K. Kawana, H. Yoshikawa, Y. Taketani, K. Yoshiike, and T. Kanda. 2001. Human papillomavirus type 16 minor capsid protein l2 N-terminal region containing a common neutralization epitope binds to the cell surface and enters the cytoplasm. J. Virol. 75:2331-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirnbauer, R., F. Booy, N. Cheng, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirnbauer, R., L. M. Chandrachud, B. W. O'Neil, E. R. Wagner, G. J. Grindlay, A. Armstrong, G. M. McGarvie, J. T. Schiller, D. R. Lowy, and M. S. Campo. 1996. Virus-like particles of bovine papillomavirus type 4 in prophylactic and therapeutic immunization. Virology 219:37-44. [DOI] [PubMed] [Google Scholar]
- 20.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self-assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, Y. L., L. A. Borenstein, R. Ahmed, and F. O. Wettstein. 1993. Cottontail rabbit papillomavirus L1 protein-based vaccines: protection is achieved only with a full-length, nondenatured product. J. Virol. 67:4154-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, Y. L., L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein. 1992. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology 187:612-619. [DOI] [PubMed] [Google Scholar]
- 23.Liu, W. J., L. Gissmann, X. Y. Sun, A. Kanjanahaluethai, M. Muller, J. Doorbar, and J. Zhou. 1997. Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology 227:474-483. [DOI] [PubMed] [Google Scholar]
- 24.Liu, X. S., I. Abdul-Jabbar, Y. M. Qi, I. H. Frazer, and J. Zhou. 1998. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology 252:39-45. [DOI] [PubMed] [Google Scholar]
- 25.Lowe, R. S., D. R. Brown, J. T. Bryan, J. C. Cook, H. A. George, K. J. Hofmann, W. M. Hurni, J. G. Joyce, E. D. Lehman, H. Z. Markus, M. P. Neeper, L. D. Schultz, A. R. Shaw, and K. U. Jansen. 1997. Human papillomavirus type 11 (HPV-11) neutralizing antibodies in the serum and genital mucosal secretions of African green monkeys immunized with HPV-11 virus-like particles expressed in yeast. J. Infect. Dis. 176:1141-1145. [DOI] [PubMed] [Google Scholar]
- 26.Ludmerer, S. W., D. Benincasa, and G. E. Mark III. 1996. Two amino acid residues confer type specificity to a neutralizing, conformationally dependent epitope on human papillomavirus type 11. J. Virol. 70:4791-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludmerer, S. W., D. Benincasa, G. E. Mark III, and N. D. Christensen. 1997. A neutralizing epitope of human papillomavirus type 11 is principally described by a continuous set of residues which overlap a distinct linear, surface-exposed epitope. J. Virol. 71:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludmerer, S. W., W. L. McClements, X. M. Wang, J. C. Ling, K. U. Jansen, and N. D. Christensen. 2000. HPV11 mutant virus-like particles elicit immune responses that neutralize virus and delineate a novel neutralizing domain. Virology 266:237-245. [DOI] [PubMed] [Google Scholar]
- 29.McClements, W. L., X. M. Wang, J. C. Ling, D. M. Skulsky, N. D. Christensen, K. U. Jansen, and S. W. Ludmerer. 2001. A novel human papillomavirus type 6 neutralizing domain comprising two discrete regions of the major capsid protein L1. Virology 289:262-268. [DOI] [PubMed] [Google Scholar]
- 30.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roden, R. B., W. H. Yutzy IV, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 32.Roden, R. B. S., N. Hubbert, R. Kirnbauer, N. D. Christensen, D. R. Lowy, and J. T. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose, R. C., W. Bonnez, C. Da Rin, D. J. McCance, and R. C. Reichman. 1994. Serological differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J. Gen. Virol. 75:2445-2449. [DOI] [PubMed] [Google Scholar]
- 34.Schiller, J. T., and A. Hidesheim. 2000. Developing HPV virus-like particle vaccines to prevent cervical cancer: a progress report. J. Clin. Virol. 19:67-74. [DOI] [PubMed] [Google Scholar]
- 35.Suzich, J. A., S. J. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. A. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volpers, C., M. Sapp, C. A. Komly, P. Richalet-Secordel, and R. E. Streeck. 1993. Development of type-specific and cross-reactive serological probes for the minor capsid protein of human papillomavirus type 33. J. Virol. 67:1927-1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 189:12-19. [DOI] [PubMed] [Google Scholar]
- 38.White, W. I., S. D. Wilson, F. J. Palmer-Hill, R. M. Woods, S. J. Ghim, L. A. Hewitt, D. M. Goldman, S. J. Burke, A. B. Jenson, S. Koenig, and J. A. Suzich. 1999. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 73:4882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesmuller, K. H., B. Fleckstein, and G. Jung. 2001. Peptide vaccines and peptide libraries. Biol. Chem. 382:571-579. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, K. N., I. H. Frazer, W. J. Liu, M. Williams, and J. Zhou. 1999. Nucleotides 1506-1625 of bovine papillomavirus type 1 genome can enhance DNA packaging by L1/L2 capsids. Virology 259:211-218. [DOI] [PubMed] [Google Scholar]
- 41.Zhao, K. N., X. Y. Sun, I. H. Frazer, and J. Zhou. 1998. DNA packaging by L1 and L2 capsid proteins of bovine papillomavirus type 1. Virology 243:482-491. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, J., X. Y. Sun, K. Louis, and I. H. Frazer. 1994. Interaction of human papillomavirus (HPV) type 16 capsid proteins with HPV DNA requires an intact L2 N-terminal sequence. J. Virol. 68:619-625. [DOI] [PMC free article] [PubMed] [Google Scholar]