Abstract
A major obstacle limiting gene therapy for diseases of the heart and skeletal muscles is an inability to deliver genes systemically to muscles of an adult organism. Systemic gene transfer to striated muscles is hampered by the vascular endothelium, which represents a barrier to distribution of vectors via the circulation. Here we show the first evidence of widespread transduction of both cardiac and skeletal muscles in an adult mammal, after a single intravenous administration of recombinant adeno-associated virus pseudotype 6 vectors. The inclusion of vascular endothelium growth factor/vascular permeability factor, to achieve acute permeabilization of the peripheral microvasculature, enhanced tissue transduction at lower vector doses. This technique enabled widespread muscle-specific expression of a functional micro-dystrophin in the skeletal muscles of dystrophin-deficient mdx mice, which model Duchenne muscular dystrophy. We propose that these methods may be applicable for systemic delivery of a wide variety of genes to the striated muscles of adult mammals.
Human mortality and quality of life are significantly affected by diseases of the striated musculature. Genetic treatments that are being developed for conditions such as heart disease, aging-associated muscle wasting and the muscular dystrophies have been limited by an inability to achieve widespread and efficient gene transfer to the heart and dispersed skeletal muscles of an adult organism1–4. For example, anesthesia, invasive surgery and hazardous cofactors are required to transduce varying fractions of the cardiomyocyte population efficiently1,5. Similarly, the transfer of genes to the muscles of individual limbs using various vectors requires either direct injection of individual muscles, or complex surgical procedures performed under anesthesia to distribute vectors via the circulation2–4,6–10. Here we describe a simple and highly efficient method to transfer genes systemically to the cardiac and skeletal muscles of adult mammals. This approach uses intravenous administration of recombinant adeno-associated virus pseudotype 6 (rAAV6-pseudotyped vectors)11, which are extremely effective at transducing skeletal muscles after intramuscular injection12.
RESULTS
Systemic transduction of skeletal muscles by rAAV6
First, we examined the potential for systemic gene transfer after intravenous administration of rAAV6 vectors at the whole-body level in young adult (6–8 wk) C57Bl/10J mice (Fig. 1). The muscles of mice examined 11 days after administration through the tail vein of ~2 × 1011 vector genomes of rAAV6 vector containing a CMV–lacZ expression cassette, did not show obvious exogenous β-galactosidase (β-gal) activity compared with the striated muscles of untreated mice, demonstrating the difficulties in achieving systemic gene transfer (Fig. 1a). Because vascular permeabilizing agents have been shown to influence extravascular dissemination of various vectors, we surveyed previously characterized1,4,5,8 compounds for enhancement of transduction efficiency. Of the agents examined (including histamine-papaverine combinations and coadministration of adenosine), systemic coadministration of vector and vascular endothelial growth factor (VEGF), a potent effector of microvascular permeability13,14, produced the greatest enhancement of reporter gene expression. Mice administered ~2 × 1011 vector genomes of rAAV6–CMV–lacZ vector combined with 10 μg of VEGF demonstrated extensive β-gal expression simultaneously in numerous axial and appendicular muscles (Fig. 1b). Mice administered a higher vector dose (~1 × 1012 vector genomes) showed even greater transduction of skeletal muscles, achieving extensive expression of β-gal throughout the entire skeletal musculature (Fig. 1c). These data demonstrate for the first time a method for achieving gene transfer throughout the skeletal muscles of an adult animal through a single intravascular administration of a recombinant vector.
Figure 1.
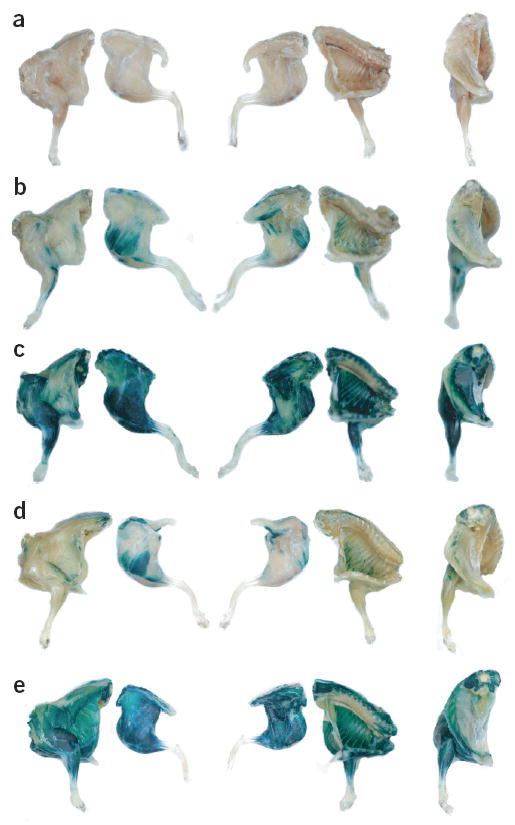
Muscle transduction (blue) following systemic rAAV6 administration. (a–e) Mice were examined at 11 (a–c) or 14 (d,e) days after administration of 2 × 1011 (a,b), 1 × 1012 (c, d) or 1 × 1013 (e) vector genomes of rAAV6–CMV–lacZ (a–c) or rAAV6–CK6–lacZ (d,e) alone (a) or with 10 μg VEGF (b–e).
An immunological reaction can sometimes be observed after foreign gene transfer to skeletal muscles, which is the result of expression of the transgene in non-muscle antigen-presenting cells15,16. The design of expression cassettes incorporating a transcription-regulating element that constrains transgene expression to skeletal muscle cells represents a method of minimizing transgene expression in non-muscle cells. The engineered regulatory element CK6 is one example of a muscle-specific transcription control element that can be incorporated into expression cassettes intended for delivery by rAAV vectors15. However, this promoter is only ~10% as active as the CMV promoter in skeletal muscles in vivo15. To assess the reporter gene activity regulated by the CK6 muscle-specific expression element in the skeletal muscles of adult mice, we administered either 1 × 1012, or 1 × 1013 vector genomes of rAAV6–CK6–lacZ vectors to mice through the tail vein. At 14 days post-treatment, increased β-gal expression was observed in axial and appendicular muscles across the bodies of mice treated with 1 × 1012 vector genomes of rAAV6–CK6–lacZ (Fig. 1d). However, the pattern of expression obtained using rAAV6–CK6–lacZ was reduced compared with mice administered an equivalent dose of rAAV6–CMV–lacZ. In fact, it was necessary to administer ~1 × 1013 vector genomes of rAAV6–CK6–lacZ and VEGF to mice to achieve a body-wide transduction comparable to that observed in mice administered one-tenth the dose of rAAV6–CMV–lacZ and VEGF (Fig. 1e).
To assess quantitatively the transduction of muscles in mice administered rAAV6 vectors through intravenous injection, individual muscles of mice from each cohort were homogenized and assayed for β-gal activity. Consistent with the earlier observations, the β-gal activity of the muscles of mice examined 11 days after administration of ~2 × 1011 vector genomes of the rAAV6–CMV–lacZ vector did not differ from that of the muscles of untreated mice (Fig. 2a). However, mice administered ~2 × 1011 vector genomes of rAAV6–CMV–lacZ vector along with 10 μg VEGF demonstrated a 10–100 fold increase in β-gal activity in cardiac and various skeletal muscles compared with mice receiving only the vector (Fig. 2a). Mice administered ~1 × 1012 vector genomes of rAAV6–CMV–lacZ demonstrated further increases in transduction levels: between 100–8,000 fold greater in cardiac and various skeletal muscles than in untreated mice (Fig. 2b). Similar transduction efficiencies were achieved with vector doses of ~1 × 1012 vector genomes in the presence and absence of VEGF, demonstrating that the mechanism of increased transduction efficiency with VEGF coadministration is less influential over the range of VEGF doses tested, as the vector dose is increased. The β-gal activity measured in the appendicular skeletal muscles of mice administered 1 × 1013 vector genomes of rAAV6–CK6–lacZ and VEGF compared favorably with levels observed in mice administered 1 × 1012 vector genomes of rAAV6–CMV–lacZ with VEGF (Fig. 2c). Notably, animals administered rAAV6–CK6–lacZ vectors showed little β-gal activity in the heart and diaphragm muscles, regardless of vector dose15, although significant transgene expression in these tissues is clearly achievable when vectors with a CMV promoter are used (Figs. 1c and 2b).
Figure 2.
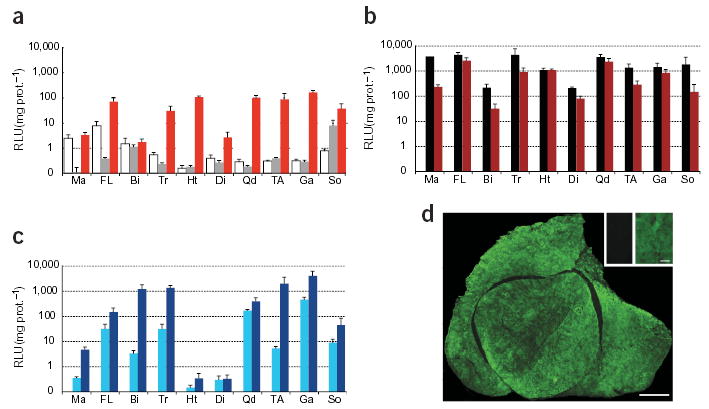
β-Gal activity in muscles after systemic rAAV6 administration. Bars represent activity (RLU: relative light units) from mice administered: (a) 2 × 1011 vector genomes rAAV6–CMV–lacZ (gray, only soleus above background P ≤0.05 with Student’s t-test) or vector with 10 μg of VEGF (red); (b) 1 × 1012 vector genomes of rAAV6–CMV–lacZ (black) or vector and 10 μg VEGF (dark red); (c) 1 × 1012 vector genomes (light blue) or 1 × 1013 vector genomes (dark blue) of rAAV6–CK6–lacZ, both with 10 μg VEGF. Data from uninjected animals (white bars) are shown in a. Data represent means ± s.e. Ma, masseter; FL, flexor digitorum profundus/carpi radialis; Bi, biceps; Tr, triceps; Ht, heart; Di, diaphragm; Qd, quadriceps; TA, tibialis anterior; Ga, gastrocnemius; So, soleus. (d), Anti-β-gal immunofluorescence microscopy of the quadriceps muscles: a mouse administered 1 × 1012 vector genomes of rAAV6–CMV–lacZ by intravenous injection (d and inset right), compared with an uninjected mouse (d, inset left). Animals examined 11 (a,b,d) or 14 (c) days post-treatment. Bars, 1 mm (d) and 100 μm.
As a single intravenous administration of rAAV6 vectors achieved marked transgene expression in skeletal muscles throughout the body of an adult animal (Fig. 2b,c), we chose to histologically examine individual muscles to determine the extent of transduction at the cellular level. Immunofluorescence microscopy highlighted widespread transgene expression throughout the muscle fibers comprising the muscles of mice administered 1 × 1012 vector genomes of rAAV6–CMV–lacZ plus VEGF (Fig. 2d). Intravenous administration of rAAV6 vectors therefore achieves not only body-wide dissemination of vector, but also relatively uniform gene transfer throughout the majority of the skeletal muscle fiber population. This is especially notable when considering that the dissemination of vectors administered by means of direct intramuscular delivery can be hampered by muscle anatomy and extracellular components such as connective tissue.
Correlation between transduction and vector genomes
In order to further investigate the effects of VEGF on the transduction of striated muscles of an adult animal after intravenous administration of rAAV6 vectors, we examined β-gal activity and vector genome copy numbers in cardiac and skeletal muscle samples obtained from mice coadministered different doses of VEGF at the time of vector administration. The β-gal activity of cardiac and skeletal muscles from mice administered ~2 × 1011 vector genomes of rAAV6–CMV–lacZ increased dose-dependently with increasing quantities of coadministered VEGF (Fig. 3a). Quantification of vector genome copy numbers in heart and tibialis anterior muscle samples demonstrated a consistent trend between reporter gene activity and vector genome copies over the range of VEGF doses examined (Fig. 3a,b). These data demonstrate that coadministration of VEGF with rAAV6 vectors, administered by means of a single intravenous injection, increases the accumulation of vector genome copies in individual muscles.
Figure 3.

β-Gal activity and vector genome copy numbers in muscles and organs after systemic administration of rAAV6 vectors with VEGF. (a,b) β-gal activity (a) and rAAV genome copy number (b) in striated muscles after intravenous injection of 2 × 1011 vector genomes of rAAV6–CMV–lacZ coadministered with 1 (yellow; genomes not determined for TA), 5 (orange), or 10 (red) μg of VEGF, compared with no VEGF administration (gray); 1 arbitrary unit = 8 × 104 vector genomes μg/DNA. (c) β-gal activity in a variety of organs after intravenous injection of 1 × 1012 vector genomes of rAAV6–CMV–lacZ alone (black) or with 10 μg of VEGF (dark red) (d) rAAV6 genomes are detectable in organs from c; 1 arbitrary unit = 9 × 106 vector genomes/μg DNA. Data from uninjected animals (white bars) are shown in a and c. Data are mean values ± s.e. TA, tibialis anterior; Br, brain; Lu, lung; Li, liver; Sp, spleen; In, intestine; Ki, kidney; Te, testes. Animals were examined 11 days post-treatment.
In contrast to the heart and skeletal muscles, elevated β-gal activity was not observed in brain, lung, liver, spleen, intestine, kidney or testes samples harvested from treated mice (Fig. 3c). However, rAAV6 vector genomes were detected in homogenates of these tissues after intravenous administration of vector with VEGF (Fig. 3d), which is consistent with previous studies17. Furthermore, hepatocytes can be transduced by intravenously-administered rAAV6 vectors containing liver-specific gene regulatory elements17. Therefore, intravenous administration of rAAV6 vectors may prove useful for transducing numerous tissue types, depending on the design of the expression cassette. Importantly, serum-based indices of organ function were comparable between cohorts receiving vehicle, vehicle with VEGF, empty capsids with VEGF and vector with VEGF, demonstrating that the intervention regimen did not induce acute organ toxicity (Supplementary Fig. 1 online).
rAAV6 vectors potently transduce the myocardium in vivo
In addition to showing uniform lacZ expression in the vast majority of skeletal muscle fibers throughout the body (Fig. 2b), mice receiving rAAV6–CMV–lacZ with VEGF also demonstrated dramatic transduction of the cardiomyocyte population (Fig. 4a). The transduction levels observed in the heart exceeded those reported for the most promising of the existing methods1,4–6, whereas skeletal muscle transduction was equal to, or exceeded, the best results obtained previously using approaches that target individual muscles in isolated limbs2–4,6–10,12. As the heart has been regarded as an extremely difficult organ in which to achieve widespread genetic transfer1,5, the extensive and uniform transduction observed in the myocardium alone is particularly encouraging. Furthermore, alternative methods of gene transfer have not been able to simultaneously target the widely dispersed axial and appendicular striated muscles in an adult mammal.
Figure 4.

Myocardial transduction (blue) in adult mice 11 days after systemic administration of 1 × 1012 vector genomes of rAAV6–CMV–lacZ and 10 μg VEGF. (a) The heart of a treated wild-type mouse examined after reaction with X-gal and counterstaining with hematoxylin and eosin. (b–i) Sections examined following reaction with X-gal and counterstaining with hematoxylin and eosin (b,d,f,h) and serial sections immunofluorescence-labeled for CD4-positive cells (red; c,e,g,i). The hearts of an untreated mouse (b,c) and a mouse administered empty vector capsids with VEGF (d,e) display regular morphology and few CD4-postive cells. (f,g) Regions of myocardium in the heart of a wild-type mouse administered 1 × 1012 vector genomes of rAAV6–CMV–lacZ and VEGF show dramatic mononuclear cell and CD4-postive cell infiltration. (h,i) Myocardium of a treated, β-gal-tolerant transgenic mouse (villin mouse19), demonstrating a lack of cellular infiltrate. Scale bars, 1 mm (a) and 100 μm (b–i).
Although dramatic transduction of the myocardium was achieved in mice administered rAAV6–CMV–lacZ (Fig. 4a), compared with mice administered vehicle (Fig. 4b,c) or an equivalent dose of rAAV6 vector capsid lacking an expression cassette (empty capsids; Fig. 4d,e), histological inspection revealed significant mononuclear cell infiltrates in the myocardium of some wild-type mice approximately 10–14 days after administration of the rAAV6–CMV–lacZ vector (Fig. 4f). Cellular infiltration was associated with the accumulation of CD4-positive cells, which typically include T-helper lymphocytes (Fig. 4g). As administration of an equivalent dose of rAAV6 empty capsids with VEGF did not induce mononuclear cell infiltrates (Fig. 4d, e), we hypothesized that the inflammatory reaction observed with CMV–lacZ in wild-type mice results from the constitutive widespread expression of a bacterial protein (β-gal)18. Indeed, administration of 1 × 1012 vector genomes of rAAV6–CMV–lacZ and VEGF to transgenic mice that express bacterial β-gal within the intestinal villi19, did not cause mononuclear cell accumulation (Fig. 4h, i), and mice treated with the rAAV6–CK6–lacZ vector did not show significant mononuclear cell accumulation or histological abnormalities in striated muscles (data not shown). These data establish that the mononuclear cell infiltrate observed in wild-type mice is not associated with viral components of the rAAV6 vector, and that high doses of rAAV6 vectors expressing β-gal from a muscle-specific promoter are tolerated well by immunocompetent adult mammals.
Rescue of a disease model by systemic rAAV6 delivery
To assess the potential for achieving systemic transduction of the skeletal musculature with a therapeutic transgene, we studied the dystrophin-deficient mdx mouse model of Duchenne muscular dystrophy20. Patients with Duchenne muscular dystrophy die prematurely from profound cardiac and respiratory muscle degeneration, owing to an inability to produce dystrophin, which stabilizes the muscle fiber architecture21. Strategic engineering of the dystrophin coding sequence has created truncated, yet highly functional, ‘microdystrophin’ cDNA cassettes that can be carried by rAAV vectors3,10. To determine whether rAAV6 could deliver these functional microdystrophins in a systemic manner, mdx mice were injected with ~1 × 1012 vector genomes of rAAV6–CK6–microdystrophin (ΔR4–23/ΔCT)3 and VEGF (Fig. 5). Eight weeks after treatment, widespread dystrophin expression was observed throughout the skeletal muscles of mice (Fig. 5a) with the exception of the diaphragm, where the CK6 promoter was not active (data not shown)15. A characteristic feature of muscles of the mdx mouse is an increased susceptibility to contraction-induced injury9,20. We and others have shown that expression of highly functional, microdystrophin proteins in the muscles of mdx mice can halt the progression of the pathology and improve the dystrophic phenotype3,10,22. The tibialis anterior limb muscles of treated mice demonstrated reduced susceptibility to injury by a protocol of eccentric contractions9 compared with untreated mdx mice (Fig. 5b), which compares favorably with data obtained following optimized, direct injection of the tibialis anterior muscle with vectors expressing truncated or full-length dystrophins9,10. Despite the significantly improved functional performance of the tibialis anterior muscles of treated mdx mice, phenotypic correction was incomplete. Some aspects of muscle function such as absolute (Po) and normalized (sPo) force-generating capacity did not differ between the muscles of treated and untreated mdx mice (mean ± standard error (s.e.): wild-type, Po 1210 ± 105 mN, sPo 246 ± 15 kN m−2; mdx, Po 1,234 ± 65 mN, sPo 182 ± 8 kN m−2; treated mdx Po 1,310 ± 151 mN, sPo 187 ± 11 kN m−2). Although near complete phenotypic correction is observed in transgenic mice expressing microdystrophin3, maximal correction of the dystrophic phenotype may only occur after progressive and extended remodeling of the skeletal musculature in the treated animal, and some aspects of the pathological phenotype that develop early in life may not be fully reversible. This hypothesis has been supported in studies demonstrating that transgenic mice expressing full-length dystrophin only after birth halt disease progression but fail to achieve the magnitude of phenotypic correction observed in mice expressing dystrophin during embryogenesis23, a result similar to the present findings. Partial mosaic expression observed in the tibialis anterior muscle at this vector dose (Fig. 5a) may also have contributed to the incomplete phenotypic correction. However, previous studies of mdx mice have demonstrated that dystrophin expression at levels greater than 20% of wild-type expression in a majority of muscle fibers, a level greatly exceeded in these studies, profoundly improves the disease phenotype3,24.
Figure 5.

Systemic delivery of microdystrophin to dystrophic mice. (a) Anti-dystrophin immunofluorescence microscopy of tibialis anterior muscles from treated mdx mice (Tmdx) administered 1 × 1012 vector genomes of rAAV6–CK6–microdystrophin and 10 μg VEGF, compared with wild-type and untreated controls. Dystrophin expression is increased in the muscles of treated compared with untreated mdx mice, but remains mosaic compared with wild-type mice. (b) Force producing capacity (Po, top) and deficit (bottom), of tibialis anterior muscles from Tmdx mice (red) compared with wild-type (black) and untreated mdx (gray) mice after consecutive eccentric contractions (LC1, LC2, respectively)9. P values with Student’s t-test between Tmdx and mdx: i = 0.078, ii ≤0.05, iii = 0.074 and iv ≤0.05. (c) Anti-dystrophin-labeled muscles from mice administered 1 × 1013 vector genomes of rAAV6–CK6–microdystrophin and VEGF. The dystrophin expression pattern observed at this vector dose is no longer mosaic, in contrast to a. Mice were examined at 8 weeks (a,b) and 6 weeks (c) after treatment. Scale bars, 100 μm.
Body-wide expression of rAAV6-delivered dystrophin
To determine whether uniform dystrophin expression could be obtained after intravascular vector delivery, an additional cohort of mdx mice were injected with a tenfold greater vector dose (~1 × 1013 vector genomes) and VEGF. Six weeks post-treatment, uniform expression of the truncated dystrophin protein was observed throughout muscles of the limbs and torso (including the intercostal muscles) of mice, at levels similar to that of the normal protein in wild-type mice (Figs. 5c and 6). This is the first study to demonstrate expression of therapeutic dystrophin-based proteins simultaneously in axial and appendicular skeletal musculature across the body of an adult dystrophic animal after vector administration. Treated dystrophic mice also displayed an ~50% reduction in serum creatine kinase levels (P < 0.05) compared with untreated dystrophic mice, demonstrating a body-wide phenotypic change indicative of reduced muscle degeneration after treatment (Fig. 6a). Despite achieving widespread transfer of an expression cassette incorporating a transgene of human origin, there was no histopathological evidence of mononuclear cell accumulation in these muscles, which express the therapeutic truncated dystrophin protein under the control of a muscle-specific transcriptional regulator (Fig. 6b). These data demonstrate that systemic administration of rAAV6 vectors incorporating a muscle-specific expression cassette can achieve sustained expression of a therapeutic protein throughout the skeletal muscles of an animal model of muscular dystrophy.
Figure 6.

Sustained expression of a therapeutic human microdystrophin protein is achieved after intravenous administration of rAAV6 vectors, without significant cellular infiltration. (a) Anti-dystrophin-labeled tibialis anterior from a treated mdx mouse administered 1 × 1013 vector genomes rAAV6–CK6–microdystrophin and 10 μg VEGF. This treatment significantly (Tmdx compared with mdx, P ≤0.05) reduced serum creatine kinase (CK) levels. Individual data are depicted as dots with group means in red. (b) Anti-dystrophin immunofluorescence microscopy (left) and hematoxylin-stained and eosin-stained sections (right) of tibialis anterior muscles from wild-type, untreated mdx (mdx) and mdx mice treated as in a (Tmdx), respectively. Yellow points function are markers identifying the same muscle fiber in left and right panels. Mice were examined 6 weeks after treatment. Scale bars, 1 mm (a) and 200 μm (b).
DISCUSSION
Achieving efficient dissemination of vectors throughout the muscles of the body has proven a major challenge to attempts to genetically manipulate striated muscle cells. The findings of the present study demonstrate for the first time that extensive transduction of the myocardium and skeletal musculature of an adult mammal is achievable by means of a single intravenous administration of rAAV6 vectors. Consistent with previous reports17, the transduction effect seems to be influenced by vector dose, and to be subject to a threshold event, as some vector doses show significant expression in the heart and skeletal muscle fibers only when coadministered with a vasculature-permeabilizing agent, in this case VEGF (Figs. 1 and 2). Widespread transduction of striated musculature was achieved without acute toxicological response using doses of vector (between 2 × 1011 and 1 × 1012 vector genomes) similar to intravenous and intramuscular doses of rAAV2 tolerated by larger animals25,26. Also, patients have been administered intramuscular injections of greater than 1 × 1014 rAAV2 vector genomes with no adverse effects27. VEGF coadministration was tolerated well by all mice, including at doses up to twofold higher than presented here (Supplementary Fig. 1 online; data not shown). The effective VEGF dose range is about the same as doses administered to larger animals, which are within an order of magnitude of treatments successfully administered to patients28. As systemic clearance of VEGF is rapid and acute, VEGF-induced vascular changes are largely short-lived28; VEGF coadministration is especially encouraging as a means to enhance systemic gene delivery after intravascular administration of vectors to conscious mammals using a reduced vector dose.
Administration of rAAV6 vectors carrying lacZ or microdystrophin regulated by the muscle-specific CK6 promoter/enhancer15 was tolerated readily by mice for the duration of this study (at least 8 weeks), highlighting the importance of maintaining tissue-specific gene expression. We have shown that systemic administration of rAAV6 vectors containing a therapeutic transgene can also achieve widespread and sustained expression of a structural protein in the vast majority of the skeletal musculature in a murine model of muscular dystrophy (Figs. 5 and 6). This level is sufficient to confer improvement of the disease phenotype (Figs. 5 and 6).
Treatment of most muscle diseases will most probably require sustained gene expression. rAAV-mediated gene expression has been demonstrated for the life of treated mice, and at the longest time points studied presently in canine subjects (beyond 4 years)27,29. Although this approach has an obvious potential application to the various forms of muscular dystrophy, there could also be potential applications where genetic transfer to the myocardium and/or skeletal muscle fibers is warranted for the treatment of other human pathologies, or for generating experimental animal models of gene expression. Potential clinical applications include transduction of the musculature to express secretable or trophic factors, such as for the treatment of cardiac pathologies1, hemophilia17,26,27 or muscle wasting associated with aging2.
METHODS
Construct cloning and vector production.
Recombinant AAV genomes containing expression cassettes for β-gal or microdystrophin (ΔR4–23/ΔCT)3 and serotype 2 inverted terminal repeats were generated by standard cloning techniques3. EagI fragments of the coding sequences were cloned into the EagI site of plasmids pDD2 (containing the CMV promoter) for β-gal and pDD344 (containing the CK6 promoter15) for both coding sequences3. The packaging/helper plasmid pDGM6, which includes the serotype 6 capsid reading frame, was generated by amplifying the capsid gene from a clone of AAV serotype 6 (ref. 30) by PCR using the oligonucleotide primers 5′- ATTTAAATCAGGTATGGCTGCCGATGGT-3′ and 5′-ATCGATTGCTATGGTGACCAGATAAGATAA-3′ encoding terminal SwaI and ClaI restriction sites and inserting the resulting DNA fragment into plasmid pDG (ref. 31) cut with the same enzymes. HEK293 cells were plated at a density of 3.2–3.8 × 106 cells on a 10-cm culture dish, 8–16 h prior to transfection with 10 μg of a vector-genome-containing plasmid and 20 μg of the packaging/helper plasmid pDGM6, by means of the calcium phosphate precipitate method to generate pseudotype 6 vectors11,30. Seventy-two hours after transfection, the media and cells were collected and homogenized through a microfluidizer (Microfluidics) prior to 0.22-μm clarification (Millipore). The vector was purified from the clarified lysate by affinity chromatography32 over a HiTrap heparin column (Amersham), and dialyzed to physiological Ringer’s solution. The purified virus was titered with a quantitative chemiluminescent slot-blot against the vector genome, visualized with the CDP-Star kit (Amersham) and a GeneGnome image acquisition/analysis system (Syngene Bio Imaging).
Manipulation of experimental animals.
All experimental manipulation of young adult (6–8 week) male wild-type C57Bl/10J, or dystrophic C57Bl/10ScSn–Dmdmdx/J mice (Jackson Labs) was approved by the Institutional Animal Care and Use Committee of the University of Washington. Individual mice (from cohorts containing 4–6 animals) were administered a 300 μL bolus injection (corresponding to ~15% of the circulating blood volume, a readily tolerable dose) through the tail vein comprising physiological Ringer’s solution containing up to 1 × 1013 vector genomes (~4 × 1014 vector genomes/kg) of rAAV6 vector, 0–20 μg (0–800 μg/kg) recombinant human VEGF-165 (R&D Systems), 0.008% mouse serum albumin (Sigma) and 2 IU (international units) (80 IU/kg) sodium heparin.
Tissue processing and analysis.
For whole-mount reaction of limb and torso segments for β-gal activity, tissues were fixed in 4% formaldehyde-supplemented PBS for 15 min before incubation at 37 °C for 8 h in PBS supplemented with 5-bromo-4-chloro-3-indolyl β-d-galactopyranoside (X-gal; Sigma), magnesium chloride, and Fe(II,III)CN (potassium ferro/ferricyanide). After completion of reaction incubation, tissues were fixed in Karnovsky’s solution at 4 °C. Histological, histochemical and immunochemical processing of muscle cryosections were completed as described previously9,33. Immunofluorescence microscopy used primary antibodies against bacterial β-gal (Chemicon), CD4+cells (Pharmingen) and the N-terminal region of dystrophin33 and the commercial Alexa 488-labeled goat-anti-rabbit and Alexa 594-labeled goat-anti-rat secondary antibodies (Molecular Probes). Protein extracts from the tissues of treated animals were assayed for β-gal activity using a luminometric kit (BD Bioscience) and measured for protein concentration with the Bradford reagent (Pierce). Serum creatine kinase levels were assayed with a kinetic NADH formation kit (Diagnostic Chemicals Limited) and serum-based chemical indices of hepatic and renal function were analyzed commercially (Phoenix Central Laboratory). Relative numbers of vector genomes were determined using real-time PCR on an ABI Prism 7700 (Perkin Elmer) with the following primer-probe combination (Applied Biosystems) targeting the lacZ open reading frame: forward, 5′-GCGTTACCCAACTTAATCGCC-3′; reverse, 5′-GCCTCTTCGCTATTACGCCA-3′; probe, 5′-FAM-CAGCACATCCCCTTTCG CCA-TAMRA-3′. Total DNA was quantified with Hoechst dye fluorescence (BioRad). The rAAV genomes are reported as relative numbers per μg of DNA minus background, with the highest copy number sample in each experiment being arbitrarily assigned a value of 1.
Functional analysis.
Eight weeks post-injection, mdx mice treated with 1 × 1012 vector genomes of rAAV6–CK6–microdystrophin (ΔR4–23/ΔCT; together with untreated mdx and wild-type control mice) were anesthetized with 2,2,2-tri-bromoethanol (Sigma) and assayed in situ for force generation and protection from contraction-induced injury as previously described9. After functional testing, animals were sacrificed and tissues were rapidly excised and processed for histology as above.
Supplementary Material
Acknowledgments
We thank S.D. Hauschka for helpful discussions; D. Duan for the AAV MCS vector plasmids pDD2 and pDD344; J. Kleinschmidt for the packaging plasmid pDG; A.D. Miller for the packaging cell line 293; J. Han for the rAAV6–CK6–lacZ construct; and M. Haraguchi and S. Oakley for technical assistance. This work was supported by grants from the National Institutes of Health and the Muscular Dystrophy Association (USA) to J.S.C.
Footnotes
Note: Supplementary information is available on the Nature Medicine website.
COMPETING INTERESTS STATEMENT The authors declare competing financial interests (see the Nature Medicine website for details).
References
- 1.Hoshijima M, et al. Chronic suppression of heart-failure progression by a pseudophosphorylated mutant of phospholamban via in vivo cardiac rAAV gene delivery. Nat Med. 2002;8:864–871. doi: 10.1038/nm739. [DOI] [PubMed] [Google Scholar]
- 2.Musaro A, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 3.Harper SQ, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 4.Greelish JP, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. doi: 10.1038/7439. [DOI] [PubMed] [Google Scholar]
- 5.Bridges CR, et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002;73:1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 6.Huard J, et al. The route of administration is a major determinant of the transduction efficiency of rat tissues by adenoviral recombinants. Gene Ther. 1995;2:107–115. [PubMed] [Google Scholar]
- 7.Cho WK, et al. Modulation of Starling forces and muscle fiber maturity permits adenovirus-mediated gene transfer to adult dystrophic (mdx) mice by the intravascular route. Hum Gene Ther. 2000;11:701–714. doi: 10.1089/10430340050015608. [DOI] [PubMed] [Google Scholar]
- 8.Zhang G, Budker V, Williams P, Hanson K, Wolff JA. Surgical procedures for intravascular delivery of plasmid DNA to organs. Methods Enzymol. 2002;346:125–133. doi: 10.1016/s0076-6879(02)46052-1. [DOI] [PubMed] [Google Scholar]
- 9.Dellorusso C, et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci USA. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watchko J, et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- 11.Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JM, et al. Viral vectors for gene transfer of micro-, mini-, or full-length dystrophin. Neuromuscul Disord. 2002;12:S23–S29. doi: 10.1016/s0960-8966(02)00078-0. [DOI] [PubMed] [Google Scholar]
- 13.Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- 14.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 15.Hauser MA, et al. Analysis of muscle creatine kinase regulatory elements in recombinant adenoviral vectors. Mol Ther. 2000;2:16–25. doi: 10.1006/mthe.2000.0089. [DOI] [PubMed] [Google Scholar]
- 16.Hartigan-O’Connor D, Kirk CJ, Crawford R, Mule JJ, Chamberlain JS. Immune evasion by muscle-specific gene expression in dystrophic muscle. Mol Ther. 2001;4:525–533. doi: 10.1006/mthe.2001.0496. [DOI] [PubMed] [Google Scholar]
- 17.Grimm D, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- 18.Yuasa K, et al. Adeno-associated virus vector-mediated gene transfer into dystrophin-deficient skeletal muscles evokes enhanced immune response against the transgene product. Gene Ther. 2002;9:1576–1588. doi: 10.1038/sj.gt.3301829. [DOI] [PubMed] [Google Scholar]
- 19.Pinson KI, Dunbar L, Samuelson L, Gumucio DL. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dyn. 1998;211:109–121. doi: 10.1002/(SICI)1097-0177(199801)211:1<109::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorevic P, Chamberlain JS. Gene therapy for muscular dystrophy - a review of promising progress. Expert Opin Biol Ther. 2003;3:803–814. doi: 10.1517/14712598.3.5.803. [DOI] [PubMed] [Google Scholar]
- 22.Sakamoto M, et al. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem Biophys Res Commun. 2002;293:1265–1272. doi: 10.1016/S0006-291X(02)00362-5. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad A, Brinson M, Hodges BL, Chamberlain JS, Amalfitano A. Mdx mice inducibly expressing dystrophin provide insights into the potential of gene therapy for Duchenne muscular dystrophy. Hum Mol Genet. 2000;9:2507–2515. doi: 10.1093/hmg/9.17.2507. [DOI] [PubMed] [Google Scholar]
- 24.Phelps SF, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 25.Arruda VR, et al. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001;4:586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- 26.Herzog RW, et al. Influence of vector dose on factor IX-specific T and B cell responses in muscle-directed gene therapy. Hum Gene Ther. 2002;13:1281–1291. doi: 10.1089/104303402760128513. [DOI] [PubMed] [Google Scholar]
- 27.Manno CS, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 28.Eppler SM, et al. A target-mediated model to describe the pharmacokinetics and hemodynamic effects of recombinant human vascular endothelial growth factor in humans. Clin Pharmacol Ther. 2002;72:20–32. doi: 10.1067/mcp.2002.126179. [DOI] [PubMed] [Google Scholar]
- 29.Herzog RW, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 30.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimm D, Kern A, Rittner K, Kleinschmidt JA. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther. 1998;9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 32.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rafael JA, et al. Forced expression of dystrophin deletion constructs reveals structure-function correlations. J Cell Biol. 1996;134:93–102. doi: 10.1083/jcb.134.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


