Abstract
1. The major serum proteins which bind halofantrine were identified by size exclusion chromatography. In addition, the binding affinity of halofantrine to human erythrocytes and serum proteins was measured by an erythrocyte partitioning technique. The influence of serum-drug binding on the distribution of halofantrine in whole blood was estimated by simulating several disease-related changes in the levels of the most important binding proteins. 2. The chromatographic resolution of serum preincubated with halofantrine allowed a quantitative analysis of binding to low density lipoproteins, high density lipoproteins, alpha 1-acid glycoprotein and albumin using the erythrocyte partitioning technique. Very low density lipoproteins did not bind halofantrine to a significant extent. 3. In whole blood halofantrine is bound to serum proteins (83%) and to erythrocytes (17%). Low density lipoproteins (affinity constant nKP = 44.4 l g-1) and high density lipoproteins (nKP = 14.4 l g-1) were the most important binding proteins in serum. alpha 1-acid glycoprotein (nKP = 4.39 l g-1) and albumin (nKP = 0.27 l g-1) had relatively low binding affinities. 4. The concentration of serum proteins influences both the fraction of unbound drug and the fraction of drug associated with the erythrocytes. Changes in serum protein concentrations often encountered in malaria are likely to increase both the unbound fraction and the fraction bound to the erythrocytes.
Full text
PDF


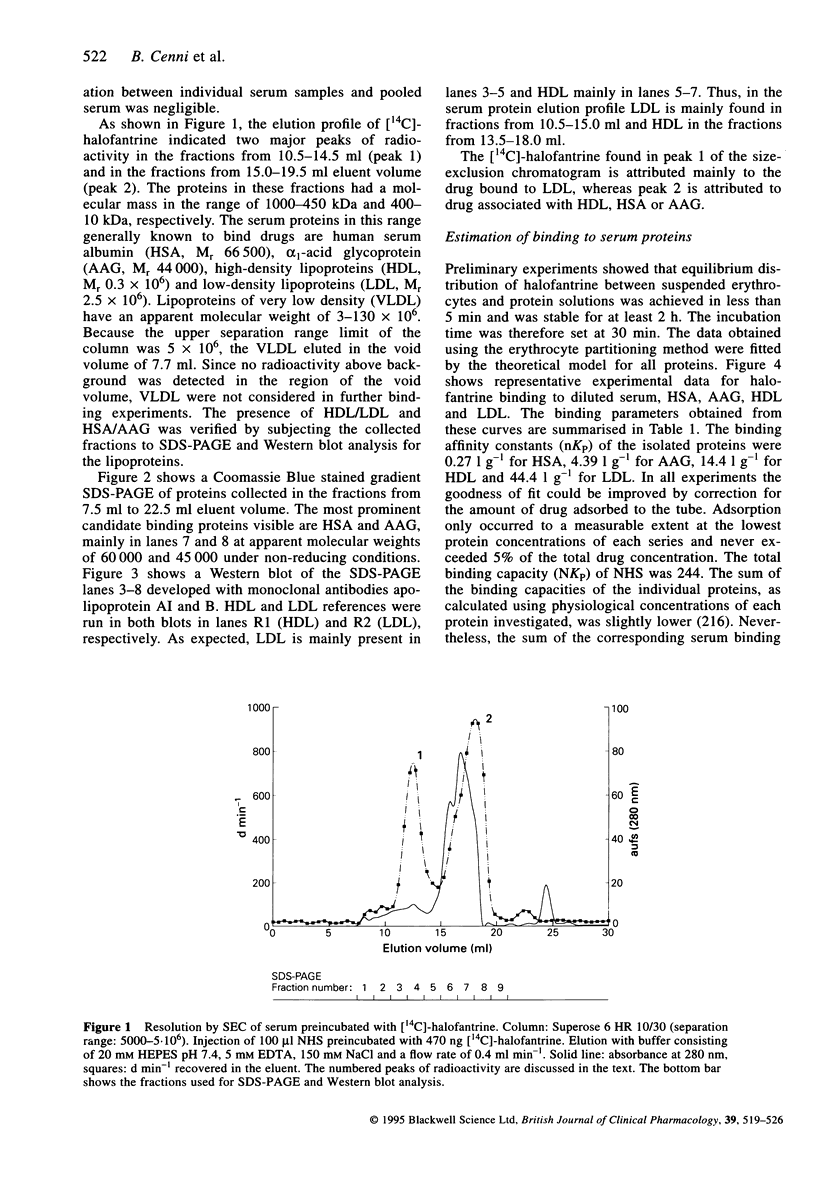
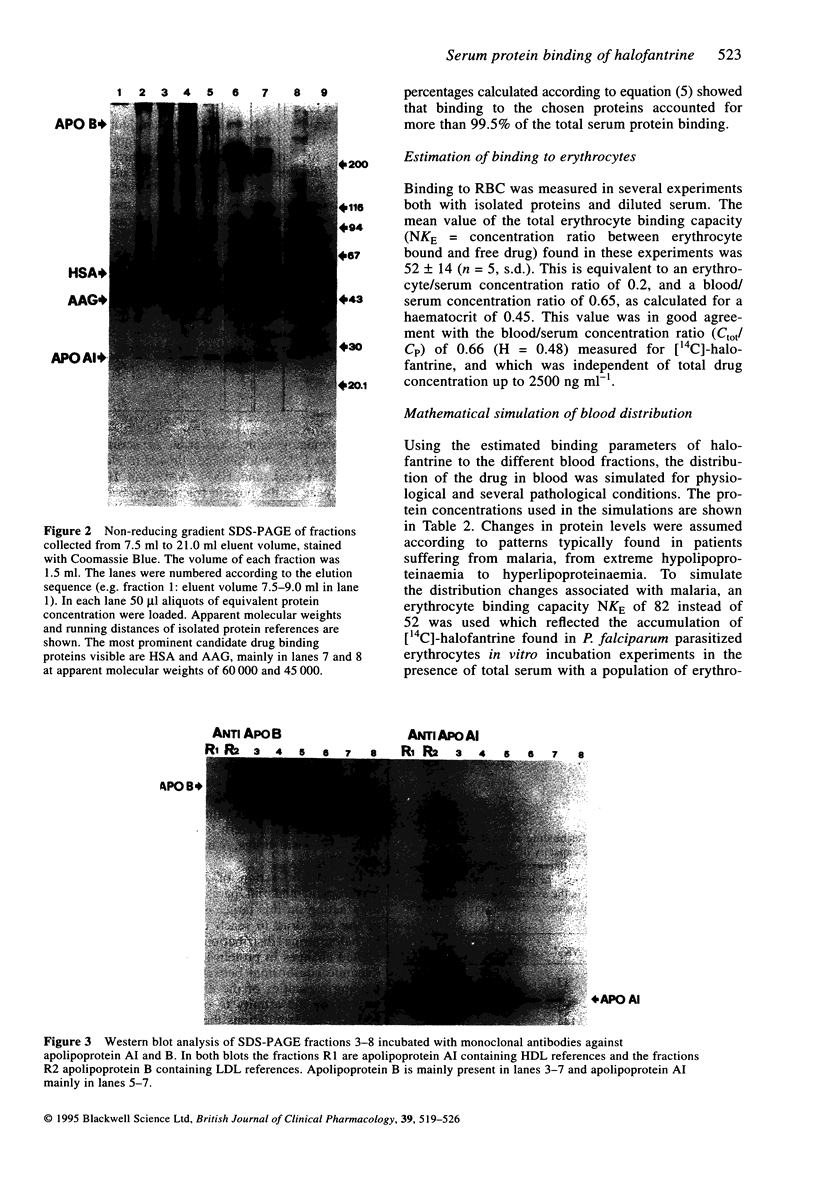
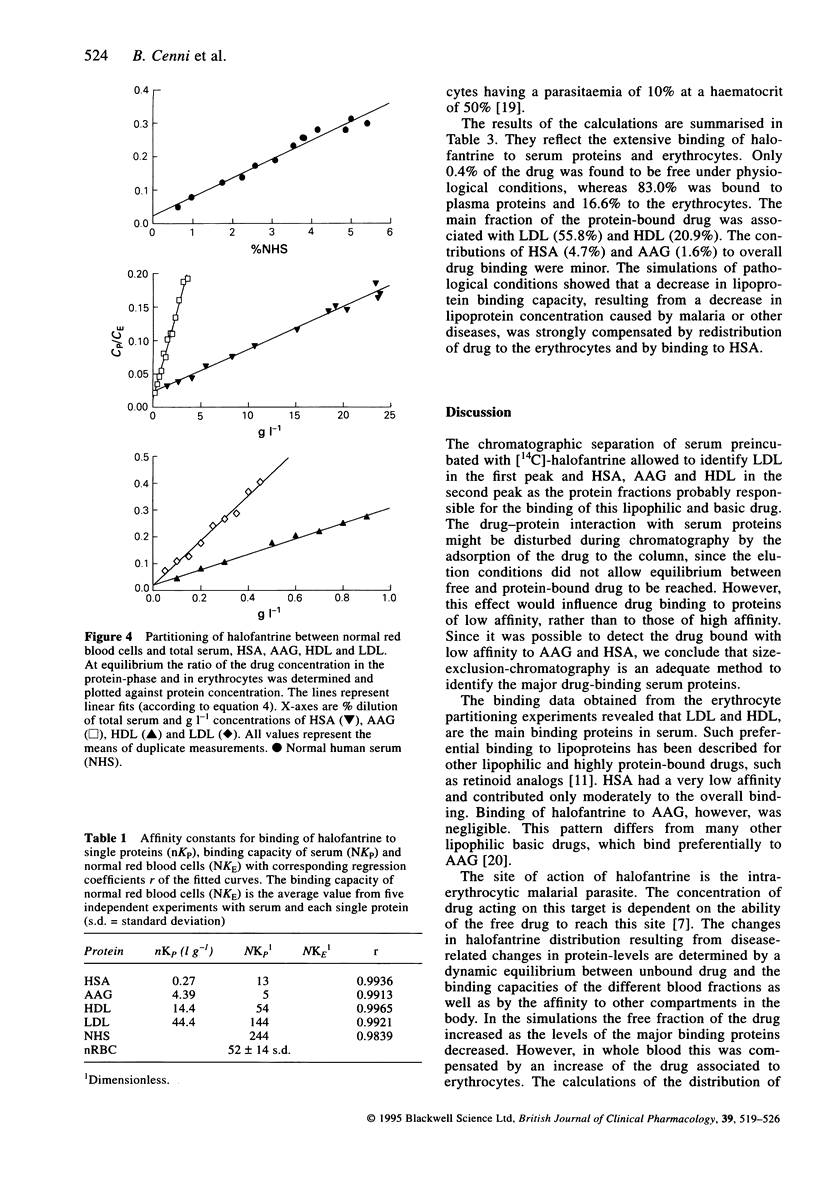

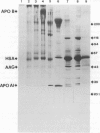
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albengres E., Urien S., Pognat J. F., Tillement J. P. Multiple binding of bepridil in human blood. Pharmacology. 1984;28(3):139–149. doi: 10.1159/000137955. [DOI] [PubMed] [Google Scholar]
- Bangchang K. N., Karbwang J., Back D. J. Primaquine metabolism by human liver microsomes: effect of other antimalarial drugs. Biochem Pharmacol. 1992 Aug 4;44(3):587–590. doi: 10.1016/0006-2952(92)90453-p. [DOI] [PubMed] [Google Scholar]
- Beevers D. G., Prince J. S. Some recent advances in non-communicable diseases in the Tropics. 1. Hypertension: an emerging problem in tropical countries. Trans R Soc Trop Med Hyg. 1991 May-Jun;85(3):324–326. doi: 10.1016/0035-9203(91)90276-5. [DOI] [PubMed] [Google Scholar]
- Graninger W., Thalhammer F., Hollenstein U., Zotter G. M., Kremsner P. G. Serum protein concentrations in Plasmodium falciparum malaria. Acta Trop. 1992 Dec;52(2-3):121–128. doi: 10.1016/0001-706x(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Harpham T., Stephens C. Urbanization and health in developing countries. World Health Stat Q. 1991;44(2):62–69. [PubMed] [Google Scholar]
- Horton R. J. Introduction of halofantrine for malaria treatment. Parasitol Today. 1988 Sep;4(9):238–239. doi: 10.1016/0169-4758(88)90136-6. [DOI] [PubMed] [Google Scholar]
- Karbwang J., Na Bangchang K., Bunnag D., Harinasuta T., Laothavorn P. Cardiac effect of halofantrine. Lancet. 1993 Aug 21;342(8869):501–501. doi: 10.1016/0140-6736(93)91631-u. [DOI] [PubMed] [Google Scholar]
- Karbwang J., Na Bangchang K. Clinical pharmacokinetics of halofantrine. Clin Pharmacokinet. 1994 Aug;27(2):104–119. doi: 10.2165/00003088-199427020-00003. [DOI] [PubMed] [Google Scholar]
- Korte R., Rehle T., Merkle A. Strategies to maintain health in the Third World. Trop Med Parasitol. 1991 Dec;42(4):428–432. [PubMed] [Google Scholar]
- Krishna S., ter Kuile F., Supanaranond W., Pukrittayakamee S., Teja-Isavadharm P., Kyle D., White N. J. Pharmacokinetics, efficacy and toxicity of parenteral halofantrine in uncomplicated malaria. Br J Clin Pharmacol. 1993 Dec;36(6):585–591. doi: 10.1111/j.1365-2125.1993.tb00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulard C., Urien S., Bastian G., Tillement J. P. Binding of retelliptine, a new antitumoral agent, to serum proteins and erythrocytes. Biochem Pharmacol. 1990 Aug 15;40(4):895–898. doi: 10.1016/0006-2952(90)90333-g. [DOI] [PubMed] [Google Scholar]
- Mberu E. K., Muhia D. K., Watkins W. M. Measurement of halofantrine and its major metabolite desbutylhalofantrine in plasma and blood by high-performance liquid chromatography: a new methodology. J Chromatogr. 1992 Oct 2;581(1):156–160. doi: 10.1016/0378-4347(92)80461-x. [DOI] [PubMed] [Google Scholar]
- Milton K. A., Hoaksey P. E., Ward S. A., Edwards G. Lack of effect of halofantrine on hepatic drug metabolism in the rat in vivo and in vitro. Biochem Pharmacol. 1990 May 15;39(10):1581–1586. doi: 10.1016/0006-2952(90)90524-o. [DOI] [PubMed] [Google Scholar]
- Mohanty S., Mishra S. K., Das B. S., Satpathy S. K., Mohanty D., Patnaik J. K., Bose T. K. Altered plasma lipid pattern in falciparum malaria. Ann Trop Med Parasitol. 1992 Dec;86(6):601–606. doi: 10.1080/00034983.1992.11812715. [DOI] [PubMed] [Google Scholar]
- Müller W. E., Stillbauer A. E. Characterization of a common binding site for basic drugs on human alpha 1-acid glycoprotein (orosomucoid). Naunyn Schmiedebergs Arch Pharmacol. 1983 Mar;322(2):170–173. doi: 10.1007/BF00512392. [DOI] [PubMed] [Google Scholar]
- Nosten F., ter Kuile F. O., Luxemburger C., Woodrow C., Kyle D. E., Chongsuphajaisiddhi T., White N. J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993 Apr 24;341(8852):1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- Pacifici G. M., Viani A. Methods of determining plasma and tissue binding of drugs. Pharmacokinetic consequences. Clin Pharmacokinet. 1992 Dec;23(6):449–468. doi: 10.2165/00003088-199223060-00005. [DOI] [PubMed] [Google Scholar]
- Rolan P. E. Plasma protein binding displacement interactions--why are they still regarded as clinically important? Br J Clin Pharmacol. 1994 Feb;37(2):125–128. doi: 10.1111/j.1365-2125.1994.tb04251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urien S., Bastian G., Lucas C., Bizzari J. P., Tillement J. P. Binding of a new vinca alkaloid derivative, S12363, to human plasma proteins and platelets. Usefulness of an erythrocyte partitioning technique. Invest New Drugs. 1992 Nov;10(4):263–268. doi: 10.1007/BF00944179. [DOI] [PubMed] [Google Scholar]
- Urien S., Claudepierre P., Meyer J., Brandt R., Tillement J. P. Comparative binding of etretinate and acitretin to plasma proteins and erythrocytes. Biochem Pharmacol. 1992 Nov 3;44(9):1891–1893. doi: 10.1016/0006-2952(92)90087-y. [DOI] [PubMed] [Google Scholar]
- Urien S., Riant P., Renouard A., Coulomb B., Rocher I., Tillement J. P. Binding of indapamide to serum proteins and erythrocytes. Biochem Pharmacol. 1988 Aug 1;37(15):2963–2966. doi: 10.1016/0006-2952(88)90282-1. [DOI] [PubMed] [Google Scholar]
- Urien S., Zini R., Lemaire M., Tillement J. P. Assessment of cyclosporine A interactions with human plasma lipoproteins in vitro and in vivo in the rat. J Pharmacol Exp Ther. 1990 Apr;253(1):305–309. [PubMed] [Google Scholar]