Abstract
Information on the establishment of immunodeficiency virus infection through transmission of infected cells is sparse. Dendritic cells (DCs) and T cells may be central to the onset and subsequent spread of infection following mucosal exposure. To directly investigate the consequences of virus being introduced by DCs or T cells, we reinjected ex vivo simian immunodeficiency virus (SIV)-loaded autologous immature DCs and T cells subcutaneously (s.c.) into healthy macaques. s.c. injection of cell-bound virus was used to mirror what may happen if virus-loaded cells pass through an epithelium or perhaps DCs and T cells that immediately entrap cell-free virus, having just crossed an epithelial barrier. Virus load in the plasma was monitored along with combined in situ hybridization and immunohistochemistry to identify the cells replicating virus in the lymphoid tissues. Both DCs and T cells transmitted infection after being pulsed with either wild-type or nef-defective (delta nef) SIVmac239. As seen in animals infected intravenously, replication of delta nef was attenuated compared to that of wild-type virus when introduced in either cell-bound form. Upon examination of the draining lymph nodes (LNs) during the first days of infection, virus-producing CD4+ T cells predominated in control animals that received s.c. cell-free virus. In dramatic contrast, both SIV-positive macrophages and T cells were detected in the LNs of monkeys infected with cell-associated SIV. Therefore, although both cell-free and cell-associated viruses are infectious, the initial cells amplifying the virus differ. This may have important implications for the subsequent dissemination of infection and/or induction of antiretroviral immunity.
Transmission of immunodeficiency viruses (human immunodeficiency virus type 1 [HIV-1] and simian immunodeficiency virus [SIV]) can occur either by free virus or by transmission of infected host cells. Dendritic cells (DCs) and T cells are among the most important cells that are able to capture immunodeficiency viruses and promote their replication (13, 22). Physiologically, DCs in an immature state capture foreign particles at the skin and mucosae and traffic them to the draining lymph nodes (LNs), where the matured DCs would induce effective antigen (Ag)-specific T- and B-cell-mediated immune responses (3). Considerable evidence exists from human and macaque studies for the ability of these sentinel cells to drive virus replication in vitro and in vivo (13, 22).
In vivo, within 2 to 3 days following application of SIV to the tonsillar (46, 47) or vaginal (52) mucosae, CD4+ T cells were identified as the major site of SIV RNA expression. However, Hu et al. (16) reported that DCs within the outer epithelium, Langerhans cells, were the first cells that were found to express SIV RNA, being detected within 18 h after vaginal exposure of macaques to the virus. These findings support a longstanding hypothesis that DCs are one of the first cells encountering viruses that breach the mucosae and then very efficiently propagate virus spread, particularly upon interaction with CD4+ T cells first locally and then in the draining lymphoid tissues.
In vitro, immature CCR5+ DCs can replicate R5 HIV-1 isolates (14), whereas mature CCR5−/low, CXCR4+ DCs are able to promote the extensive amplification of R5 and X4 HIV-1 as long as CD4+ T cells are present but support limited replication in the absence of T cells (reviewed in references 13 and 22). Similarly, SIV replicates well in autologous DC-T-cell mixtures from macaque blood, skin, and mucosal sites (20, 34, 40). Either virus-loaded DCs or T cells introduce infectious virus to the DC-T-cell mixtures, and virus growth requires no additional external stimulus. The T cells do not need to enter the cell cycle for the virus to amplify (39, 41), much like what has been observed in vivo (45, 48, 49). Virus replication in the immature DC-T-cell milieu has also been shown to be dependent on the presence of nef (34, 38). However, mature DCs promote replication of wild-type virus and an attenuated version of the virus with a deletion in the nef gene (delta nef) (34). This directly reflects the attenuated growth of the virus in vivo (23, 43), which mimics the prolonged survival of a cohort of HIV-infected individuals who exhibit minimal immune destruction after being infected for many years with nef-defective virus (8, 24, 27, 28). This also provides a primary cell system that is representative of the cellular locale encountered by the virus as it crosses the mucosae (22). Therefore, both cellular and viral components influence the level of replication in the DC-T-cell milieu.
This study set out to investigate whether wild-type versus delta nef SIV could be transmitted by virus-loaded immature DCs or CD4+ T cells in vivo and to determine what cell types are responsible for the early virus replication in this setting. We exposed immature DCs or CD4+ T cells to wild-type or delta nef virus in vitro, before subcutaneously (s.c.) reinjecting the virus-carrying cells into the donor animal. This strategy aimed at approximating the events following the DC or T-cell capture of virus that has just breached an epithelial barrier or the scenario that such virus-carrying cells traversed the epithelia. All animals became infected after injection of cell-associated virus s.c. or of cell-free virus s.c. or intravenously (i.v.). Delta nef virus-infected animals exhibited lower viremia, and fewer virus-positive cells were detected in the lymphoid tissues. Strikingly, there was a considerable increase in the involvement of macrophages in the early replication and spread of infection with cell-associated virus, unlike the almost exclusive virus-producing CD4+ T cells detected after injection of cell-free virus. This suggests that the initial mode of virus dissemination differs for cell-free versus cell-associated virus and may influence the design of strategies aimed at preventing infection.
MATERIALS AND METHODS
Culture medium and cytokines.
RPMI 1640 (Cellgro; Fisher Scientific, Springfield, N.J.) containing 10 mM HEPES (GIBCO-BRL, Life Technologies, Grand Island, N.Y.), 2 mM l-glutamine (GIBCO-BRL), 50 μM 2-mercaptoethanol (Sigma Chemical Company, St. Louis, Mo.), penicillin (100 U/ml)-streptomycin (100 μg/ml) (GIBCO-BRL), and 1% heparinized human plasma was used throughout these studies. 174xCEM cells (obtained from the NIH AIDS Research and Reference Reagent Program) were maintained in culture medium containing 10% fetal calf serum (Sigma).
Animals.
Prior to use, the adult male and female rhesus macaques (Macaca mulatta) used for this study were confirmed to be negative for antibodies (Abs) to SIV, type D retroviruses, and simian T-cell leukemia virus type 1. The macaques were housed at the Tulane Regional Primate Research Center. Animal care operations in compliance with the regulations detailed under the Animal Welfare Act, and in the Guide for the Care and Use of Laboratory Animals were observed. Animals were anesthetized with ketamine-HCl (10 mg/kg) prior to all procedures. Intravenous inoculation was achieved by injecting 102 50% tissue culture infectious doses (TCID50) of wild-type SIVmac239 or 2.35 × 104 TCID50 of delta nef virus as per an established protocol (7). As indicated, animals were injected with autologous cell-associated virus (see below) s.c. at three sites (total of 0.5 ml) approximately 1 to 2 cm from palpated LNs of the inguinal or axillar regions. Control animals received 102 or 105 TCID50 of cell-free wild-type or delta nef virus injected s.c at three sites proximal to an inguinal LN. Skin punch biopsies and LN samples were aseptically taken from the injection site at 1, 3, 7, and 14 days postinfection (p.i.). Part of each tissue sample was kept frozen or fixed in formalin for combined immunohistochemistry (IH)-in situ hybridization (ISH) analysis, while other parts of the tissue were used to prepare single-cell suspensions (17, 18) to set up in cocultures to detect infectious virus (below).
Generation of DCs and T cells.
Monocyte-derived DCs were generated from adherent monocytes (36). Eighty milliliters of heparinized blood was obtained from healthy anesthetized animals and the peripheral blood mononuclear cells (PBMCs) were collected over a Ficoll-Hypaque density gradient (Pharmacia, Uppsala, Sweden). A total of 1.2 × 107 to 1.5 × 107 PBMCs were plated per well of a six-well plate (Becton Dickinson Labware, Franklin Lakes, N.J.) and incubated for 1 to 2 h at 37°C. After removal of nonadherent cells, fresh culture medium containing 1000 U of recombinant human granulocyte-macrophage colony-stimulating factor (Immunex, Seattle, Wash.) per ml and 100 U of recombinant human interleukin-4 (R&D Systems, Minneapolis, Minn.) per ml was added. Cytokines were replenished every other day by replacing 0.3 ml of medium with 0.5 ml of medium with additional cytokines. After 7 days, immature DCs were collected and contaminating CD2+ T and CD20+ B cells were depleted by using three rounds of magnetic Dynabeads (Dynal, Oslo, Norway) (34).
CD4+ T-cell-enriched fractions were obtained from PBMCs isolated from 80 ml of heparinized blood samples by removing the HLA-DR+ and CD8+ subsets with Dynabeads (34). PBMCs were stained with a mouse anti-human CD8 monoclonal Ab (MAb) (Becton Dickinson Immunocytometery Systems [BDIS], San Jose, Calif.), washed, and mixed with goat anti-mouse immunoglobulin (Ig)-coated beads as well as an equal amount of anti-HLA-DR-coated beads. The cell-bead mixtures were incubated with rotation for 20 min at 4°C. This was repeated twice more upon addition of fresh beads.
The phenotype and purity of DCs and T cells were monitored by flow cytometry using a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) and analyzed with Cell Quest Software. DCs were analyzed by using fluorescein isothiocyanate-conjugated anti-HLA-DR (BDIS) combined with phycoerythrin-conjugated IgG; anti-CD2 (Dako, Carpinteria, Calif.); anti-CD20, -CD25, and -CD80 (BDIS); anti-CD83 (Coulter Corp, Miami, Fla.); or anti-CD86 (PharMingen, San Diego, Calif.). T cells were evaluated by using MAbs against CD4 (Ortho, Raritan, N.J.), CD8, HLA-DR, and CD2.
In vitro loading of DCs and T cells with virus.
Purified DCs and CD4+ T cells (a maximum of 106 cells per 1.5-ml Eppendorf tube in 500 μl of medium) were exposed to SIVmac239 or delta nef SIVmac239 at a multiplicity of infection (MOI) of 0.1. After 1.5 h at 37°C the cells were washed four times with 0.5 to 1 ml of cold medium by using an Eppendorf centrifuge (five spins at 2,500 rpm for 2 to 3 min) to remove unbound virus (12, 41), counted (by trypan blue exclusion), and placed back into culture overnight. Immature DCs were kept in medium containing granulocyte-macrophage colony-stimulating factor and interleukin-4. The following morning, the cells were collected and washed four times again, and the counted cells were pelleted in cold medium. We have shown previously that this extensive washing procedure efficiently removes the bulk (if not all) of the cell-free virus (as determined by quantitative reverse transcription-PCR analysis of the supernatant washes for viral gag RNA) (12). Subjecting the cells to another round of this washing procedure after the overnight cultures further reduced the likelihood of any cell-free virus carryover. The cells were kept on ice before being injected within the next 6 h. Before reinjection, the tubes were recentrifuged one more time and the cells were resuspended in 1 ml of RPMI and transferred to a 2-ml syringe. In the initial studies, the DCs from two animals (F154 and L780) were pulsed with SIVmac239 (MOI of 0.1) and then cultured overnight in the presence of 10 μg of tetanus toxoid (TT) (Staten Seruminstitut, Copenhagen, Denmark) per ml.
Aliquots of the virus-pulsed cells were cocultured with the SIV-permissive 174xCEM cell line to verify the presence of transmissible virus, as described previously (22). In brief, virus-loaded cells were mixed with the 174xCEM cells (104 virus-carrying cells per 105 174xCEM cells), and virus replication was monitored over the next days by measuring reverse transcriptase (RTase) activity (5). Where possible, additional aliquots of virus-pulsed cells were cultured alone (105 cells per well) or in the presence of 174xCEM cells (104 virus-carrying cells per 105 174xCEM cells) for 2 days before SIV gag DNA levels were monitored by PCR (see below). When cell numbers permitted, samples of virus-loaded cells were analyzed directly by PCR to monitor viral DNA loads.
IH and ISH.
IH was performed prior to ISH. To unmask Ags, dewaxed paraffin sections (5 μm thick) were boiled in 0.01 M buffered sodium citrate solution (pH 6) for 2 min. Cryostat sections were fixed in 2% paraformaldehyde for 10 min. The sections were incubated with primary Abs (Table 1) to defined cellular Ags according to the manufacturers' instructions. Binding of Abs was visualized either by the alkaline phosphatase-anti-alkaline phosphatase method using New fuchsin as the chromogen or by the peroxidase technique. The sections were either counterstained with hemalaun and mounted or subjected to ISH.
TABLE 1.
Antibodies used for immunohistochemistry
| Antigen | Clone | Source | Isotype | Cells identified |
|---|---|---|---|---|
| CD3 | NAa | Dako | Rabbit Ig | T cells |
| CD4 | Leu3A | BD | IgG1 | T cells, macrophages |
| NCL-CD4-1F6 | Novocastra | IgG1 | T cells, macrophages | |
| CD68 | KP1 | Dako | IgG1 | Macrophages |
| Sialoadhesin (CD169) | 2D7 | P. Crocker, University of Dundee | IgG1 | Macrophages |
| CD1a | SK9 | BD | IgG2b | Langerhans cells |
| Fascin, p55 | 55K | AIDS Research and Reference Reagent Program | IgG1 | Mature DCs |
| DC-LAMP (CD208) | 104.G4 | S. Saeland, Schering Plough | IgG1 | Mature DCs |
NA, not applicable. Rabbit polyclonal anti-CD3 was visualized by the peroxidase-antiperoxidase method.
ISH was performed on either paraffin or cryostat sections as described previously (42). Briefly, frozen sections were fixed in 4% paraformaldehyde, and dewaxed paraffin sections were boiled in a domestic pressure cooker in citrate buffer (pH 6.0) for 5 min prior to hybridization. The sections were hybridized to a 35S-labeled, single-stranded antisense RNA probe of SIVmac239 (Lofstrand Labs, Gaithersburg, Md.) overnight at 45°C. As a negative control, sections were hybridized with a 35S-labeled sense probe. The sections were then dipped into photo emulsion (NTB2; Kodak, Rochester, N.Y.), exposed for 3 to 7 days, developed, counterstained with hemalaun, and mounted. To determine the frequency of SIV RNA-positive cells per square millimeter of section, we used a microscope (Axiophot; Carl Zeiss Inc., Jena, Germany) equipped with Plan Neofluar for transmitted and incident light, a 3CD color camera, and a PC-based image analysis system (KS 400; Kontron, Esching, Germany).
Coculture assays for the detection of infectious virus from infected animals.
Aliquots of PBMCs or LN cells (LNCs) (17, 18) were collected at various times after infection, and equal numbers of PBMCs or LNCs (2 × 106 to 5 × 106) were cultured with equal numbers of 174xCEM cells in 5 ml of medium in T25 flasks for up to 3 weeks. When there were fewer than 1.5 × 106 PBMCs available, cocultures were set up in 24-well plates. Cultures were split two or three times per week. Syncytium formation was monitored by microscopy, and virus amplification was confirmed in culture supernatants at days 14 and 21 by the detection of p27 Ag with a commercially available enzyme-linked immunosorbent assay (ELISA) (Zeptometrix, Buffalo, N.Y.) according to the manufacturer's protocol (31).
Anti-SIV Ab assays.
Plasma samples obtained at the indicated times after infection were monitored for the presence of SIV envelope Abs by using an established protocol (44).
Detection of virus protein and RNA in the plasma.
Plasma was separated from heparinized blood samples and stored at −70°C. SIV p27 Ag levels in plasma were measured by ELISA (Zeptometrix). In addition, the numbers of viral RNA copies were determined by bDNA analyses with a lower detection limit of 1,500 copies per ml of plasma (Bayer Reference Testing Laboratory, Bayer Diagnostics, Berkley, Calif.) (32).
PCR analysis of SIV gag DNA.
Using an established protocol (51), proviral DNA was detected by PCR with primers detecting late gag sequences (6). Virus-pulsed cells (105 total) were transferred into a 0.5-ml microcentrifuge tube (CNO650-GT; National Scientific, San Rafael, Calif.) and centrifuged 2 to 3 min at 3,000 rpm (MicroSpin 12S; Sorvall Instruments, Dupont), and the cells were washed once in cold phosphate-buffered saline for 2 to 3 min at 3,000 rpm. The supernatant was carefully aspirated, and the pellet was resuspended in 50 μl of hypotonic lysis buffer (10 mM Tris [pH 8.0], 1 mM EDTA, 0.001% Triton X-100-sodium dodecyl sulfate in sterile double-distilled water [ddH2O]) containing 600 μg of proteinase K (Boehringer Mannheim, Roche Molecular Biochemicals, Indianapolis, Ind.) per ml. The cells were then incubated for 1 h at 56°C, followed by 15 min at 95°C, to inactivate the protease. After lysis, aliquots of the DNA were stored at −20°C. To amplify the gag gene sequence, the primers DSgag1 (plus strand; 5′ATG GGC GCG AGA AAC TCC GTC TTG3′) and DBgag5 (minus strand; 5′TCC AAC AGG CTT TCT GCT AAT CC3′) were used. DNA (equivalents of 2 × 104 cells) was added to 50 μl of a PCR mixture containing 20 pmol of each primer, 1× reaction buffer (Promega, Madison, Wis.), 3 mM MgCl2, 200 μM each deoxynucleoside triphosphate (Boehringer Mannheim), 2 U of Taq DNA polymerase (M1861; Promega) and 4 μCi of [32P]dCTP (NEG013H; NEN, Boston, Mass.) in ddH2O. Two drops of mineral oil were added to the samples, and amplification was carried out in a DNA thermal cycler (Perkin-Elmer Cetus, Norwalk, Conn.). Amplification involved three cycles of 94°C for 1 min, 56°C for 30 s, and 72°C for 30 s followed by 26 cycles of 95°C for 10 s, 58°C for 30 s, and 72°C for 30 s. PCR products were separated on an 8% acrylamide gel run in 0.5× Tris-borate-EDTA. Gels were dried and exposed to XAR-5 film (Kodak, Rochester, N.Y.). For normalization of the PCR products, the cellular gene for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified from each sample, using a modified version of an established PCR protocol (50). In brief, to amplify the GAPDH gene sequence, the following primers were used: GAP1 (plus strand; TGG TAT CGT GGA AGG ACT CAT GGT) and GAP2 (minus strand; GGA AGG AAA TTA TGG GAA AGC CAG). Fifty microliters of a reaction mix containing 50 pM each primer, 8 mM MgCl2, 2.5 μCi of [32P]dCTP (NEN), 120 μM cold deoxynucleoside triphosphates, ddH2O, and 2 U of Taq DNA polymerase was added to 2 μl of each DNA sample (approximately 4 × 103 cells). Two drops of mineral oil were added to the samples, and the reaction was carried out in 22 cycles of 1 min at 94°C and 2 min at 67°C. Amplified products were visualized with an 8% acrylamide gel (see above).
RESULTS
Transmission of infection via s.c. injected SIV-carrying DCs.
SIV-pulsed DCs, while hardly replicating virus themselves, are able to transmit virus to T cells in vitro, thereby initiating robust viral replication (13, 22). To investigate whether s.c. administered DC-borne virus would transmit infection in vivo, immature DCs were obtained from two healthy macaques, pulsed with SIVmac239 (MOI of 0.1), washed thoroughly to remove cell-free virus, and cultured overnight in the presence of 10 μg of TT per ml. The TT was included in order to verify that functional DCs had reached the draining LNs, where they should prime a TT-specific T-cell response, as we have seen in healthy animals with TT-pulsed, uninfected DCs (unpublished observations). On the following day, the cells were collected, washed again, and counted, and 1 × 106 to 2 × 106 autologous DCs were reinjected at three sites proximal to an inguinal LN. The largest numbers of DCs able to be injected were used in this pilot experiment.
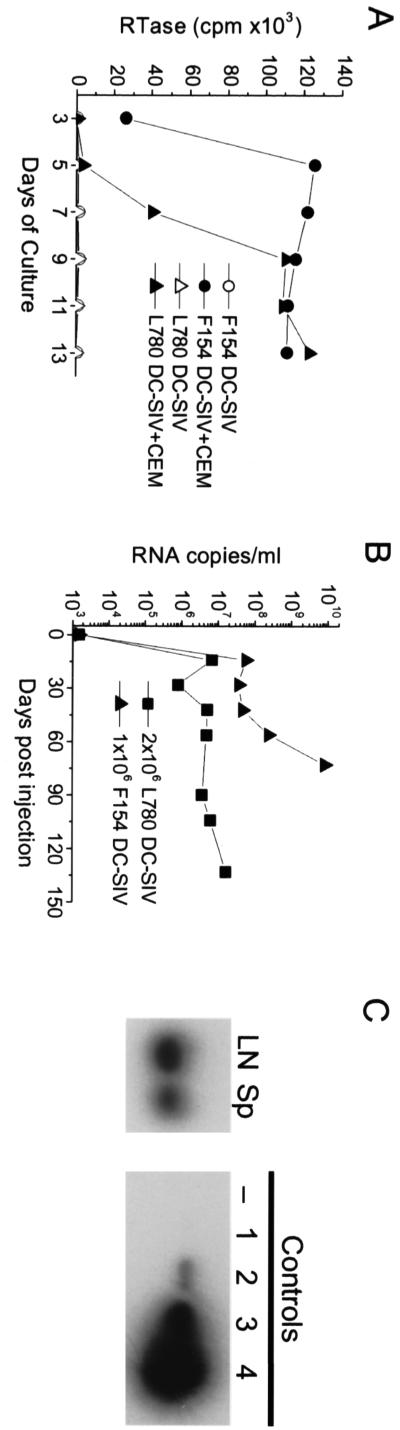
Aliquots of the virus-loaded cells were able to rapidly amplify virus in vitro upon coculture with 174xCEM cells (Fig. 1A), confirming the presence of infectious virus in the cell preparations used for reinjection. Both animals exhibited significant plasma viremia within 2 weeks of the s.c. administration of the 1 × 106 to 2 × 106 virus-carrying DCs, which did not rebound to lower set point levels (Fig. 1B). With dramatically increasing viremia, animal F154 had to be euthanized after 73 days due to clinical symptoms suggestive of disease progression. Autopsy confirmed lesions of immunodeficiency disease, and high levels of virus (approximately 1010 RNA copies/ml) were detected in the blood (Fig. 1B) and in the lymphoid tissues as verified by PCR (estimates of ≥102 to 103 SIV gag copies per 105 spleen cells and LNCs, respectively) (Fig. 1C). F154 was likely a rapid progressor, exhibiting the extremely high viremia coincident with an elevated plasma SIV p27 Ag value at week 3 and an absence of a detectable anti-SIV Ab response (even at necropsy). Subsequently, animal L780 died within 7 months after developing simian AIDS. However, even with considerable virus levels and the presence of SIV p27 Ag in the plasma, L780 did develop an anti-SIV Ab response.
FIG. 1.
Immature DCs exposed to SIVmac239 in vitro transmit infection after s.c. reinjection. (A and B) Immature monocyte-derived DCs from two healthy macaques (F154 and L780) were exposed to SIVmac239 (MOI of 0.1), washed, and cultured overnight in the presence of 10 μg of TT per ml. The next day, the cells were washed again, recounted, and monitored for their ability to transmit infection in vitro (A) and in vivo (B). (A) Virus-loaded DCs were mixed with 174xCEM cells (104 DCs with 105 174xCEM [CEM] cells [F154 DC-SIV+CEM and L780 DC-SIV+CEM]) or plated alone (F154 DC-SIV and L780 DC-SIV) and cultured for approximately 2 weeks. Starting at day 3 of culture, supernatants were sampled every other day and monitored for the presence of RTase activity. The results are expressed as cpm (103) of RTase activity per μl of culture supernatant. (B) SIV and TT-bearing DCs (L780, 2 × 106 DC-SIV; F154, 1 × 106 DC-SIV) were s.c. reinjected into the donor animals at three sites proximal to an inguinal LN. Blood samples collected over the ensuing weeks were analyzed for virus RNA levels by bDNA analysis. The numbers of RNA copies per milliliter of plasma are shown. (C) LN and spleen (Sp) cell suspensions isolated from tissues obtained at necropsy of animal F154 were assessed for virus levels by SIV gag PCR. Aliquots of 105 cells were sampled, and the SIV gag DNA was amplified. Standard curve controls representing 0 (lane −), 101 (lane 1), 102 (lane 2), 103 (lane 3), and 104 (lane 4) SIV gag copies were included to estimate SIV gag copy numbers in each test sample.
Therefore, s.c. injection of autologous immature DCs that are carrying wild-type SIV resulted in the rapid and efficient infection of macaques. In standard T-cell proliferation assays on PBMCs (10) with the same working batch of TT applied to separate concurrent in vitro studies (21) as well as to prime naive macaques, no TT-specific T-cell priming was detected in these infected animals at several time points after reinjection of the SIV- and TT-carrying DCs. In each immune assay, aliquots of the PBMCs were also stimulated with the superantigen SEB to verify that the cells were functional. This is in contrast to the TT-specific priming seen when DC-borne TT was used to prime healthy humans (11) and macaques (unpublished observations) in the absence of infection. Assuming that the reinjected DCs bearing SIV and TT were functional, it is possible that the T cells responding to the TT provided a perfect niche in which the wild-type virus rapidly replicated (as can be seen in vitro [22]), consequently destroying the TT-responding cells and rendering the animals unprimed to TT. However, the definitive experiments necessary to address this issue were not the focus of these studies. In sum, the rapid infection resulting from the application of virus-carrying DCs does not correlate with the induction of strong immune responses to coadministered Ags.
Transmission of wild-type and delta nef infection by DCs and T cells.
Having established the model approach in which 1 × 106 to 2 × 106 DCs loaded with virus in vitro could efficiently transmit wild-type infection upon s.c. reinjection, we extended this to examine whether fewer DCs or T cells (3 × 105) could transmit both wild-type and delta nef viruses. Based on our earlier in vitro findings that either DCs or T cells can introduce infectious virus and that vigorous virus replication required nef (34), we were especially interested in whether immature DCs or resting CD4+ T cells would be able to transmit delta nef virus in vivo.
Immature DCs (11 animals) or CD4+ T cells (6 animals) obtained from healthy rhesus macaques were infected with wild-type virus (DCs from five animals and T cells from four animals) or delta nef (DCs from six animals and T cells from two animals) at an MOI of 0.1, washed, and incubated overnight. The cells were then washed again before being injected s.c. proximal to the inguinal or axillary LNs of the donor animals. A minimum of 3 × 105 cells were injected at each inguinal or axillary site (Table 2). Six of the animals (two inoculated with DCs infected with wild-type virus, two inoculated with DCs infected with delta nef virus, and two inoculated with T cells infected with wild-type virus) (Table 2) were injected s.c. with 3 × 105 cells at three or four different sites close to the inguinal and axillary LNs (two inguinal and two axillary locations and a maximum of 1.2 × 106 cells per animal).
TABLE 2.
Transmission of infection by injected cell-free or cell-associated wild-type and nef-defective SIVmac239
| Animal | Inoculuma | Plasma p27 Agb | Anti-SIV Abb | Time of death p.i.c |
|---|---|---|---|---|
| G544 | 3 × 105 wt-DCs | + | + | 13 mo |
| AR 82 | 3 × 105 wt-DCs | + | − | 19 wk |
| H917 | 3 × 105 wt-DCs | + | + | 8 mo |
| H522 | 1.2 × 106 wt-DCs | + | NDe | 3 mo |
| I686d | 9 × 105 wt-DCs | + | ND | 3 mo |
| M846 | 3 × 105 delta-DCs | + | + | 17 mo |
| K080 | 3 × 105 delta-DCs | + | + | 27 mo |
| P741 | 3 × 105 delta-DCs | + | + | 37 mo |
| P318 | 3 × 105 delta-DCs | + | + | 28 mo |
| AT 53 | 1.2 × 106 delta-DCs | + | ND | 3 mo |
| C577 | 1.2 × 106 delta-DCs | + | ND | 3 mo |
| L721 | 3 × 105 wt-T cells | + | + | 15 mo |
| L702 | 3 × 105 wt-T cells | + | + | 6 mo |
| J696 | 1.2 × 106 wt-T cells | + | ND | 3 mo |
| J995 | 1.2 × 106 wt-T cells | + | ND | 3 mo |
| M177 | 3 × 105 delta-T cells | + | − | 24 mo |
| L507 | 3 × 105 delta-T cells | + | + | Alive 3 yr |
| AR 73 | 1 × 105 delta, s.c. | + | − | 3 mo |
| N992 | 1 × 102 delta, s.c. | + | + | Alive 3 yr |
| M806 | 1 × 105 wt, s.c. | + | + | 13 mo |
| AR 79 | 1 × 102 wt, s.c. | + | + | 4.5 mo |
| AR 70 | 1 × 105 wt, s.c. | + | + | 3 mo |
| G402 | 1 × 105 wt, s.c. | + | + | 7 mo |
| AT 88 | 1 × 102 wt, i.v. | ND | − | 11 wk |
| AR 80 | 1 × 102 wt, i.v. | ND | + | 19 mo |
| AT 62 | 2.35 × 104 delta, i.v. | ND | + | Alive 3.5 yr |
| AT 84 | 2.35 × 104 delta, i.v. | ND | + | 19 mo |
| AR 83 | 2.35 × 104 delta, i.v. | ND | + | Alive 3.5 yr |
| AR 85 | 2.35 × 104 delta, i.v. | ND | + | 13 mo |
wt-DCs, wild-type virus-infected DCs; delta-DCs; delta nef virus-infected DCs.
Plasma p27 Ag was detected within 1 to 3 weeks of infection, and anti-SIV envelope Abs we detected by weeks 8 to 12 p.i., varying as expected between donors. Positivity was confirmed in at least one follow-up measurement, and negative results reflect animals that never seroconverted during that testing period.
Animals were euthanized if they appeared to be ailing, and necropsy samples were analyzed where possible. H522, I686, AT 53, C577, J696, and J995 were euthanized and necropsied after 3 months.
I686 received only 3 × 105 cells at three sites. Note that the i.v. delta nef virus-infected animals AT 62, AT 84, AR 83, and AR85 were i.v. challenged with 100 TCID50 of wild-type virus 15 weeks after their initial infection with delta nef virus for use in other ongoing immune studies. In other continuing studies, animals M846, K080, P318, P741, M177, L507, and N992 were similarly i.v. challenged with wild-type virus approximately 11 months after injection of cell-associated or cell-free delta nef virus. All times of death are relative to the initial infection with delta nef virus.
ND, not done.
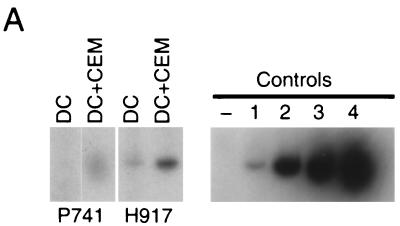
The presence of infectious virus in each virus-pulsed cell preparation was verified in vitro by coculturing an aliquot of the virus-carrying cells with permissive 174xCEM cells and microscopically monitoring syncytium formation in the cocultures or SIV p27 production by ELISA. When possible, PCR analysis for SIV gag DNA was performed and revealed that undetectable or 10 to 100 copies of SIV gag DNA was associated with 3 × 105 reinjected cells. Figure 2A shows two examples (animals P741 [delta nef virus-loaded DCs] and H917 [wild-type virus-loaded DCs]), in which aliquots of the injected virus-pulsed DCs were cultured alone (105 DCs) or with 174xCEM cells (104 DCs with 105 174xCEM cells) for 2 days to amplify the virus prior to PCR analysis for SIV gag DNA. Amplification of the negligible signals seen in the purified DCs alone is evident after 2 days of coculture of 1/10 of the DCs with 174xCEM cells (Fig. 2A).
FIG. 2.
PCR confirmation of infection via DC-associated and cell-free s.c. administered virus. (A) Immature DCs from two healthy macaques (P741 and H917) were exposed to 0.1 MOI of delta nef (P741) or wild-type (H917) SIV and washed, and an aliquot (of the cells injected into the animals [Fig. 3]) was cultured alone (DC) or with 174xCEM cells (DC+CEM) for 2 days (at a DC/174xCEM ratio of 1:10). After culture, the cells were collected and the SIV gag DNA levels evaluated by PCR. The standard curve of controls described for Fig. 1 is included. (B) PBMCs were prepared from blood samples taken 1, 4, and 6 weeks after injection of animals with delta nef virus-infected DCs (K080 and M846), wild-type virus-infected DCs (AR 82 and G544), or cell-free wild-type virus (M806) (Table 2). SIV gag DNA levels were detected by PCR analysis, confirming the presence of infection in all animals compared to the standard curve controls (Fig. 1).
Control animals were infected with s.c or i.v. administered cell-free virus (Table 2). DC- and T-cell-associated virus likely comprises fractions of virus that have infected the cells, as well as virions (potentially infectious) that are bound to the cell surface or internalized into intracellular compartments (12). With an MOI of 0.1 for the cell infections, the maximum amount of cell-associated virus that could have been injected with 3 × 105 cells was 3 × 104 infectious doses (or 1.2 × 105 for animals receiving 1.2 × 106 cells). While the PCR data suggest that a maximum of only 100 copies of SIV DNA would be associated with the injected cells, our recent studies using real time reverse transcription-PCR for SIV gag RNA also suggest that an average of 34% of the input virus can be captured by immature DCs (12), and this gives no indication as to what proportion of that would be infectious. Consequently, four animals were injected s.c. with 102 or 105 TCID50 of wild-type or delta nef virus in an attempt to cover the extreme situations of viral transmission. Animals receiving wild-type (102 TCID50) or delta nef (2.35 × 104 TCID50) SIV i.v. were also included as comparative controls of cell-free virus infection (7).
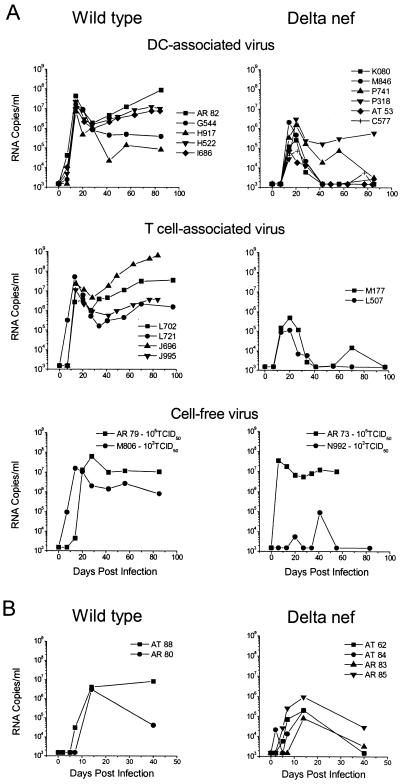
After injection of cell-free or cell-associated virus, the animals had regular blood samples taken over the next weeks and LN samples biopsied at the indicated time points p.i. In the plasma, Ab responses as well as plasma viremia measurements (SIV RNA measured by bDNA and SIV Ag levels measured by ELISA) were determined. Virus was detected in the PBMCs by PCR, with the virus DNA levels increasing over time (see, e.g., Fig. 2B, weeks 1, 4, and 6 p.i.), and its infectivity was confirmed in coculture assays. Each animal became infected within days of exposure, with plasma p27 Ag being detected within 1 to 3 weeks p.i. (Table 2) and the peak viremia occurring within 2 weeks p.i. for animals receiving wild-type virus-infected cells and at 2 to 3 weeks p.i. for those receiving delta nef virus-infected cells (Fig. 3). Overall, animals injected with cell-associated wild-type virus showed peak levels of approximately 2 × 106 to 6 × 107 virus copies/ml of plasma, compared to only 7 × 104 to 3 × 106 virus copies/ml in those infected with delta nef virus-infected cells (Fig. 3A). On average, the plasma virus levels of animals infected with delta nef virus-pulsed DCs or T cells then decreased to significantly lower set point levels (six of them often had <1,500 copies, and one had <105 copies, which later dropped to <4 × 103 copies) than those seen with wild-type infections (persisting at between 105 and 109 copies). One exception among the delta nef virus-infected animals (animal P318) did develop an anti-SIV Ab response (Table 2), but its plasma virus load diminished only about 1 log unit after the peak level and remained at around 2 × 105 to 5 × 105 copies/ml over the next weeks (Fig. 3A).
FIG. 3.
Transmission of infection by cell-associated versus cell-free virus. (A) Immature monocyte-derived DCs or CD4+ T cells were isolated from 17 healthy macaques (as indicated) and exposed to an MOI of 0.1 of SIVmac239 wild-type or SIVmac239 delta nef virus. After an overnight incubation, the cells were collected, washed, and recounted, and 3 × 105 to 1.2 × 106 SIV-bearing DCs or T cells were reinjected into the donor animals proximal to the inguinal or axillary LNs (Table 2). Individual animal numbers are listed in each graph. Four other animals were s.c. injected with the indicated doses of cell-free virus. (B) Six healthy macaques were infected i.v. with either 102 TCID50 of wild-type SIVmac239 or 2.35 × 104 TCID50 of delta nef SIVmac239. Plasma viremia was measured by bDNA analysis for up to 100 days after injection of cell-associated or cell-free virus to reveal the number of SIV RNA copies per milliliter of plasma.
Similarly, s.c. injection of cell-free SIV resulted in peak infections within 2 to 3 weeks in three of the four animals (Fig. 3A). All three of these animals exhibited peak virus loads of between 107 and 108 copies and maintained high set point values of around 106 to 107 copies; this was so even in AR 73, an animal injected with 105 TCID50 of delta nef virus. There appeared to be little difference between animals receiving 102 or 105 TCID50 of wild-type virus, with both also exhibiting high SIV Ag levels and anti-SIV Ab responses in the plasma (Table 2). Interestingly, the dramatic (possibly misleading) infection of AR 73 with nef-defective virus may be attributed to the fact that this animal appeared to be a rapid progressor, never developing detectable anti-SIV Ab responses (Table 2), even at necropsy. In contrast, animal N992, infected with the low dose of delta nef virus, spiked with only low-level viremia of up to105 copies per ml after 6 weeks, with virus remaining undetectable for much of the time during this period.
As expected, the i.v. infected animals that were included as additional internal controls for the wild-type and delta nef infections exhibited peak virus loads within 2 weeks which dropped to the various set point levels thereafter (Fig. 3B) and had infectious virus detectable within 5 days of infection in their PBMCs and LNCs; PBMC samples from all animals were SIV gag PCR positive. However, one wild-type virus-infected animal (AT 88) developed rapidly progressing immunodeficiency syndrome with sustained high plasma viremia. AT 88 died from simian AIDS at week 12 without developing a detectable Ab response to SIV. One animal, AR 85, that received delta nef virus had approximately 1-log-unit-higher virus levels than the other delta nef virus-infected animals and did not show as rapid a decrease in viremia after the initial peak at 2 weeks. Interestingly, while the other delta nef virus-infected animals remained healthy, AR 85 developed immunodeficiencies and died at 59 weeks after infection.
Thus, both cell-free and cell-associated wild-type as well as delta nef SIV are infectious when administered s.c., and the latter infection can be achieved with as few as 3 × 105 virus-carrying DCs or T cells. Moreover, cell-borne delta nef virus results in a similarly attenuated acute viremia as seen in i.v. infection with cell-free virus.
Increased involvement of infected macrophages following cell-associated virus transmission.
Both DCs and T cells are able to transmit infection of either delta nef or wild-type SIV upon s.c. injection (Fig. 2 and 3; Table 2). We were interested to ascertain how each infectious source is amplified during acute infection. CD4+ T cells have previously been shown to be the major source of virus after infection with cell-free wild-type SIV (47, 52) or delta nef SIV (46) applied to the mucosal surfaces, but is the initial focus of infection distinct if the virus is delivered in a cell-bound or cell-free form?
To address this question, ISH and IH were performed on the frozen LN samples collected during the first 14 days of infection. Irrespective of the infectious inoculum, only after 14 days were there sufficient numbers of SIV-positive cells in the tissues to be phenotyped (Tables 3 and 4). CD3+ T cells were characterized by staining of small cells for CD4. DC subsets were identified by the expression of CD1a for the LC-like DCs (26) or DC-LAMP (CD208), which is a lysosome-associated membrane protein expressed by activated DCs (9). An Ab against the lysosomal marker CD68 was used to mark macrophages (36). Although they may be hard to detect in vivo, DCs can also express CD68 (33). Therefore, macrophages were more carefully defined by using an Ab against sialoadhesin (CD169), a lectin with Ig superfamily domains that binds sialic acid and is expressed exclusively by a subset of macrophages (30, 35). CD169 is not expressed by macaque skin- or monocyte-derived DCs (unpublished observations). Large CD4+ cells remain unidentified, as they may represent activated T cells, macrophages, or possibly even DCs.
TABLE 3.
SIV RNA expression in the LNs of i.v. infected macaques at 14 days p.i.
| Animal | Inoculum (TCID50, i.v.)a | No. of RNA-positive cells perb:
|
|
|---|---|---|---|
| Section | mm2 | ||
| AT 88 | 1 × 102, wt | 348.5 | 52 |
| AR 80 | 1 × 102, wt | 234.5 | 16.1 |
| AT 62 | 2.35 × 104, delta | 17.25 | 3.15 |
| AT 84 | 2.35 × 104, delta | 8.2 | 0.4 |
| AR 83 | 2.35 × 104, delta | 15 | 0.72 |
| AR 85 | 2.35 × 104, delta | 132 | 6.5 |
wt, wild-type virus; delta, delta nef virus.
Average numbers of RNA-positive cells (by ISH) at day 14. LNs were biopsied at days 2, 5, 7, and 14. However, negligible numbers of SIV-positive cells were detected before day 14 (ranging from 0.08 to 2.25 SIV-positive cells per mm2 or 0.5 to 38 SIV-positive cells per section at day 7).
TABLE 4.
Increased numbers of CD169-positive, SIV RNA-expressing cells in LNs 14 days after s.c. reinjection of cell-associated virus
| Animal | Inoculum (s.c.)c | No. of RNA-positive cells pera:
|
% of RNA-positive cells expressing CD169b | |
|---|---|---|---|---|
| Section | mm2 | |||
| I686 | wt-DCs | 890 | 24.18 | 49.36 |
| H522 | wt-DCs | 853 | 29 | 25.61 |
| C577 | delta-DCs | 135 | 10.5 | NDd |
| AT 53 | delta-DCs | 20 | 0.9 | ND |
| J696 | wt-T cells | 1,495 | 48.14 | 28.78 |
| J995 | wt-T cells | 1,758 | 29.42 | 50.52 |
| AR 70 | 105 TCID50 wt | 185 | 6.16 | 9.5 |
| G402 | 105 TCID50 wt | 147 | 16.4 | 1.5 |
LNs were biopsied at days 1, 3, 7, and 14 after injection of cell-associated virus and 14 days after infection with cell-free virus. As with the i.v. infected animals, few SIV-positive cells were detected before day 14 (i.e., at day 7 the numbers of SIV-positive cells ranged from 0.0 to 2.4 per mm2 or 0 to 36 per section).
CD169 stains were performed on day 14 samples where sufficient RNA-positive profiles were available to phenotype.
Animals were s.c. injected with DC- or T-cell-associated wild-type (wt) or delta nef (delta) virus or with 105 TCID50 of cell-free wild-type virus.
ND, not done, as there were too few RNA-positive cells.
A summary of the ISH data obtained for the control i.v. infected animals at 14 days p.i. is provided in Table 3. The distribution of virus-positive cells was predominantly within the T-cell areas of the LNs, with very little (if any) trapping of virions (immune complexed) in the germinal centers being detected at this early stage. While very few virus-positive cells were detectable before day 14, considerably higher (at least 10 times greater) numbers of SIV ISH-positive profiles were detected in wild-type virus-infected animals. Only AR 85, the delta nef virus-infected animal with plasma virus levels comparable to those of the wild-type virus-infected animals (Fig. 3), showed similar numbers of ISH-positive cells (Table 3). Much like as seen after cell-free infection via the mucosa (47, 52), combined ISH and IH analysis of the tissues confirmed that CD4+ T cells were the main source of virus production in both the wild-type (Fig. 4, animal AT 88) and delta nef virus-infected animals. No CD169+ cells (Fig. 4, animal AR 80) and rarely any CD68+ cells (Fig. 4, animal AT 88) were found to be SIV positive. Therefore, while there were considerably fewer virus-producing cells detected in the LNs after i.v. infection with the attenuated delta nef compared to the wild-type virus, CD4+ T cells (and not macrophages or DCs) were the main source of virus production in each case.
FIG. 4.
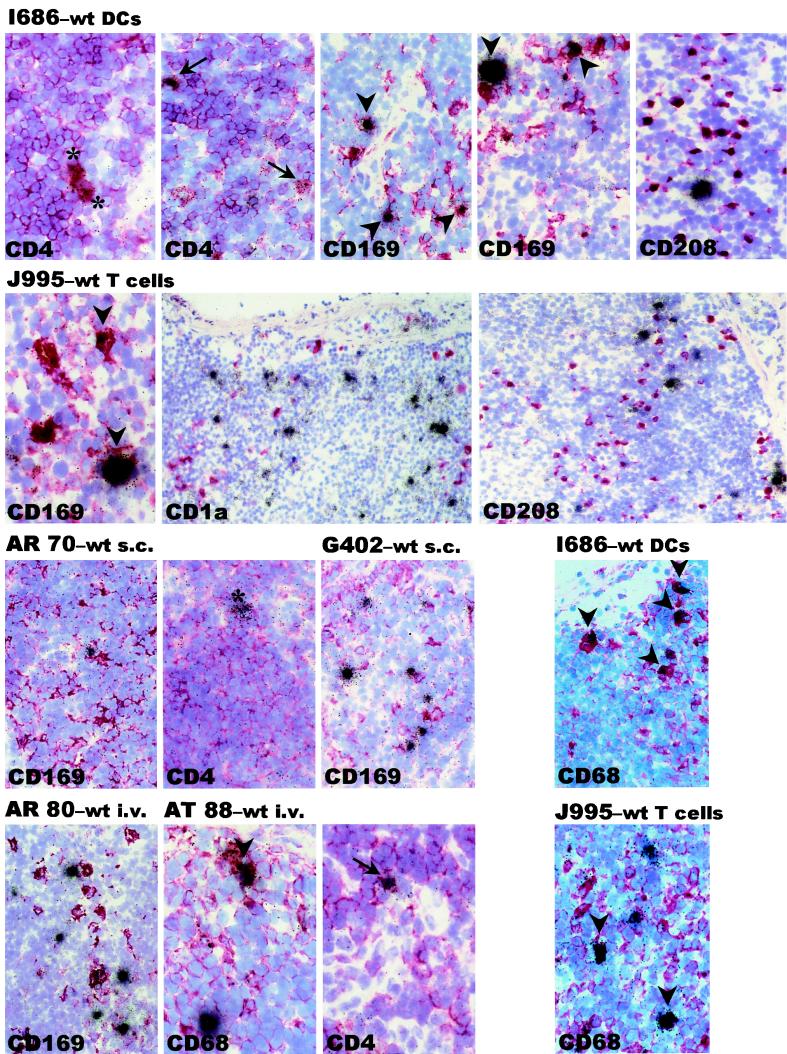
Increased numbers of virus-infected macrophages in the LNs of animals receiving cell-associated virus. Combined ISH and IH data for the draining LNs of six representative animals, biopsied 14 days after injection of cell-associated versus cell-free SIV, are shown. Animal I686 received wild-type virus (wt)-infected DCs, J995 received wild-type virus-infected T cells, AR 70 and G402 were injected with cell-free wild-type virus, and AR 80 and AT 88 were i.v. infected with wild-type virus. Frozen tissues were processed for IH against the indicated markers (red), counterstained with hemalaun (purple-blue), and followed by ISH for SIV RNA (black grains). Small CD4+ T cells (arrows, I686), large CD4+ cells (macrophages or activated T cells; asterisks, I686), and CD169+ and CD68+ macrophages (arrowheads, I686 and J995) that are positive for SIV are apparent in animals injected with either DC- or T-cell-associated SIV. SIV-positive cells are negative for the DC markers CD208 (I686 and J995) and CD1a (J995). The SIV-positive cells in AR 70 and G402 lacked CD169 but expressed CD4 (asterisk). The CD4+ cell expressing SIV in the example shown for AR 70 appears to be intermediate in size and is thus difficult to definitively identify as either a T cell or macrophage. SIV-positive cells in the cell-free SIV-infected i.v. controls (AR 80 and AT 88) are smaller CD4+ cells (arrow) with few CD68+ cells (arrowhead) that lack CD169. Magnifications, ×40 (CD1a and CD208 panels of J995) and ×100 (all other panels).
Similarly, we detected the heaviest infections in the T-cell areas of the day 14 LNs of animals s.c. injected with either cell-free or cell-associated virus (Table 4). Although punch biopsies were taken of each injection site on days 1, 3, 7, and 14 p.i. (coincident with the LN biopsies), we were unable to detect any virus-producing cells indicative of virus expansion directly at the injection sites. In the LNs virus-positive cells were rare before day 7 p.i. Again, considerably fewer virus-positive cells were apparent in animals receiving delta nef virus-pulsed DCs (Table 4). The higher plasma viral loads in the wild-type- compared to the delta nef virus-infected monkeys generally correlated with many fewer ISH-positive cells in the lymphoid tissues of the latter. Unfortunately, due to the limited numbers of SIV RNA-positive cells in the frozen tissues from the delta nef virus-infected animals (Table 4), we were unable to confidently identify the virus-positive cells in these tissues.
As observed after i.v. infection with cell-free virus, CD4+ cells were the major source of virus production in the tissues from animals s.c. infected with cell-free wild-type SIV (Fig. 4, animal AR 70). There were no SIV-positive CD1a+ or CD208+ DCs detected and very few (1 to 10%) SIV-positive, CD169+ macrophages (Fig. 4, AR 70 and G402). The numbers of SIV-positive cells and what percentages of these are CD169+ macrophages are summarized in Table 4. In striking contrast, the LNs of animals infected with cell-associated wild-type SIV included SIV-positive CD4+ T cells (Fig. 4, animal I686) and significant numbers of SIV-positive macrophages identified by using both CD169 and CD68 staining (Fig. 4, animals I686 and J995). As many as 25 to 50% of the SIV RNA-positive cells were CD169+ macrophages (Table 4). Some large CD4+ cells that may be activated T cells, macrophages, or DCs were detected (Fig. 4, animal I686). The possibility that the large CD4+ cells are DCs seems unlikely, since we observed no SIV-positive CD208+ or CD1a+ cells (Fig. 4, animals I686 and J995), also decreasing the likelihood that the SIV-positive CD68+ cells may include immature DCs. This was independent of the cell type that introduced the infectious virus. Figure 4 represents an overview highlighting the increased frequency of infected CD169+ macrophages in animals receiving cell-associated virus. Without performing three- or four-color confocal microscopy on thin sections to definitively identify the virus-positive cells (25), we cannot exclude the possibility that the CD169+ SIV-positive cells may represent SIV-positive T cells clustered closely to CD169+ macrophages. However, this seems unlikely due to the significant differences between the animals exposed to virus in the cell-free versus cell-associated forms, as one would expect that such observations would be made in tissues from both groups of animals. Therefore, unlike what is seen with when cell-free virus is administered via the i.v. or s.c. (see above) or mucosal (46) routes, s.c. injected cell-borne virus appears to result in increased infection of macrophages in addition to the T cells during the early stages of infection.
DISCUSSION
Both DCs and T cells can transmit SIV and HIV in vitro (13, 22) and likely drive the early stages of virus spread following mucosal exposure (16, 46, 47, 52). However, little is known about their potential to introduce virus and initiate infections in vivo. The present study provides direct evidence for the ability of DCs and T cells to efficiently transmit wild-type and nef-defective SIVmac239 after being pulsed with relatively small amounts of virus ex vivo. As expected from the i.v. infections (see, e.g., Fig. 3B and Table 3), wild-type virus introduced s.c. in either the cell-free or cell-associated form resulted in more robust virus infection and heavier virus loads than in delta nef virus-infected animals (Fig. 3A and Table 4). The need for nef to drive virus replication in the immature DC-T-cell milieu (34, 38) is probably being manifest here following s.c. delivery of virus into a similar cellular locale. Just as we observed in vitro (34), this occurs irrespective of whether the virus is added to the local milieu as free virus or is introduced by either immature DCs or CD4+ T cells. Of note, although the levels of virus replication were lower in animals receiving delta nef virus-pulsed cells, this virus did replicate to greater extents than we saw in our in vitro assay (34) and did not require the almost 250-times-more virus often used for cell-free i.v. infection.
In vitro, very little replication of the attenuated virus occurred in immature DC-T-cell mixtures, in contrast to that in the presence of mature DCs or if the immature DCs introduced the same amounts of wild-type virus (34). The differences between the levels of delta nef virus replication in vitro and in vivo could be due to possible maturation of the delta nef virus-bearing immature DCs after s.c. injection (4). The matured DCs could thereby overcome the need for nef for efficient viral replication as seen in vitro. However, while it could be different in vivo, the fact that activation or maturation of delta nef virus-carrying immature DCs in vitro did not result in enhancement of delta nef virus replication argues against this possibility (D. Messmer et al., submitted for publication). In contrast, activation of the T cells in such cultures readily rescued delta nef virus replication. Therefore, it is possible that in vivo there is at least low-level T-cell activation (not necessarily virus specific) that could facilitate greater amplification of immature DC-borne delta nef virus. Furthermore, should the virus-carrying immature DCs meet memory CD4+ T cells, such as those positioned at the body surfaces, this would only exacerbate virus spread, as observed in vitro (13) and in vivo (49, 50).
The transmission of infection by ex vivo virus-loaded DCs or T cells probably involves virus that has infected the cells as well as virus that is simply bound on the cell surface and/or trapped within intracellular compartments (12, 15, 37). T cells capture immunodeficiency viruses primarily via CD4 and chemokine coreceptors, while DCs can capture viruses via CD4, chemokine coreceptors, and members of the C-type lectin receptor family (13). The variable expression of these molecules by different DC subsets as well as by DCs at different stages of activation makes it likely that virus will utilize different receptors depending on the DC subset it meets and the route of transmission. Irrespective of the cell-cell interactions driving infection, this is the first study showing significant viral replication in non-T cells at an early stage of infection specifically following transmission of cell-associated virus. Previous studies utilizing cell-free virus applied to the mucosae highlight how DCs can capture and possibly replicate virus early (16) but that CD4+ T cells take over to further amplify virus levels (46, 47, 52). Comparably, in the present study, cell-free virus injected either i.v. or s.c. replicated predominantly in the CD4+ T-cell compartment, and infected macrophages probably contributed little to the infection (Table 4 and Fig. 4). In striking contrast, when virus was administered in CD4+ T-cell- or DC-associated forms, early virus replication was detected in both macrophages and CD4+ T cells. This shift in virus-producing cells to include up to 50% of CD169+ macrophages occurred with T-cell- or DC-borne virus. Virus-positive DCs could not be detected in significant numbers, suggesting that DCs favor transferring the virus to more permissive cell types while potentially providing signals (especially to resting T cells) to drive virus growth.
The mechanism for the enhanced macrophage tropism of wild-type SIV after inoculation in cell-bound form is under investigation. Recently, Igarashi et al. (19) reported that high numbers of infected macrophages could be detected only after the depletion of CD4+ T cells as a result of infection with the highly pathogenic SHIVDH12R. In our study, however, the LNs of animals inoculated with cell-bound virus did not reveal any major loss of CD4+ T cells that might identify a similar phenomenon. The fact that cell-free SIVmac239 replicated predominantly in CD4+ T cells while cell-associated SIVmac239 grew in both macrophages and T cells implies that it is the form of introducing the virus that determined the fate of the virus in this setting. It is possible that the s.c. injection of cell-borne SIV caused the secretion of chemokines and/or proinflammatory cytokines that lead to the recruitment of macrophages (and possibly T cells) and perhaps a minor inflammation at the site of injection. In this situation, the infected cells could simply hand over the virus to the attracted cells, which subsequently could migrate to the draining LNs and there amplify infection in concert with CD4+ T cells. On the other hand, although DCs not carrying SIV readily migrate to the draining LNs (reference 4 and unpublished observations) some (or all) of the injected virally infected cells could die at the site of injection or after having migrated to the draining LNs, and the resulting cellular debris could be picked up by macrophages or resident DCs. Both macrophages and DCs can pick up the remainder of apoptotic or necrotic cells via receptor-mediated endocytosis (1, 2). A recent report revealed that uptake of apoptotic debris augments HIV infection of macrophages in vitro (29), supporting the notion that a similar mechanism may contribute to enhanced macrophage infection in vivo. In the LNs, virus-laden macrophages could directly foster virus growth, while DCs carrying virus could transmit infection to neighboring CD4+ T cells. It is of course possible that the injected virus-carrying cells migrated directly to the draining LNs, where they interacted with the cellular milieu to augment virus growth. This further supports the idea of cell-cell communication needed for viral amplification, much as is observed in cocultures of infected DCs and T cells (13, 22). In vitro studies are under way to dissect the events dictating cell-cell transmission of virus.
Due to low numbers of SIV-positive cells in the tissues even after 14 days after delta nef virus infection (Table 4) we were unable to phenotype the cells that were the initial focus of virus replication in these settings. Thus, we still need to ascertain if there are differences between the cells fostering virus growth after cell-associated wild-type versus delta nef virus infection. However, recent studies applying cell-free delta nef virus to the mucosa (46) resulted in T-cell infection similar to that seen with wild-type virus (47). In addition, we cannot say whether the same profiles would apply to the most acute stages of infection or whether DCs may contribute at these earlier time points before virus is transmitted and exacerbated in the more permissive CD4+ T cells and macrophages.
This study provides the first report that SIV infection can readily be transmitted with virus carried by defined cell types, i.e., CD4+ T cells and DCs. By investigating the sites of early viral replication, we have revealed significant differences in the cells forging virus growth in a setting mimicking cell-bound versus cell-free virus that has breached the body surface. The pronounced involvement of macrophages following cell-associated infection might have considerable impact on the subsequent induction of immune responses and on the development of strategies to prevent virus transmission.
Acknowledgments
All animals used in this study were housed in the Tulane Regional Primate Research Center, and additional thanks go to Richard Rockar and Marion Ratterree at the Tulane Regional Primate Research Center for expert care and handling of our macaques. We are grateful to Peter J. Dailey and Casey Wingfield, Bayer Reference Testing Laboratory, Bayer Diagnostics, Berkley, Calif., who performed the bDNA analyses. We sincerely thank Judy Adams for assistance with graphics and Gudrun Groβschupff, Petra Meyer, and Birgit Raschdorff for excellent technical assistance. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 174xCEM from Peter Cresswell and the fascin MAb (55 kDa) from Dako Corporation.
This work was funded by amfAR, National Institutes of Health grants AI44335 and AI40877, the Irma T. Hirschl Trust, the Campbell Foundation, and the Rockefeller Foundation (to M.P.); National Institutes of Health grant AI40874 (to R.M.S.); the H. W. and J. Hector Foundation (to R.I.); and grant QLRT-PL 1999-01215 from the European Community (to K.T.-R., P.R., and R.I.).
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Albert, M. L., S. F. A. Pearce, L. M. Francisco, B. Sauter, P. Roy, R. L. Silverstein, and N. Bhardwaj. 1998. Immature dendritic cells phagocytose apoptotic cells via avb5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Barratt-Boyes, S. M., M. I. Zimmer, L. A. Harshyne, E. M. Meyer, S. C. Watkins, S. Capuano III, M. Murphey-Corb, L. D. Falo, Jr., and A. D. Donnenberg. 2000. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J. Immunol. 164:2487-2495. [DOI] [PubMed] [Google Scholar]
- 5.Cameron, P. U., P. S. Freudenthal, J. M. Barker, S. Gezelter, K. Inaba, and R. M. Steinman. 1992. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257:383-387. [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti, L., M. Guyader, M. Alizon, M. D. Daniel, R. C. Desrosiers, P. Tiollais, et al. 1987. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature 328:543-547. [DOI] [PubMed] [Google Scholar]
- 7.Connor, R. I., D. C. Montefiori, J. M. Binley, J. P. Moore, S. Bonhoeffer, A. Gettie, E. A. Fenamore, K. E. Sheridan, D. D. Ho, P. J. Dailey, and P. A. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, V. A. Lawson, S. Crowe, A. Maerz, S. Sonza, J. Learmont, J. S. Sullivan, A. Cunningham, D. Dwyer, D. Dowton, and J. Mills. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 9.de Saint-Vis, B., J. Vincent, S. Vandenabeele, B. Vanbervliet, J.-J. Pin, S. Ait-Yahia, S. Patel, M.-G. Mattei, J. Banchereau, S. Zurawski, J. Davoust, C. Caux, and S. Lebecque. 1998. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity 9:325-336. [DOI] [PubMed] [Google Scholar]
- 10.Dhodapkar, M., R. M. Steinman, M. Sapp, H. Desai, C. Fossella, J. Krasovsky, S. M. Donahoe, P. R. Dunbar, V. Cerundolo, D. F. Nixon, and N. Bhardwaj. 1999. Rapid generation of broad T-cell immunity in humans after single injection of mature dendritic cells. J. Clin. Investig. 104:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhodapkar, M. V., J. Krasovsky, R. M. Steinman, and N. Bhardwaj. 2000. Mature dendritic cells boost functionally superior T cells in humans without foreign helper epitopes. J. Clin. Investig. 105:R9-R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank, I., M. J. Piatak, H. Stoessel, N. Romani, D. Bonnyay, J. D. Lifson, and M. Pope. 2002. Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): Differential intracellular fate of virions in mature and immature DCs. J. Virol. 76:2936-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank, I., and M. Pope. 2002. The enigma of dendritic cell-immunodeficiency virus interplay. Curr. Mol. Med. 2:229-248. [DOI] [PubMed] [Google Scholar]
- 14.Granelli-Piperno, A., E. Delgado, V. Finkel, W. Paxton, and R. M. Steinman. 1998. Immature dendritic cells selectively replicate M-tropic human immunodeficiency virus type 1, while mature cells efficiently transmit both M- and T-tropic virus to T cells. J. Virol. 72:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grewe, C., A. Beck, and H. R. Gelderblom. 1990. HIV: early virus-cell interactions. J. AIDS 3:965-974. [PubMed] [Google Scholar]
- 16.Hu, J., M. B. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu, J., C. J. Miller, U. O'Doherty, P. A. Marx, and M. Pope. 1999. The dendritic cell-T cell milieu of the lymphoid tissue of the tonsil provides a locale in which SIV can reside and propagate at chronic stages of infection. AIDS Res. Hum. Retrovir. 15:1305-1314. [DOI] [PubMed] [Google Scholar]
- 18.Hu, J., M. Pope, U. O'Doherty, C. Brown, and C. J. Miller. 1998. Immunophenotypic characterization of SIV-infected cells in cervix, vagina and draining lymph nodes of chronically infected rhesus macaques. Lab. Investig. 78:435-451. [PubMed] [Google Scholar]
- 19.Igarashi, T., C. R. Brown, Y. Endo, A. Buckler-White, R. Plishka, N. Bischofberger, V. Hirsch, and M. A. Martin. 2001. Macrophage are the principal reservoir and sustain high virus loads in rhesus macaques after the depletion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ignatius, R., F. Isdell, U. O'Doherty, and M. Pope. 1998. Dendritic cells from skin and blood of macaques both promote SIV replication with T cells from different anatomical sites. J. Med. Primatol. 27:121-128. [DOI] [PubMed] [Google Scholar]
- 21.Ignatius, R., K. Mahnke, M. Rivera, K. Hong, F. Isdell, R. M. Steinman, M. Pope, and L. Stamatatos. 2000. Presentation of proteins encapsulated into sterically stabilized liposomes by dendritic cells initiates CD8+ T cell responses in vivo. Blood 96:3505-3513. [PubMed] [Google Scholar]
- 22.Ignatius, R., R. M. Steinman, A. Granelli-Piperno, D. Messmer, C. Stahl-Hennig, K. Tenner-Racz, P. Racz, I. Frank, L. Zhong, S. Schlesinger-Frankel, and M. Pope. 2001. Dendritic cells during infection with HIV-1 and SIV, p. 487-504. In M. T. Lotze and A. W. Thomson (ed.), Dendritic cells, 2nd ed. Academic Press, London, United Kingdom.
- 23.Johnson, R. P., and R. C. Desrosiers. 1998. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr. Opin. Immunol. 10:436-443. [DOI] [PubMed] [Google Scholar]
- 24.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 25.Kornack, D. R., and P. Rakic. 2001. Cell proliferation without neurogenesis in adult primate neocortex. Science 294:2127-2130. [DOI] [PubMed] [Google Scholar]
- 26.Krenacs, L., L. Tiszalvicz, T. Krenacs, and L. Boumsell. 1993. Immunohistochemical detection of CD1A antigen in formalin-fixed and paraffin-embedded tissue sections with monoclonal antibody 010. J. Pathol. 171:99-104. [DOI] [PubMed] [Google Scholar]
- 27.Learmont, J., B. Tindall, L. Evans, A. Cunningham, P. Cunningham, J. Wells, R. Penny, J. Kaldor, and D. A. Cooper. 1992. Long-term symptomless HIV-1 infection in recipients of blood products from a single donor. Lancet 340:863-867. [DOI] [PubMed] [Google Scholar]
- 28.Learmont, J. C., A. F. Geczy, J. Mills, L. J. Ashton, C. H. Raynes-Greenow, R. J. Garsia, W. B. Dyer, L. McIntyre, R. B. Oelrichs, D. I. Rhodes, N. J. Deacon, and J. S. Sullivan. 1999. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1. A report from the Sydney Blood Bank Cohort. N. Engl. J. Med. 340:1715-1722. [DOI] [PubMed] [Google Scholar]
- 29.Lima, R. G., J. Van Weyenbergh, E. M. Saraiva, M. Barral-Netto, B. Galvao-Castro, and D. C. Bou-Habib. 2002. The replication of human immunodeficiency virus type 1 in macrophages is enhanced after phagocytosis of apoptotic cells. J. Infect. Dis. 185:1561-1566. [DOI] [PubMed] [Google Scholar]
- 30.Martinez-Pomares, L., N. Platt, A. J. McKnight, R. P. da Silva, and S. Gordon. 1996. Macrophage membrane molecules: markers of tissue differentiation and heterogeneity. Immunobiology 195:407-416. [DOI] [PubMed] [Google Scholar]
- 31.Marx, P. A., R. W. Compans, A. Gettie, J. K. Staas, R. M. Gilley, M. J. Mulligan, G. V. Yamshchikov, D. Cheng, and J. H. Eldridge. 1993. Protection against vaginal SIV transmission with microencapsulated vaccine. Science 260:1323-1327. [DOI] [PubMed] [Google Scholar]
- 32.Marx, P. A., A. I. Spira, A. Gettie, P. J. Dailey, R. S. Veazey, A. A. Lackner, C. J. Mahoney, C. J. Miller, L. E. Claypool, D. D. Ho, and N. J. Alexander. 1996. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2:1084-1089. [DOI] [PubMed] [Google Scholar]
- 33.Mehlhop, E. R., L. A. Villamide, I. Frank, A. Gettie, C. Santisteban, D. Messmer, R. Ignatius, J. D. Lifson, and M. Pope. 2002. Enhanced in vitro stimulation of rhesus macaque dendritic cells for activation of SIV-specific T cell responses. J. Immunol. Methods 260:219-234. [DOI] [PubMed] [Google Scholar]
- 34.Messmer, D., R. Ignatius, C. Santisteban, R. M. Steinman, and M. Pope. 2000. The decreased replicative capacity of SIVmac239 Δnef is manifest in cultures of immature dendritic cells and T cells. J. Virol. 74:2406-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munday, J., H. Floyd, and P. R. Crocker. 1999. Sialic acid binding receptors (siglecs) expressed by macrophages. J. Leukoc. Biol. 66:705-711. [DOI] [PubMed] [Google Scholar]
- 36.O'Doherty, U., R. Ignatius, N. Bhardwaj, and M. Pope. 1997. Generation of monocyte-derived cells from the precursors in rhesus macaque blood. J. Immunol. Methods 207:185-194. [DOI] [PubMed] [Google Scholar]
- 37.Pauza, C. D., and T. M. Price. 1988. Human immunodeficiency virus infection of T cells and monocytes proceeds via receptor-mediated endocytosis. J. Cell Biol. 107:959-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petit, C., F. Buseyne, C. Boccaccio, J. P. Abastado, J. M. Heard, and O. Schwartz. 2001. Nef is required for efficient HIV-1 replication in cocultures of dendritic cells and lymphocytes. Virology 286:225-236. [DOI] [PubMed] [Google Scholar]
- 39.Pope, M., M. G. H. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 40.Pope, M., D. Elmore, D. Ho, and P. Marx. 1997. Dendritic cell-T cell mixtures, isolated from the skin and mucosae of macaques, support the replication of SIV. AIDS Res. Hum. Retrovir. 13:819-827. [DOI] [PubMed] [Google Scholar]
- 41.Pope, M., S. Gezelter, N. Gallo, L. Hoffman, and R. M. Steinman. 1995. Low levels of HIV-1 in cutaneous dendritic cells promote extensive viral replication upon binding to memory CD4+ T cells. J. Exp. Med. 182:2045-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reimann, K. A., K. Tenner-Racz, P. Racz, D. C. Montefiori, Y. Yasutomi, W. Lin, B. J. Ransil, and N. L. Letvin. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J. Virol. 68:2362-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinclair, E., P. Barbosa, and M. B. Feinberg. 1997. The nef gene products of both simian and human immunodeficiency viruses enhance virus infectivity and are functionally interchangeable. J. Virol 71:3641-3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith, S. M., B. Holland, C. Russo, P. J. Dailey, P. A. Marx, and R. I. Connor. 1999. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res. Hum. Retrovir. 15:1691-1701. [DOI] [PubMed] [Google Scholar]
- 45.Spina, C. A., H. E. Prince, and D. D. Richman. 1997. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J. Clin. Investig. 99:1774-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stahl-Hennig, C., R. M. Steinman, P. Ten Haaft, K. Uberla, N. Stolte, S. Saeland, K. Tenner-Racz, and P. Racz. 2002. The simian immunodeficiency virus Δnef vaccine, after application to the tonsils of rhesus macaques, replicates primarily within CD4+ T cells and elicits a local perforin-positive CD8+ T-cell response. J. Virol. 76:688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 48.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 49.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vesanen, M., C. E. Stevens, P. E. Taylor, P. Rubinstein, and K. Saksela. 1996. Stability in controlling viral replication identifies long-term nonprogressors as a distinct subgroup among human immunodeficiency virus type 1-infected persons. J. Virol. 70:9035-9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willerford, D. M., M. J. J. Gale, R. E. Benveniste, E. A. Clark, and W. M. Gallatin. 1990. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J. Immunol. 144:3779-3783. [PubMed] [Google Scholar]
- 52.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]