Abstract
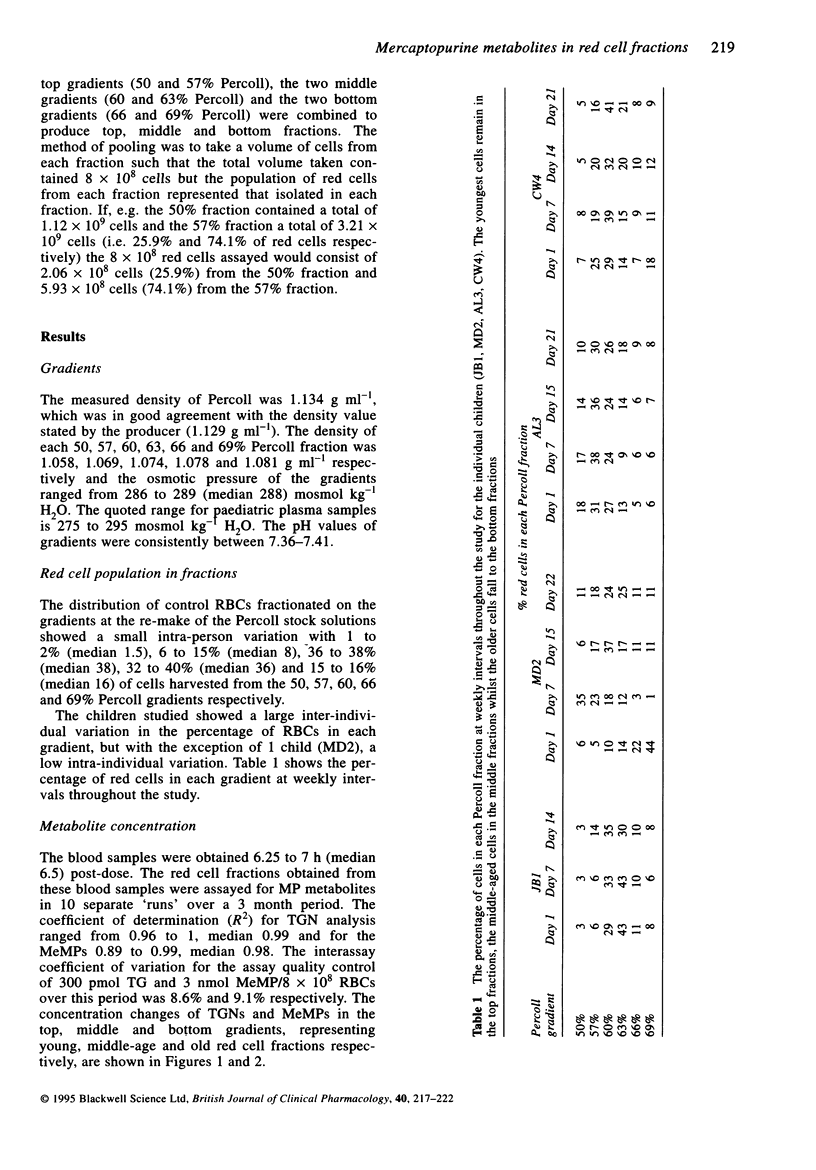
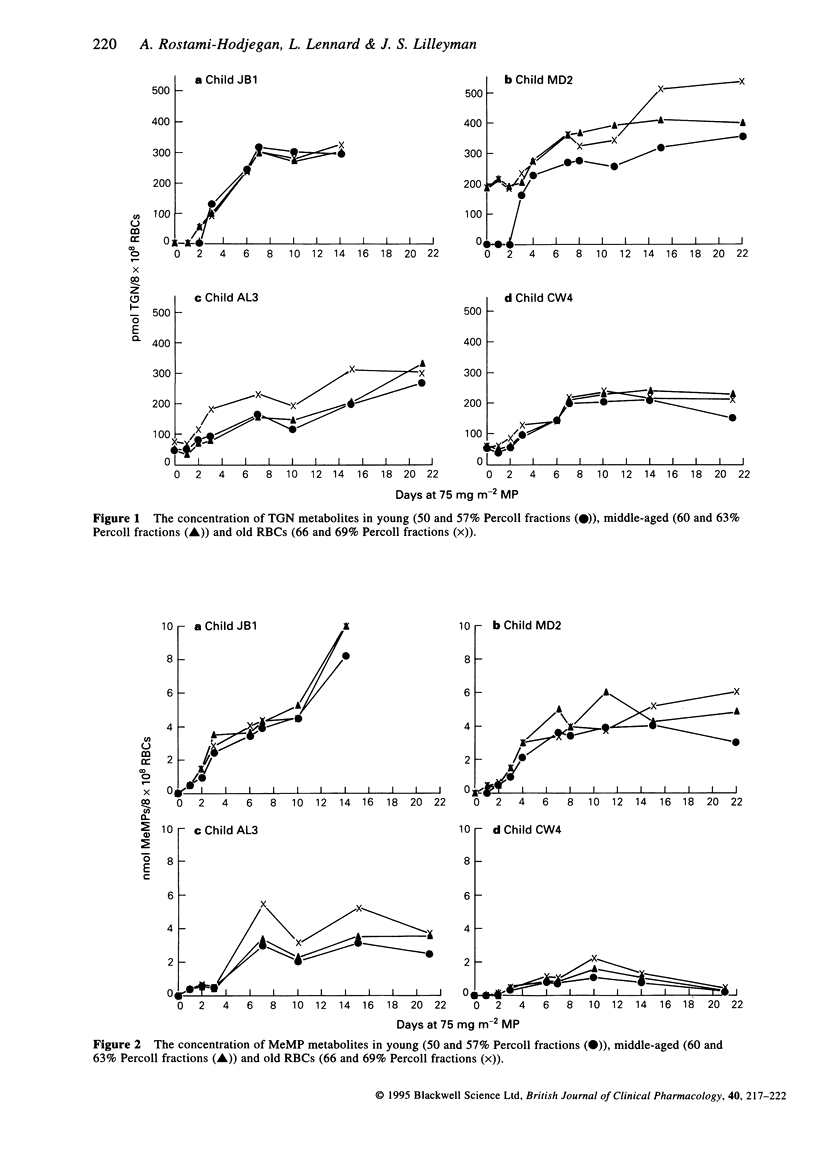
1. Red blood cells from four children with lymphoblastic leukemia were age fractionated on Percoll density gradients into 'young', 'middle-aged' and 'old' cells. 2. The rates of accumulation of the mercaptopurine (MP) metabolites thioguanine nucleotides (TGNs) and methylmercaptopurine nucleotides (MeMPs) were measured in the cell fractions from the start of MP continuing chemotherapy. 3. TGNs and MeMP metabolites were present in all the red cell fractions after 3 days oral MP. There was no significant difference between the metabolite concentrations measured in either young, middle-aged or old cells (Mann-Whitney P = 1.0 to 0.12). 4. These observations suggest that MP metabolites do not enter red cells at the stem cell level at the start of therapy. 5. With respect to the monitoring of therapy, these results suggest that the concentration of TGNs after 7 to 10 days MP could be used to predict eventual steady-state concentrations using a simple model.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. A., Black D. A., Human L., Harley E. H. Oxypurine cycle in human erythrocytes regulated by pH, inorganic phosphate, and oxygen. J Clin Invest. 1988 Sep;82(3):980–986. doi: 10.1172/JCI113707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. A., Human L. Regulation of 5-phosphoribosyl 1-pyrophosphate and of hypoxanthine uptake and release in human erythrocytes by oxypurine cycling. J Biol Chem. 1990 Apr 25;265(12):6562–6568. [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Chalmers A. H., Atkinson M. R., Burgoyne L. A., Murray A. W. Evidence for the utilization of preformed purines by rat lymphoid tissues: implication to immunosuppression by 6-thiopurine analogues. Aust J Exp Biol Med Sci. 1971 Apr;49(2):177–183. doi: 10.1038/icb.1971.16. [DOI] [PubMed] [Google Scholar]
- Koepke J. F., Koepke J. A. Reticulocytes. Clin Lab Haematol. 1986;8(3):169–179. doi: 10.1111/j.1365-2257.1986.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Lena N., Imbert A. M., Brunet P., Cano J. P., Carcassonne Y. Kinetics of methotrexate and its metabolites in red blood cells. Cancer Drug Deliv. 1987;4(2):119–127. doi: 10.1089/cdd.1987.4.119. [DOI] [PubMed] [Google Scholar]
- Lennard L., Gibson B. E., Nicole T., Lilleyman J. S. Congenital thiopurine methyltransferase deficiency and 6-mercaptopurine toxicity during treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1993 Nov;69(5):577–579. doi: 10.1136/adc.69.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Keen D., Lilleyman J. S. Oral 6-mercaptopurine in childhood leukemia: parent drug pharmacokinetics and active metabolite concentrations. Clin Pharmacol Ther. 1986 Sep;40(3):287–292. doi: 10.1038/clpt.1986.178. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Are children with lymphoblastic leukaemia given enough 6-mercaptopurine? Lancet. 1987 Oct 3;2(8562):785–787. doi: 10.1016/s0140-6736(87)92511-6. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S., Van Loon J., Weinshilboum R. M. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990 Jul 28;336(8709):225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- Lennard L., Lilleyman J. S. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989 Dec;7(12):1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- Lennard L., Maddocks J. L. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983 Jan;35(1):15–18. doi: 10.1111/j.2042-7158.1983.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Lennard L., Rees C. A., Lilleyman J. S., Maddocks J. L. Childhood leukaemia: a relationship between intracellular 6-mercaptopurine metabolites and neutropenia. Br J Clin Pharmacol. 1983 Oct;16(4):359–363. doi: 10.1111/j.1365-2125.1983.tb02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennard L., Singleton H. J. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr. 1992 Nov 27;583(1):83–90. doi: 10.1016/0378-4347(92)80347-s. [DOI] [PubMed] [Google Scholar]
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- Lilleyman J. S., Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet. 1994 May 14;343(8907):1188–1190. doi: 10.1016/s0140-6736(94)92400-7. [DOI] [PubMed] [Google Scholar]
- Mager J., Dvilansky A., Razin A., Wind E., Izak G. Turnover of purine nucleotides in human red blood cells. Isr J Med Sci. 1966 May-Jun;2(3):297–301. [PubMed] [Google Scholar]
- Parks R. E., Jr, Crabtree G. W., Kong C. M., Agarwal R. P., Agarwal K. C., Scholar E. M. Incorporation of analog purine nucleosides into the formed elements of human blood: erythrocytes, platelets, and lymphocytes. Ann N Y Acad Sci. 1975 Aug 8;255:412–434. doi: 10.1111/j.1749-6632.1975.tb29249.x. [DOI] [PubMed] [Google Scholar]
- Piomelli S., Seaman C. Mechanism of red blood cell aging: relationship of cell density and cell age. Am J Hematol. 1993 Jan;42(1):46–52. doi: 10.1002/ajh.2830420110. [DOI] [PubMed] [Google Scholar]
- Salvo G., Caprari P., Samoggia P., Mariani G., Salvati A. M. Human erythrocyte separation according to age on a discontinuous "Percoll" density gradient. Clin Chim Acta. 1982 Jul 1;122(2):293–300. doi: 10.1016/0009-8981(82)90290-x. [DOI] [PubMed] [Google Scholar]
- Schrøder H. Methotrexate pharmacokinetics in age-fractionated erythrocytes. Cancer Chemother Pharmacol. 1986;18(3):203–207. doi: 10.1007/BF00273386. [DOI] [PubMed] [Google Scholar]
- Stet E. H., De Abreu R. A., Bökkerink J. P., Blom H. J., Lambooy L. H., Vogels-Mentink T. M., de Graaf-Hess A. C., van Raay-Selten B., Trijbels F. J. Decrease in S-adenosylmethionine synthesis by 6-mercaptopurine and methylmercaptopurine ribonucleoside in Molt F4 human malignant lymphoblasts. Biochem J. 1994 Nov 15;304(Pt 1):163–168. doi: 10.1042/bj3040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidd D. M., Paterson A. R. A biochemical mechanism for the delayed cytotoxic reaction of 6-mercaptopurine. Cancer Res. 1974 Apr;34(4):738–746. [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- da Costa M., Iqbal M. P. The transport and accumulation of methotrexate in human erythrocytes. Cancer. 1981 Dec 1;48(11):2427–2432. doi: 10.1002/1097-0142(19811201)48:11<2427::aid-cncr2820481115>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


