Abstract
The thymus is responsible for de novo production of CD4+ and CD8+ T cells and therefore is essential for T-cell renewal. The goal of this study was to assess the impact of simian immunodeficiency virus (SIV) infection on the production of T cells by the thymus. Levels of recent thymic emigrants within the peripheral blood were assessed through quantification of macaque T-cell receptor excision circles (TREC). Comparison of SIV-infected macaques (n = 15) to uninfected macaques (n = 23) revealed stable or increased TREC levels at 20 to 34 weeks postinfection. Further assessment of SIV-infected macaques (n = 4) determined that TREC levels decreased between 24 and 48 weeks postinfection. Through the assessment of longitudinal time points in three additional SIVmac239-infected macaques, the SIV infection was divided into two distinct phases. During phase 1 (16 to 30 weeks), TREC levels remained stable or increased within both the CD4 and CD8 T-cell populations. During phase 2 (after 16 to 30 weeks), TREC levels declined in both T-cell populations. As has been described for human immunodeficiency virus (HIV)-infected patients, this decline in TREC levels did at times correlate with an increased level of T-cell proliferation (Ki67+ cells). However, not all TREC decreases could be attributed to increased T-cell proliferation. Further evidence for thymic dysfunction was observed directly in a SIVmac239-infected macaque that succumbed to simian AIDS at 65 weeks postinfection. The thymus of this macaque contained an increased number of memory/effector CD8+ T cells and an increased level of apoptotic cells. In summary, reduced levels of TREC can be observed beginning at 16 to 30 weeks post-SIV infection and correlate with changes indicative of dysfunction within the thymic tissue. SIV infection of macaques will be a useful model system to elucidate the mechanisms responsible for the thymic dysfunction observed in HIV-infected patients.
The thymus is responsible for de novo production of CD4+ and CD8+ cells from T-cell progenitors. Naïve T cells from the thymus comprise a diverse T-cell receptor repertoire that is critical for the maintenance of a competent immune system (20, 23, 33). In normal healthy adults, the importance of a functional thymus is not clear; however, the thymus may be critical in human immunodeficiency virus (HIV) disease, in which T cells are being destroyed in large numbers (22, 59). The evidence to date suggests that HIV infection impairs thymic function (1, 4, 16, 24, 27, 29, 36, 39, 46). Infants infected with HIV, particularly those with detectable levels of virus at birth, have morphological evidence of thymic dysfunction (16, 39). Experiments in the SCID-hu mouse model have determined that specific thymocyte subsets are infected and illustrate how HIV infection may impair thymopoiesis (1, 24, 36). Understanding the effect of HIV on the thymus is crucial to the design of strategies to enhance immune reconstitution in infected patients treated with antiviral therapy.
There are no practical ways to distinguish phenotypically between cells that have recently emigrated from the thymus and long-lived naïve cells in the periphery. However, the episomal DNA molecules formed during excisional rearrangement of the T-cell receptor genes within the thymus can be used as an additional marker for recent thymic emigrants in the peripheral blood (26, 55, 56). These products, termed T-cell receptor rearrangement excision circles (TREC), are stable (30), do not duplicate during mitosis, and are subsequently diluted out with each cell division (50, 55). In HIV-infected patients, particularly children, TREC levels are decreased in both the CD4+ and CD8+ T-cell subsets (8-10, 61). There is a correlation between the degree to which TREC levels decline and the rate of disease progression (14). In addition, following administration of highly active antiretroviral therapy to HIV-infected adults, TREC levels were generally found to increase (10, 47). Highly active antiretroviral therapy-treated patients who exhibit a failure of thymic T-cell production may exhibit a good virological response that is not accompanied by a substantial increase in CD4+ T cells (51).
Infection of rhesus macaques with simian immunodeficiency virus (SIV) can result in the onset of simian AIDS, generally within 6 months to 2 years postinfection (21, 41, 45, 58). SIV-infected cells within the thymus can be observed by 7 days postinfection (60). The majority of infected thymocytes and thymic macrophages are present within the thymic medulla, where mature thymocytes reside (28, 60). A study addressing thymic changes during the acute phase of infection indicated that evidence of thymic dysfunction during days 7, 14, and 21 postinfection are partially reversed by day 50 postinfection (60). The thymic function found at 50 days postinfection is unlikely to be long lived, as SIV infection generally results in thymic involution and histopathogenic lesions (2, 28, 60).
Our goal was to assess thymic function in SIV-infected macaques without the requirement that the macaques be sacrificed. In previous studies, we demonstrated that the TREC assay could be used to assess levels of recent thymic emigrants in the peripheral blood of both rhesus macaques and sooty mangabeys (44). Here, TREC levels were assessed in SIV-infected macaques in both cross-sectional and longitudinal analyses. These analyses enabled the SIV disease course to be divided into two distinct phases. Phase 1 (16 to 30 weeks) involves stable or increasing TREC levels, and phase 2 (after 16 to 30 weeks) is characterized by a steady decline in TREC levels. Assessment of the thymus at necropsy identified an increased number of memory/effector CD8+ T cells and an increased level of apoptotic/terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL)-positive cells, similar to findings observed during HIV infection (17, 48). In summary, reduced levels of TREC within the peripheral blood can be observed beginning at 16 to 30 weeks post-SIV infection and correlate with changes indicative of dysfunction within the thymic tissue.
MATERIALS AND METHODS
SIV infection of rhesus macaques (Macaca mulatta).
Uninfected macaques assessed in the cross-sectional analysis have been described previously (44). The SIV-positive macaques assessed in the cross-sectional analysis were being used in ongoing studies at the New England Regional Primate Research Center (n = 4), the California Regional Primate Research Center (n = 3), and Bioqual Research Laboratories (Rockville, Md.) (n = 8). These macaques were infected intravenously with either SIVmac239 (n = 4; ages 0.7, 1.5, 1.5, and 1.5 years), SIVmac251 (n = 3; ages 2, 3, and 4 years), or SIVE660 (n = 8; ages 3, 3.5, 4, 4, 4, 4, 4, and 6.5 years) (37).
Peripheral blood samples were obtained from these infected macaques at 20 to 34 weeks postinfection. Samples from four of the 4-year-old SIVE660-infected macaques were obtained at both 24 and 48 weeks postinfection. In addition, three macaques (RSm5 [RM1], RTq5 [RM2], and RUh5 [RM3]) were infected with 2.5 × 105 50% tissue culture infectious doses (determined in CEMx174 cells) of SIVmac239 at the Yerkes Regional Primate Research Center and followed longitudinally. All animals were maintained in accordance with National Institutes of Health guidelines, and appropriate approvals were obtained from the local Animal Care and Use committees.
Plasma viral RNA.
The SIV plasma viral loads were measured by real-time PCR as described previously (49).
Measurement of TREC in sorted cells.
The sorting of peripheral blood mononuclear cells (PBMC) by magnetic-activated cell sorting (Miltenyi Corp.) into CD8+ and CD8-depleted T-cell populations (to obtain CD4+ TREC levels) was performed as previously described (44). TREC levels in Fig. 1 and 2 were obtained by quantitative competitive PCR as previously described (38, 44). The TREC analysis presented in Fig. 4 was quantified by real-time PCR as described for humans (11). The sequences of the primers (5′-CACATCCCTTTCAACCATGCT-3′ and 5′-GCCAGCTGCAGGGTTTAGG-3′) (amplifying a 108-bp fragment) and probe (5′-FAM-ACGCCTCTGGTTTTTGTAAAGGTGCTCACT-QSY-3′ [Megabases Inc., Chicago, Ill.]) were altered from the human sequences to match macaque sequences.
FIG. 1.
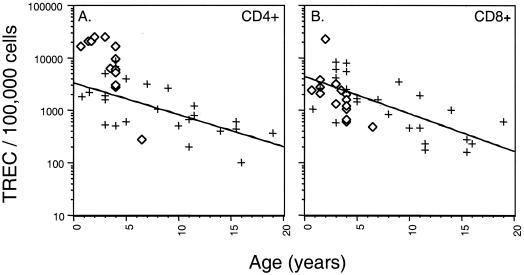
Analysis of TREC levels during SIV infection in macaques. TREC levels within 100,000 CD4+ (A) and CD8+ (B) cells are depicted for 23 uninfected (+) and 15 SIV-infected (⋄) animals. At the time of sampling, the SIV-positive macaques had been infected for 6 to 8 months. Three different viral strains were examined, SIVmac239 (four animals), SIVmac251 (three animals), and SIVE660 (eight animals). SIV-positive TREC levels were highest in the youngest macaques and declined with age. No apparent isolate-specific differences in TREC levels were observed between the SIVmac239-, SIVmac251-, and SIVE660-infected macaques.
FIG. 2.
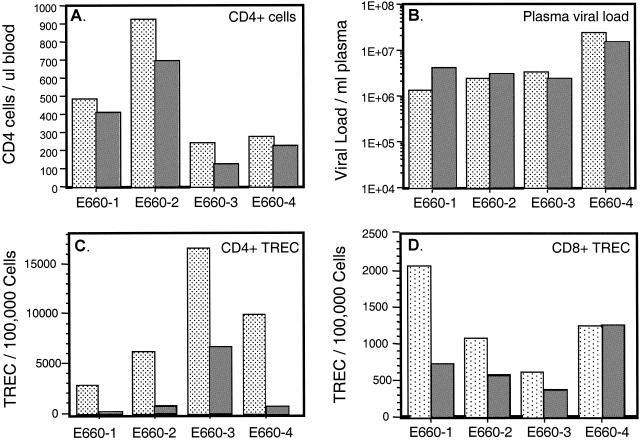
TREC levels were assessed within SIVE660-infected macaques at 25 weeks (dotted columns) and 48 weeks (shaded columns) postinfection. The level of CD4+ T cells in blood (A) and the SIV viral load per milliliter of blood plasma (B) are shown for each macaque. TREC levels were determined by quantitative competitive PCR (38, 44) and are presented for the CD4+ (C) and CD8+ (D) T cells.
FIG. 4.
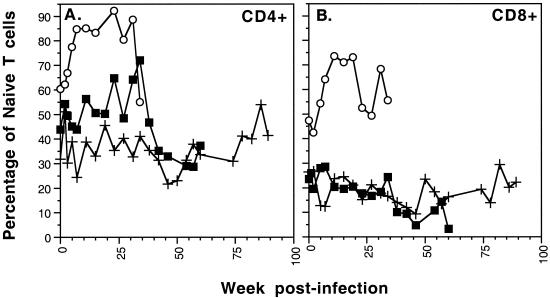
Longitudinal analysis of naïve T-cell levels in SIVmac239-infected macaques RM1 (○), RM2 (▪), and RM3 (+). The percentages of CD4+ (A) and CD8+ (B) cells with a naïve phenotype (CD45RA+ CD62L+) are plotted for each macaque.
All samples and standards were assessed in duplicate. TREC DNA standards ranged from 102 to 107 TREC molecules and were used to determine TREC levels within a cell lysate containing 100,000 cells. Lysates were assessed to ensure that each contained similar amounts of PCR-amplifiable DNA with the Taqman glyceraldehyde-3-phosphate dehydrogenase control reagent (PE Biosystems). The addition of the glyceraldehyde-3-phosphate dehydrogenase normalization increases our confidence that the TREC changes observed in the CD8+ populations are not due to differences in the preparation of the cells or inhibitors in the cell lysate. Real-time PCR was performed on the ABI Prism 7700 sequence detector (Applied Biosystems).
Monoclonal antibodies used for flow cytometry.
The antibodies used for immunophenotyping of rhesus peripheral blood mononuclear cells were anti-CD4 (clone SK3); anti-CD8 (clone SK1); anti-CD45RA (clone L48) and anti-CD62L (clone SK11) (Becton Dickinson Immunocytometry Systems, San Jose, Calif.); and anti-CD3ɛ (clone SP34) (Pharmingen). In addition, antibodies used for immunophenotyping of thymocytes were anti-CCR5 (clone 3A9) (Pharmingen), anti-CD95 (clone DX2) (Becton Dickinson Immunocytometry Systems), and anti-CD11a (clone 25.3) (Immunotech). For intracellular Ki67 staining, anti-Ki67 monoclonal antibody (clone R0840) (Dako Corp.) directed toward the nuclear antigen Mib-1 was used. The antibodies were conjugated to the following fluorophores: fluorescein isothiocyanate (CD45RA, CD3, and CD11a), phycoerythrin (CD62L, CCR5, CD4, and Ki67), peridinin chlorophyll protein (CD8), and allophycocyanin (CD4 and CD95).
Isolation and immunophenotyping of PBMC.
PBMC were isolated via a Ficoll Hypaque gradient and assessed with four fluorometric markers to determine the absolute number of specific cell subpopulations. Staining was performed in 1× phosphate-buffered saline (PBS), 1% bovine serum albumin and 0.1% NaN3 (fluorescence-activated cell sorting [FACS] buffer). Antibodies were added to 500,000 to 1,000,000 cells and incubated for 20 min on ice in the dark. For intracellular Ki67 staining, PBMC were first stained with anti-CD4, anti-CD8, and anti-CD3. They were then fixed and permeabilized with Becton Dickinson FACS lysing solution with 0.025% Tween 20, washed twice in FACS buffer, and stained with the Ki67 monoclonal antibody. After the antibody incubations were completed, the cells were washed in FACS buffer and fixed in 1% paraformaldehyde.
Isolation and immunophenotyping of thymocytes.
Thymocytes were obtained from macaque RM2 at necropsy (4.5 years of age) and macaques 30379 and 30381 (3.5 years of age). Macaques 30379 and 30381 had been exposed to an oral inoculation (SIVmac251) 2 days prior to necropsy and did not contain detectable virus within the thymus. Thymic tissue was subjected to mechanical disruption and filtration through a 70-μm nylon mesh (Falcon). After collection, thymocytes were placed in FACS buffer, and the appropriate antibodies were added to 500,000 to 1,000,000 cells. After the antibody incubations were completed, the cells were washed in FACS buffer and fixed in 1% paraformaldehyde.
Flow cytometry.
Flow cytometric analysis of samples was performed on 50,000 acquired events with a FACScalibur flow cytometer (Becton Dickinson). Analyses of these data were undertaken with Paint-A-Gate software (Becton Dickinson). Cells were gated with forward and side scatter by a generous lymphocyte gate, so that dividing lymphocytes were also included.
In situ TUNEL assay and immunohistochemistry.
Prior to infection, a small biopsy sample was obtained from the thymus of animals RM1, RM2, and RM3. Thymus samples obtained at biopsy or necropsy were placed in Streck tissue fixative buffer (Streck Laboratories, Inc., Omaha, Neb.) and then paraffin embedded. Sections (5 μm) were placed onto slides, the paraffin was removed in xylene, and the samples were rehydrated in 100, 95, then 70% ethanol and finally washed in PBS (pH 7.4). Tissue sections were then placed on ice in a citrate buffer with Triton X-100 (Sigma) for 5 min. After three washes in PBS, the TUNEL reaction mixture (In Situ Cell Death Detection Kit; Roche, Indianapolis, Ind.) was applied to the tissues and incubated at 37°C in a humidity chamber as per the manufacturer's instructions.
Following the TUNEL reaction, cells were washed in PBS and subjected to a 10-min incubation either with anticytokeratin antibody (Dako Corp., catalog no. A0575) and a biotinylated anti-rabbit immunoglobulin antibody (Lsab 2; Dako Corp.) or with anti-CD68 biotin-conjugated antibody (Dako Corp., clone KP1) at room temperature. The tissue was then incubated with streptavidin RPE (Dako Corp.) for 10 min prior to the application of a 1:1,000 dilution of 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes). All sections were mounted in FluorSave (Calbiochem, San Diego, Calif.) and assessed with multicolor analysis on a digital light microscope (Zeiss Axiovert 100 M). Images were captured and analyzed with Open Lab (Improvision, Inc.).
Statistical methods.
Separate analyses were conducted for CD4+ and CD8+ T cells (Fig. 1). Each analysis employed the Wilcoxon rank sum test with age as a covariate.
RESULTS
Cross-sectional analysis of TREC levels within SIV-infected macaques.
A cross-sectional assessment of TREC levels in SIV-infected macaques at 20 to 34 weeks postinfection was undertaken. To ensure that the results were representative of different pathogenic SIV isolates, we assessed SIVmac239-, SIVmac251-, and SIVE660-infected macaques. TREC levels were measured in both CD4+ and CD8+ T cells isolated by magnetic bead separation as described previously (44).
Within the CD4+ T cells, the TREC levels were significantly higher than those observed within CD4+ T cells from the uninfected macaques (P < 0.01) (Fig. 1A). The CD4 TREC level varied depending on the age of the SIV-positive macaque. The youngest macaques assayed, 0.7 to 3 years of age (SIVmac239 [n = 3] and SIVmac251 [n = 2]), contained the highest levels of CD4 TREC (mean, 21,845 TREC/100,000 cells). The SIV-positive macaques between 3.5 and 4 years of age (SIVE660 [n = 6] and SIVmac251 [n = 1]) contained a lower level of CD4 TREC (mean, 7,112 TREC/100,000 cells). Finally, the SIVE660-infected macaque that was 6.5 years of age contained the lowest CD4 TREC level observed (280 TREC/100,000 cells).
The CD8 TREC levels within the SIV-positive macaques also declined with the age of the macaque (Fig. 1B). However, in contrast to the results for the CD4 TREC, the CD8 TREC levels were slightly lower than the TREC levels observed in the uninfected macaques (Fig. 1B). Although this is suggestive, the Wilcoxon rank sum test did not assign statistical significance to the difference in the level of CD8 TREC in the SIV-positive macaques compared to the uninfected macaques (P = 0.09). Therefore, cross-sectional TREC analysis within the CD4+ and CD8+ T-cell populations at 20 to 34 weeks postinfection did not provide any indication of thymic dysfunction within the SIV-positive macaques.
We hypothesized that 20 to 34 weeks postinfection may be too early after infection to observe a decrease in thymic output as determined by TREC levels in peripheral T cells. Therefore, an additional blood sample was obtained from four of the SIVE660-infected macaques at 48 weeks postinfection (each 4 years of age). These macaques exhibited declining CD4 T-cell levels and sustained high plasma viremia between 24 and 48 weeks postinfection (Fig. 2A and B). Over this 24-week time period, the CD4+ TREC levels decreased in each macaque (Fig. 2C). The CD8+ TREC level decreased within three macaques and remained unchanged in the fourth (Fig. 2D). These findings indicate that the levels of recent thymic emigrants, as determined by TREC levels, are declining between 24 and 48 weeks postinfection. Although this result is suggestive of a decline in thymic output during this time period, it is possible that increased levels of T-cell proliferation are influencing TREC levels in these SIV-positive macaques.
Longitudinal assessment of macaques infected with SIVmac239.
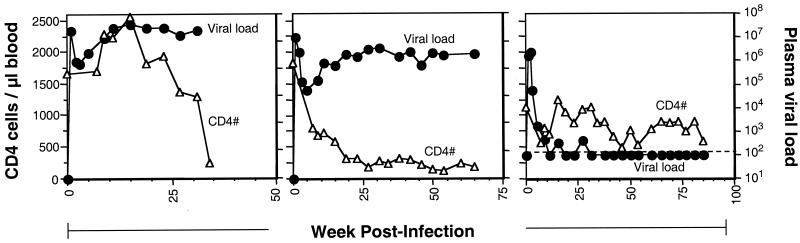
Assessment of SIVE660-infected macaques at 24 and 48 weeks postinfection indicated the importance of longitudinal samples in assessment of changes in peripheral blood TREC levels. We subsequently inoculated three macaques intravenously with a SIVmac239 viral stock so that they could be monitored longitudinally and levels of T-cell proliferation could be assessed (Fig. 3). The three SIVmac239-inoculated macaques represented a range of disease outcomes. Macaque RM1 succumbed to simian AIDS at 35 weeks postinfection (Fig. 3A). RM2 displayed high viral loads and low CD4+ T-cell counts throughout the disease course until it succumbed to simian AIDS at 65 weeks postinfection (Fig. 3B). Macaque RM3 is a slow progressor with low viral load and slowly declining CD4+ T-cell numbers at 120 weeks postinfection (Fig. 3C).
FIG. 3.
Longitudinal analyses of SIVmac239-infected macaques RM1 (A), RM2 (B), and RM3 (C). The number of CD4+ T cells (▵) and plasma viral load (•) assessed within the peripheral blood are shown for each macaque. The dotted line represents the lower level of detection for plasma viral load.
Levels of naïve (CD45RA/CD62L) T cells were observed to fluctuate throughout the disease course (Fig. 4). The percentage of T cells exhibiting a naïve phenotype increased (CD4 and CD8 in RM1, CD4 in RM2) or remained stable (CD8 in RM2, CD4 and CD8 in RM3) at early times postinfection. After approximately 35 weeks of infection, the percentage of naïve cells declined within the CD4 and CD8 subsets of RM2. This decrease in the percentage of naïve T cells may be due to alteration in T-cell proliferation, virus-induced cell death (direct and/or indirect), or alterations in thymic output.
Phase 1: TREC levels during the first 16 to 30 weeks of SIV infection are stable or increasing.
Assessment of TREC levels in the SIVmac239-infected macaques enabled the SIV infection to be divided into two distinct phases. Phase 1 encompassed the first 16 to 30 weeks, during which time the TREC levels were observed to fluctuate but generally remained stable or increased compared to the preinfection TREC levels (Fig. 5). The increased level of TREC within both CD4+ (RM1, RM2, and RM3) and CD8+ (RM1 and RM3) T cells during phase 1 was not expected based on the studies of HIV-infected patients (9, 10, 61). The increasing levels of TREC can in part be explained by an increase in the percentage of T cells with a naïve phenotype in RM1's CD4, RM1's CD8, and RM2's CD4 cells (Fig. 4). An increase in the percentage of naïve T cells would be predicted to increase TREC levels because this cell type contains the majority of TREC molecules (10). Second, a decrease in T-cell proliferation in the presence of stable thymic output would also be predicted to increase TREC levels (9, 10, 19).
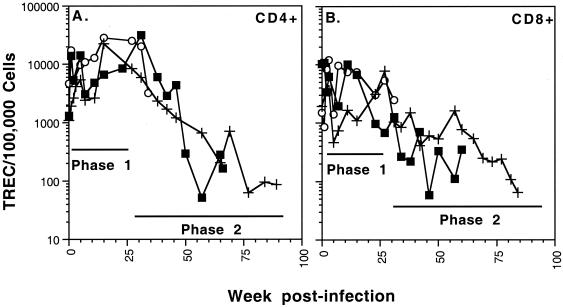
FIG. 5.
Longitudinal analysis of TREC levels in SIVmac239-infected macaques RM1 (○), RM2 (▪), and RM3 (+). The TREC levels were assessed in CD4+ (A) and CD8+ (B) cells. TREC levels were determined by real-time Taqman PCR analysis. Phase 1 (stable or increasing TREC levels) and phase 2 (decreasing TREC levels) are indicated on each graph.
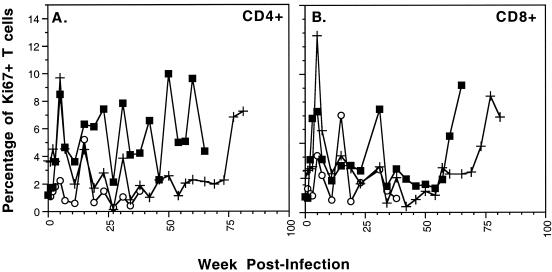
Here, T-cell proliferation was assessed with antibodies to Ki67, a nuclear protein expressed in proliferating cells (12, 13). In uninfected macaques, we observed Ki67 levels between 1 and 4% in the CD4 and 1 and 3% in the CD8 T cells. In the SIV-infected macaques, Ki67 levels were stable or elevated during phase 1. Therefore, we did not observe a general decrease in T-cell proliferation correlating with the increased TREC levels (Fig. 6). In summary, phase 1 of the infection was defined by stable or increasing TREC levels within the peripheral T cells, with no evidence of thymic dysfunction.
FIG. 6.
Longitudinal analysis of SIVmac239-infected macaques RM1 (○), RM2 (▪), and RM3 (+) to assess expression of the Ki67 antigen as an indicator of cellular proliferation. The percentage of cells expressing Ki67 in the peripheral blood within CD4+ cells (A) and CD8+ cells (B) is depicted for each macaque. Ki67+ T-cell levels preinfection ranged from 1 to 4% in the CD4+ cells and 1 to 3% in the CD8+ cells.
Phase 2: TREC levels decline after 16 to 30 weeks of infection.
In humans who have undergone a total thymectomy, TREC levels begin to decrease after about 100 days (14 weeks) (42). Similarly, a decrease in TREC levels was observed after 16 to 30 weeks in the SIVE660- and SIVmac239-infected macaques (H423, H424, H425, 18896, RM2, and RM3 [Fig. 2C and D; Fig. 5]). This TREC decrease marked the beginning of phase 2 of the infection and could be observed for both the CD4+ and CD8+ T cells. The start of phase 2 occurred in the CD4+ cells at 30 (RM2) and 16 (RM3) weeks and in the CD8+ cells at 16 (RM2) and 30 (RM3) weeks postinfection.
TREC levels will be influenced by the influx of TREC-containing T cells into the peripheral blood, the proliferation rate of the T cells, and the percentage of naïve T cells within the periphery. A recent study of HIV-infected patients determined that the loss of TREC during HIV infection is a result of both decreased thymic output and increased proliferation of the TREC-containing cells (9). In the SIV-infected macaques, T-cell proliferation levels (Ki67+) were elevated at some phase 2 time points for both RM2 and RM3 (Fig. 6). It is likely that increased cellular proliferation is contributing, at least in part, to the declining TREC levels at these times. However, there are numerous time points at which TREC levels are declining and yet the frequency of Ki67+ lymphocytes remains low (i.e., RM3 CD4+ cells at 24 to 70 weeks postinfection [Fig. 5A and 6A]). Therefore, an increased level of cellular proliferation is not sufficient to fully explain the phase 2 TREC decline, indicating that decreased thymic output is also contributing to the phase 2 TREC decline.
Assessment of thymus tissue following SIV infection.
RM2 succumbed to an AIDS-related lymphoma, wasting, and diarrheal disease at 65 weeks postinfection. At necropsy, thymic tissue was obtained and assessed. Previous studies have determined that SIV can be detected at early times postinfection (7 days) (60). The infected cells are located predominantly within the thymic medulla, where the more mature single CD4+ and CD8+ thymocytes reside (60). Here SIV gag-specific PCR verified that SIV was present in the thymic tissue after 65 weeks of infection. Serial dilutions of RM2 thymocyte DNA determined that SIV viral load was relatively low, 1 to 10 proviral copies within 4,000 thymocytes.
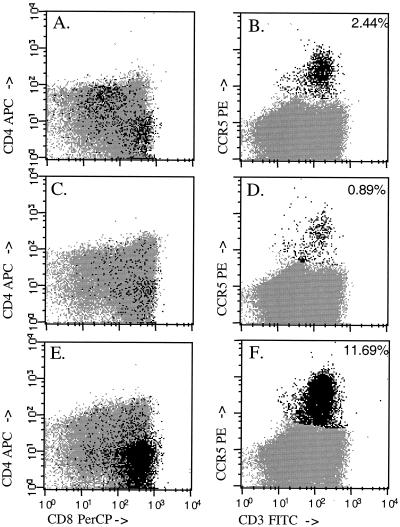
During an HIV infection, an influx of lymphocytes, predominantly CD8+ memory/effector cells, is observed within the thymus (17, 43). To assay for the presence of memory/effector T cells in the thymus of RM2, the thymocytes were assessed by flow cytometry. Memory/effector T cells were identified by the coexpression of CD3 and the chemokine receptor CCR5 (3) (Fig. 7). The dot plots depicting the CCR5+ and CD3+ thymocytes from two control macaques and for RM2 obtained at necropsy are shown in Fig. 7. Within the unfractionated thymocytes, 2.44 and 0.89% of the cells expressed CCR5 and CD3, respectively, in the control macaques. This percentage was 11.69% for the thymocytes isolated from RM2. These CCR5+ CD3+ T cells were predominantly CD8+ CD4− (Fig. 7E) and are likely to represent CD8+ memory cells. In addition, the memory/effector cell markers CD95+ (Fas) and CD11a+ (LFA1) were also used. The increase in CD95+ CD11a+ CD8+ cells at 65 weeks postinfection provided further evidence for an increase in the percentage of memory/effector CD8+ T cells in the RM2 thymus (data not shown).
FIG. 7.
Phenotypic assessment of T cells within the thymus. Thymocytes from two control macaques and RM2 at necropsy were labeled with anti-CD4, anti-CD8, anti-CD3, and anti-CCR5 monoclonal antibodies. Dot plots indicating the anti-CD4 and anti-CD8 reactivity (A, C, and E) or the anti-CCR5 and anti-CD3 reactivity (B, D, and F) are depicted here. The percentage of cells staining positive (black) for CCR5 is indicated in the upper right of each panel. The majority of CCR5+ cells within macaque RM2 were CD8+ and CD3+ (E and F). FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP, peridinin chlorophyll protein; APC, allophycocyanin.
HIV and SIV infection can result in an increase in the level of cells undergoing apoptosis within the thymus (40, 60). In situ analysis was used to assess the location and quantity of apoptotic cells within thymic tissue obtained from RM2 prior to infection and at necropsy. The TUNEL assay identifies low-molecular-weight DNA within the nucleus as well as the cytoplasm of cells undergoing apoptosis (34). Thymocyte maturation occurs within the epithelial space of the thymus, identified by anticytokeratin antibodies (Fig. 8) (17, 18).
FIG. 8.
Identification of apoptotic cells in the thymus of macaque RM2. The TUNEL assay was used to identify cells containing low-molecular-weight DNA fragments (green) indicative of apoptosis. An anticytokeratin antibody was used to identify thymic epithelium and the Hassall's corpuscles (HC), some of which are indicated (red). Cells were also stained with DAPI (blue) to identify all nuclei. Streck tissue fixative buffer-fixed paraffin-embedded thymic tissue from macaque RM2 obtained prior to infection (A) or 65 weeks postinfection at necropsy (B) was assessed. Prior to infection, approximately 1% of the area within the thymic cortex stained positive for TUNEL (A), while approximately 4% of the area stained positive in RM2 at necropsy (B). The area of the tissue staining positive for TUNEL was determined by using Open Lab (Improvision, Inc.).
Prior to infection, approximately 1% of the area of RM2's thymus was TUNEL positive (identified in green) (Fig. 8A). A similar level of apoptosis was observed within two control macaques (data not shown). In contrast, approximately 4% of the area of RM2's thymic tissue at necropsy contained TUNEL-positive cells (Fig. 8B). The paucity of Hassall's corpuscles at necropsy further indicated that the thymic architecture had been affected by the infection. Compared to the surrounding thymocytes, many of the TUNEL-positive cells in Fig. 8 were large and irregularly shaped. By using anti-CD68 antibodies, the large TUNEL-positive cells were identified as macrophages (yellow/orange indicating CD68+, TUNEL-positive cells) (Fig. 9). CD68+, TUNEL-positive cells (yellow or orange) were identified within both the RM2 thymus prior to infection (Fig. 9A) and at necropsy (Fig. 9B). These macrophages may have obtained their low-molecular-weight DNA by engulfing dead or dying thymocytes (35, 57). Although many of the TUNEL-positive cells were located among the immature thymocytes in the cortex, apoptotic cells were also observed within the thymic medulla.
FIG. 9.
Phenotypic assessment of TUNEL-positive cells in the thymus of macaque RM2. Streck tissue fixative buffer-fixed, paraffin-embedded thymic tissue from macaque RM2 obtained prior to infection (A) or 65 weeks postinfection at necropsy (B) was assessed. The TUNEL assay was used to identify cells containing low-molecular-weight DNA fragments (green) indicative of apoptosis. Macrophages were identified by the antibody to CD68 (red). Cells staining positive for both TUNEL and anti-CD68 are depicted in yellow and orange. Cells were also stained with DAPI (blue) to identify all nuclei. The arrow and inset indicate a macrophage (yellow/orange) surrounding what appears to be an apoptotic T cell.
DISCUSSION
HIV and SIV are capable of infecting thymocytes, resulting in thymocyte depletion and thymic atrophy (2, 8, 17, 39, 40, 48, 52). In the present study, we used measurement of TREC levels in the peripheral blood as a means to evaluate thymic function. TREC analysis enabled the SIV infection to be divided into two distinct phases. Phase 1 involved stable or increasing TREC levels within both the CD4 and CD8 T-cell populations. Within the SIVmac239-infected macaques, phase 1 lasted throughout the first 16 to 30 weeks. The cross-sectional analysis of SIV-positive macaques (Fig. 1) assessed samples at 20 to 34 weeks postinfection. The TREC levels were similar to or higher than the levels in the uninfected macaques, possibly because these macaques were likely at the end of phase 1 or the very beginning of phase 2.
The observation that TREC levels were sometimes elevated within the peripheral T cells, particularly in the CD4+ cells, was not expected based on the studies of HIV-infected patients (9, 10, 61). Previous studies have assessed thymic tissue from SIV-infected macaques through 50 days postinfection. These studies have identified alterations in the thymocyte subsets, increased thymocyte proliferation, altered thymic architecture, and an increased level of thymocyte apoptosis at the earliest time points (days 7, 14, and 21 postinfection) (40, 60). However, at 50 days postinfection, many of these changes had resolved, indicating that the thymus could function during an SIV infection (40, 60). Alternatively, the increase in TREC levels may not be due to any changes within the thymus but rather to an increase in the percentage of cells that contain TREC in the peripheral blood (naïve phenotype). The increased percentage of cells with a naïve phenotype (Fig. 4A) may result from an increase in the number of cells in the naïve cell pool or a decrease in the number of cells in the memory cell pool. A specific decrease in CD4+ memory/effector T cells has been documented following SIV infection (25, 54).
In conclusion, there are two hypotheses that might explain the stable or increasing TREC levels during phase 1: thymic output is maintained or increased to compensate for the T-cell destruction, or thymic output is reduced, but this decrease is not detectable in the periphery until after 16 to 30 weeks of infection.
A decline in TREC levels during HIV infection has been attributed to both decreased thymic output and increased proliferation of peripheral T cells (9, 10, 19, 61). Within the SIV-infected macaques, TREC levels did not decline until the second phase of infection, after 16 to 30 weeks. This decrease was observed within the four SIVE660-infected macaques (Fig. 2) as well as two of the SIVmac239-infected macaques, RM2 and RM3 (RM1 succumbed to AIDS prior to the start of phase 2). The decline in TREC levels did not appear to be dependent on high viral loads, as it declined within the slow progressor RM3 with undetectable viral loads for most of the disease course.
We have now followed macaque RM3 for 120 weeks, and TREC levels in this animal are still low, ranging from undetectable (<20 TREC/100,000 cells) to 200 TREC/100,000 cells within the CD4 and CD8 T cells. However, the decline in TREC levels during phase 2 of the SIV infection does not in itself indicate decreased thymic output. The use of the proliferation marker Ki67 offers a means by which the proliferation status of CD4 and CD8 T cells can be assessed. Our data did not demonstrate a direct association between low TREC levels and increased Ki67 expression. Therefore, dilution of TREC molecules through proliferation is not sufficient to explain all observed phases of TREC decline. This finding is similar to data obtained from HIV-infected patients (9) and suggests that both T-cell proliferation and thymic output are contributing to the decreased peripheral blood TREC levels observed in SIV-infected macaques.
An earlier cross-sectional study assessed PBMC α1 circles (TREC) in macaques infected at 3 months to 3 years of age and did find a detectable but limited (approximately 0.3 log units) impact of SIV infection in macaques (5). In addition, the previous study also assessed α1 circles (TREC) at longitudinal time points in six SIVmac251-infected macaques. Three macaques exhibited the phase 1-phase 2 pattern observed here, and three macaques contained stable α1 circle (TREC) levels for 400 to 800 days (5). Therefore, an infection by SIV does not always result in low peripheral blood TREC levels in macaques. Both viral (strain, dose, and infection route) and host factors are likely influencing the levels of recent thymic emigrants within the peripheral blood of SIV-infected macaques.
Quantification of TREC levels within the peripheral blood represents an indirect assessment of thymic output during SIV infection. Thymic tissue was assessed directly from a macaque that progressed to disease over 65 weeks (RM2). Previous studies have observed increased levels of infiltrating lymphocytes within the perivascular space around the thymic epithelium (18). We used flow cytometry to observe an increase in the level of memory/effector CD8+ T cells in the SIV-infected thymus. These memory/effector CD8+ T cells may have been located within the perivascular space or within the epithelial space, which was the predominant tissue observed following in situ analysis.
Here, increased levels of apoptosis within the epithelial space were observed. These apoptotic cells were particularly evident within the thymic cortex, where the immature thymocytes reside. Many of the apoptotic cells were CD68+ macrophages, which may be undergoing apoptosis due to an SIV infection (60) or through an indirect mechanism. However, it is likely that the macrophages in the thymus may be becoming TUNEL-positive due to their role as scavengers responsible for removal of the apoptotic thymocytes. In mice, thymic macrophages have been demonstrated to acquire low-molecular-weight DNA from the apoptotic thymocytes and become TUNEL positive (35, 57). The increased level of apoptotic T cells within the thymic cortex is unlikely to be directly due to viral infection.
Similar to findings in other studies, levels of SIV DNA were quite low (1 to 10 proviral copies/4,000 thymocytes) (60). Therefore, an indirect mechanism may be affecting the thymocytes, resulting in an increased level of apoptosis. Rosenzweig and colleagues assessed the thymic tissue from macaques through 50 days of an SIV infection and observed an increase in Fas and Fas ligand, two proteins involved in apoptotic induction (40). In addition, there was a decrease in Bcl-2, a protein that protects cells from apoptosis-inducing signaling (40). It is possible that shifts in the cytokines that affect thymic function (52) may be impacting the expression of these apoptosis-related proteins.
T cells generated from thymopoiesis would be predicted to have a broad T-cell repertoire capable of mounting a T-cell response to novel antigens. In contrast, the peripheral expansion of T cells leads to the expression of limited repertoires with limited abilities to identify novel antigens (31-33). The findings presented here demonstrate declining TREC levels (indirect measure of thymic dysfunction) and increased levels of memory/effector CD8+ T cells and apoptosis (direct assessment of thymic dysfunction) in the SIV-positive macaque thymus. Similar findings have been observed for HIV (17, 48, 52) as well as at early time points post-SIV infection (40, 60). It is likely that a reduced thymic output is contributing to the skewed T-cell receptor repertoire observed during HIV (7, 15) and SIV (6) infection. A limited T-cell receptor repertoire would be predicted to impair the ability of the immune system to respond to a wide range of antigens, possibly resulting in the opportunistic infections and cancers characteristic of AIDS.
There are a number of mechanisms by which HIV and SIV may be inducing thymic dysfunction, including the direct killing of thymocytes (4, 27, 46), killing of the dendritic cells required for normal thymocyte development (53), damaging of the thymic epithelial cells required for normal thymopoiesis (46), and inhibition of thymocyte signaling. SIV infection of macaques will be a useful animal model for distinguishing the influence of each of these mechanisms in lentivirus-induced thymic dysfunction.
Acknowledgments
We thank Ziad Peerwani for excellent technical assistance, Ross Payne and Lee Thao for assisting with the in situ immunohistochemistry, Stephanie Ehnert and Dan Anderson for assisting with the macaque experiments undertaken at the Yerkes Regional Primate Center, and Koen Van Rompay at the California Regional Primate Center and Amitinder Kaur and Marie-Claire Gauduin at the New England Regional Primate Center for helpful discussion and comments. We thank William Frawley for statistical analysis and Victor Garcia, Alagar Muthukumar, Courtney Matthews, Guido Silvestri, and Laurie Davis for careful reading of the manuscript.
This work was supported by NIH grants AI35522 (D.L.S.), DE12926 (D.L.S.), RR00168 (New England Regional Primate Research Center), and RR00169 (California Regional Primate Research Center) and by the Elizabeth Glaser Pediatric AIDS Foundation (D.L.S.). R.P.J. and A.A.L. are Elizabeth Glaser Scientists of the Pediatric AIDS Foundation. J.M.M. is supported by a molecular microbiology training grant from the University of Texas Southwestern Medical Center.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Baskin, G. B., M. Murphey Corb, L. N. Martin, B. Davison Fairburn, F. S. Hu, and D. Kuebler. 1991. Thymus in simian immunodeficiency virus-infected rhesus monkeys. Lab. Investig. 65:400-407. [PubMed] [Google Scholar]
- 3.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonyhadi, M., L. Rabin, S. Salimi, D. Brown, J. Kosek, J. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti, L. A., S. R. Lewin, L. Zhang, A. Gettie, A. Luckay, L. N. Martin, E. Skulsky, D. D. Ho, C. Cheng-Mayer, and P. A. Marx. 2000. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J. Virol. 74:1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z. W., Z. C. Kou, C. Lekutis, L. Shen, D. Zhou, M. Halloran, J. Li, J. Sodroski, d. Lee-Parritz, and N. L. Letvin. 1995. T-cell receptor VB repertoire in an acute infection of rhesus monkeys with simian immunodeficiency viruses and a chimeric simain-human immunodeficiency virus. J. Exp. Med. 182:21-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connors, M., J. Kovacs, S. Krevat, J. Gea-Banacloche, M. Sneller, M. Flanigan, J. Metcalf, R. Walker, J. Falloon, M. Baseler, R. Stevens, I. Feuerstein, H. Masur, and H. Lane. 1997. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat. Med. 3:533-540. [DOI] [PubMed] [Google Scholar]
- 8.Correa, R., and M. A. Munoz-Fernandez. 2001. Viral phenotype affects the thymic production of new T cells in HIV-1-infected children. AIDS 15:1959-1963. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., M. R. Betts, B. J. Hill, S. J. Little, R. Lempicki, J. A. Metcalf, J. Casazza, C. Yoder, J. W. Adelsberger, R. A. Stevens, M. W. Baseler, P. Keiser, D. D. Richman, R. T. Davey, and R. A. Koup. 2001. Evidence for increased T-cell turnover and decreased thymic output in HIV infection. J. Immunol. 167:6663-6668. [DOI] [PubMed] [Google Scholar]
- 10.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 11.Douek, D. C., R. A. Vescio, M. R. Betts, J. M. Brenchley, B. J. Hill, L. Zhang, J. R. Berenson, R. H. Collins, and R. A. Koup. 2000. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 355:1875-1881. [DOI] [PubMed] [Google Scholar]
- 12.Gerdes, J., H. Lemke, H. Baisch, H. H. Wacker, U. Schwab, and H. Stein. 1984. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 133:1710-1715. [PubMed] [Google Scholar]
- 13.Gerdes, J., U. Schwab, H. Lemke, and H. Stein. 1983. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 31:13-20. [DOI] [PubMed] [Google Scholar]
- 14.Goedert, J. J., T. R. O'Brien, A. Hatzakis, and L. G. Kostrikis. 2001. T-cell receptor excision circles and HIV-1 2-LTR episomal DNA to predict AIDS in patients not receiving effective therapy. AIDS 15:2245-2250. [DOI] [PubMed] [Google Scholar]
- 15.Gorochov, G., A. Neumann, A. Kereveur, C. Parizot, T. Li, C. Katlama, M. Karmochkine, G. Raguin, B. Autran, and P. Debre. 1998. Perturbation of CD4+ and CD8+ T-cell repertoires during progression to AIDS and regulation of the CD4+ repertoire during antiviral therapy. Nat. Med. 4:215-221. [DOI] [PubMed] [Google Scholar]
- 16.Grody, W., S. Fligiel, and F. Naeim. 1985. Thymus involution in the acquired immunodeficiency syndrome. Am. J. Clin. Pathol. 84:85-95. [DOI] [PubMed] [Google Scholar]
- 17.Haynes, B. F., L. P. Hale, K. J. Weinhold, D. D. Patel, H. X. Liao, P. B. Bressler, D. M. Jones, J. F. Demarest, K. Gebhard-Mitchell, A. T. Haase, and J. A. Bartlett. 1999. Analysis of the adult thymus in reconstitution of T lymphocytes in HIV-1 infection. J. Clin. Investig. 103:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haynes, B. F., M. L. Markert, G. D. Sempowski, D. D. Patel, and L. P. Hale. 2000. The role of the thymus in immune reconstitution in aging, bone marrow transplantation, and HIV-1 infection. Annu. Rev. Immunol. 18:529-560. [DOI] [PubMed] [Google Scholar]
- 19.Hazenberg, M. D., S. A. Otto, J. W. Cohen Stuart, M. C. Verschuren, J. C. Borleffs, C. A. Boucher, R. A. Coutinho, J. M. Lange, T. F. Rinke de Wit, A. Tsegaye, J. J. van Dongen, D. Hamann, R. J. de Boer, and F. Miedema. 2000. Increased cell division but not thymic dysfunction rapidly affects the T-cell receptor excision circle content of the naive T-cell population in HIV-1 infection. Nat. Med. 6:1036-1042. [DOI] [PubMed] [Google Scholar]
- 20.Heitger, A., N. Neu, H. Kern, E. R. Panzer-Grumayer, H. Greinix, D. Nachbaur, D. Niederwieser, and F. M. Fink. 1997. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood 90:850-857. [PubMed] [Google Scholar]
- 21.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson, B. D., D. C. Douek, S. Killian, L. E. Hultin, D. D. Scripture-Adams, J. V. Giorgi, D. Marelli, R. A. Koup, and J. A. Zack. 1999. Generation of functional thymocytes in the human adult. Immunity 10:569-575. [DOI] [PubMed] [Google Scholar]
- 24.Kaneshima, H., L. Su, M. L. Bonyhadi, R. I. Connor, D. D. Ho, and J. M. McCune. 1994. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J. Virol. 68:8188-8192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur, A., C. L. Hale, S. Ramanujan, R. K. Jain, and R. P. Johnson. 2000. Differential dynamics of CD4+ and CD8+ T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J. Virol. 74:8413-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong, F.-K., C.-L. Chen, and M. Cooper. 1998. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity 8:97-104. [DOI] [PubMed] [Google Scholar]
- 27.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 28.Lackner, A. A., P. Vogel, R. A. Ramos, J. D. Kluge, and M. Marthas. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am. J. Pathol. 145:428-439. [PMC free article] [PubMed] [Google Scholar]
- 29.Linder, J. 1987. The thymus gland in secondary immunodeficiency. Arch. Pathol. Lab. Med. 111:1118-1122. [PubMed] [Google Scholar]
- 30.Livak, F., and D. Schatz. 1996. T-cell receptor α locus V(D)J recombination by-products are abundant in thymocytes and mature T cells. Mol. Cell. Biol. 16:609-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackall, C., T. Fleisher, M. Brown, M. Andrich, C. Chen, I. Feuerstein, I. Magrath, L. Wexler, D. Dimitrov, and R. Gress. 1997. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 89:3700-3707. [PubMed] [Google Scholar]
- 32.Mackall, C., F. Hakin, and R. Gress. 1997. T-cell regeneration: all repertoires are not created equal. Immunol. Today 18:245-251. [DOI] [PubMed] [Google Scholar]
- 33.Mackall, C. L., C. V. Bare, L. A. Granger, S. O. Sharrow, J. A. Titus, and R. E. Gress. 1996. Thymic-independent T-cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J. Immunol. 156:4609-4616. [PubMed] [Google Scholar]
- 34.Mori, C., N. Nakamura, Y. Okamoto, M. Osawa, and K. Shiota. 1994. Cytochemical identification of programmed cell death in the fusing fetal mouse palate by specific labelling of DNA fragmentation. Anat. Embryol. (Berlin) 190:21-28. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura, M., H. Yagi, T. Ishii, S. Kayaba, H. Soga, T. Gotoh, S. Ohtsu, M. Ogata, and T. Itoh. 1997. DNA fragmentation is not the primary event in glucocorticoid-induced thymocyte death in vivo. Eur. J. Immunol. 27:999-1004. [DOI] [PubMed] [Google Scholar]
- 36.Namikawa, R., H. Kaneshima, M. Lieberman, I. L. Weissman, and J. M. McCune. 1988. Infection of the SCID-hu mouse by HIV-1. Science 242:1684-1686. [DOI] [PubMed] [Google Scholar]
- 37.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Piatak, M., Jr., K. C. Luk, B. Williams, and J. D. Lifson. 1993. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. BioTechniques 14:70-81. [PubMed] [Google Scholar]
- 39.Rosenzweig, M., D. P. Clark, and G. N. Gaulton. 1993. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS 7:1601-1605. [DOI] [PubMed] [Google Scholar]
- 40.Rosenzweig, M., M. Connole, A. Forand-Barabasz, M. P. Tremblay, R. P. Johnson, and A. A. Lackner. 2000. Mechanisms associated with thymocyte apoptosis induced by simian immunodeficiency virus. J. Immunol. 165:3461-3468. [DOI] [PubMed] [Google Scholar]
- 41.Rudensey, L. M., J. T. Kimata, R. E. Benveniste, and J. Overbaugh. 1995. Progression to AIDS in macaques is associated with changes in the replication, tropism, and cytopathic properties of the simian immunodeficiency virus variant population. Virology 207:528-542. [DOI] [PubMed] [Google Scholar]
- 42.Sempowski, G. D., L. P. Hale, J. S. Sundy, J. M. Massey, R. A. Koup, D. C. Douek, D. D. Patel, and B. F. Haynes. 2000. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J. Immunol. 164:2180-2187. [DOI] [PubMed] [Google Scholar]
- 43.Sempowski, G. D., and B. F. Haynes. 2002. Immune reconstitution in patients with HIV infection. Annu. Rev. Med. 53:269-284. [DOI] [PubMed] [Google Scholar]
- 44.Sodora, D. L., D. C. Douek, G. Silvestri, L. Montgomery, M. Rosenzweig, T. Igarashi, B. Bernacky, R. P. Johnson, M. B. Feinberg, M. A. Martin, and R. A. Koup. 2000. Quantification of thymic function by measuring T-cell receptor excision circles within peripheral blood and lymphoid tissues in monkeys. Eur. J. Immunol. 30:1145-1153. [DOI] [PubMed] [Google Scholar]
- 45.Sodora, D. L., A. Gettie, C. J. Miller, and P. A. Marx. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retroviruses. 14(Suppl. 1):S119-S123. [PubMed] [Google Scholar]
- 46.Stanley, S. K., J. M. McCune, H. Kaneshima, J. S. Justement, M. Sullivan, E. Boone, M. Baseler, J. Adelsberger, M. Bonyhadi, J. Orenstein, et al. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp Med. 178:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steffens, C. M., K. Y. Smith, A. Landay, S. Shott, A. Truckenbrod, M. Russert, and L. Al-Harthi. 2001. T-cell receptor excision circle (TREC) content following maximum HIV suppression is equivalent in HIV-infected and HIV-uninfected individuals. AIDS 15:1757-1764. [DOI] [PubMed] [Google Scholar]
- 48.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 49.Suryanarayana, K., T. A. Wiltrout, G. M. Vasquez, V. M. Hirsch, and J. D. Lifson. 1998. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res. Hum. Retroviruses 14:183-189. [DOI] [PubMed] [Google Scholar]
- 50.Takeshita, S., M. Toda, and H. Ymagishi. 1989. Excision products of the T-cell receptor gene support a progressive rearrangement model of the α/δ locus. EMBO J. 8:3261-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Teixeira, L., H. Valdez, J. M. McCune, R. A. Koup, A. D. Badley, M. K. Hellerstein, L. A. Napolitano, D. C. Douek, G. Mbisa, S. Deeks, J. M. Harris, J. D. Barbour, B. H. Gross, I. R. Francis, R. Halvorsen, R. Asaad, and M. M. Lederman. 2001. Poor CD4 T-cell restoration after suppression of HIV-1 replication may reflect lower thymic function. AIDS 15:1749-1756. [DOI] [PubMed] [Google Scholar]
- 52.Uittenbogaart, C. H., W. J. Boscardin, D. J. Anisman-Posner, P. S. Koka, G. Bristol, and J. A. Zack. 2000. Effect of cytokines on HIV-induced depletion of thymocytes in vivo. AIDS 14:1317-1325. [DOI] [PubMed] [Google Scholar]
- 53.Valentin, H., M. T. Nugeyre, F. Vuillier, L. Boumsell, M. Schmid, F. Barre-Sinoussi, and R. A. Pereira. 1994. Two subpopulations of human triple-negative thymic cells are susceptible to infection by human immunodeficiency virus type 1 in vitro. J. Virol. 68:3041-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veazey, R. S., I. C. Tham, K. G. Mansfield, M. DeMaria, A. E. Forand, D. E. Shvetz, L. V. Chalifoux, P. K. Sehgal, and A. A. Lackner. 2000. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4+ T cells are rapidly eliminated in early SIV infection in vivo. J. Virol. 74:57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Verschuren, M., I. Wolvers-Tettero, T. Breit, J. Noordzij, E. van Wering, and J. van Dongen. 1997. Preferential rearrangements of the T cell receptor-δ-deleting elements in human T cells. J. Immunol. 158:1208-1216. [PubMed] [Google Scholar]
- 56.Verschuren, M., I. Wolvers-Tettero, T. Breit, and J. van Dongen. 1998. T-cell receptor Vδ−Jα rearrangements in T-cell receptor-δ gene deletion. Immunology 93:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wack, A., H. M. Ladyman, O. Williams, K. Roderick, M. A. Ritter, and D. Kioussis. 1996. Direct visualization of thymocyte apoptosis in neglect, acute and steady-state negative selection. Int. Immunol. 8:1537-1548. [DOI] [PubMed] [Google Scholar]
- 58.Watson, A., J. Ranchalis, B. Travis, J. McClure, W. Sutton, P. R. Johnson, S. L. Hu, and N. L. Haigwood. 1997. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J. Virol. 71:284-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei, X., S. Ghosh, M. Taylor, V. Johnson, E. Emini, P. Deutsch, J. Lifson, S. Bonhoeffer, M. Nowak, B. Hahn, M. Saag, and G. Shaw. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 60.Wykrzykowska, J., M. Rosenzweig, R. Veazey, M. Simon, K. Halvorsen, R. Desrosiers, R. Johnson, and A. Lackner. 1998. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J. Exp. Med. 187:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, L., S. R. Lewin, M. Markowitz, H. H. Lin, E. Skulsky, R. Karanicolas, Y. He, X. Jin, S. Tuttleton, M. Vesanen, H. Spiegel, R. Kost, J. van Lunzen, H. J. Stellbrink, S. Wolinsky, W. Borkowsky, P. Palumbo, L. G. Kostrikis, and D. D. Ho. 1999. Measuring recent thymic emigrants in blood of normal and HIV-1-infected individuals before and after effective therapy. J. Exp. Med. 190:725-732. [DOI] [PMC free article] [PubMed] [Google Scholar]