Abstract
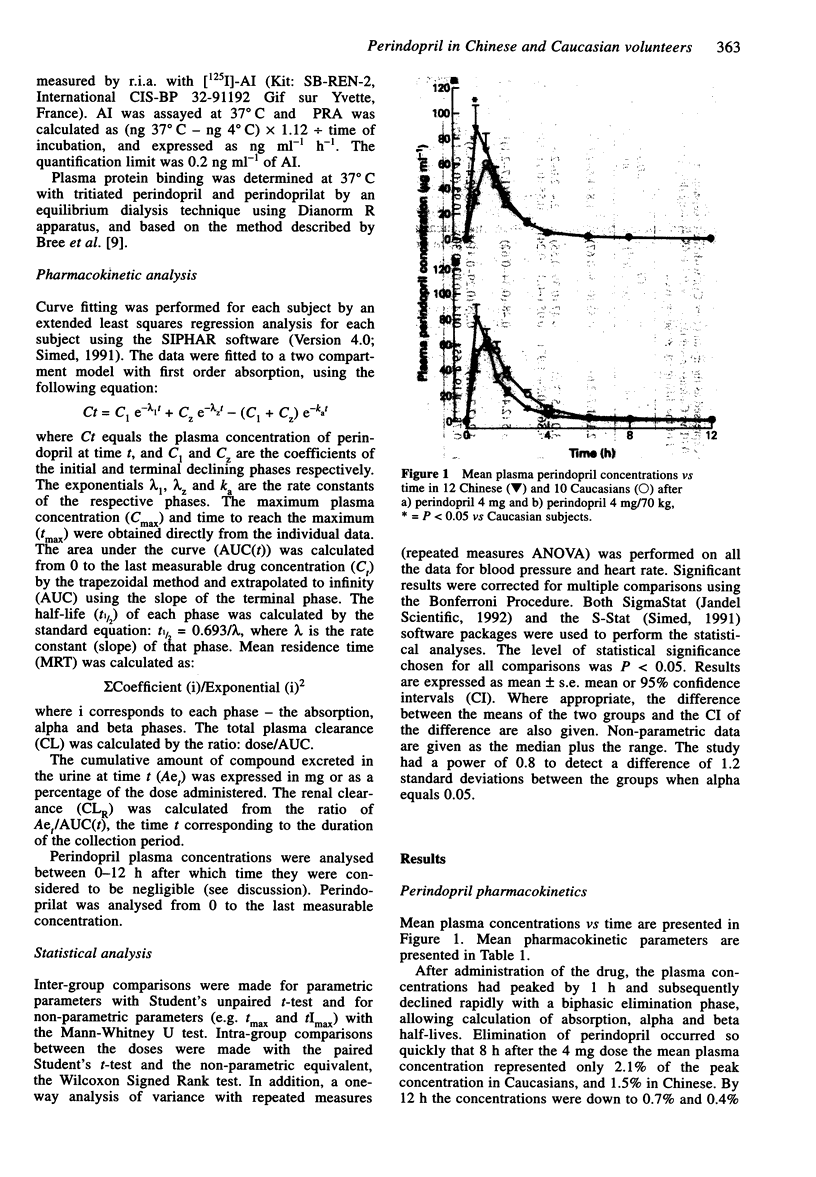
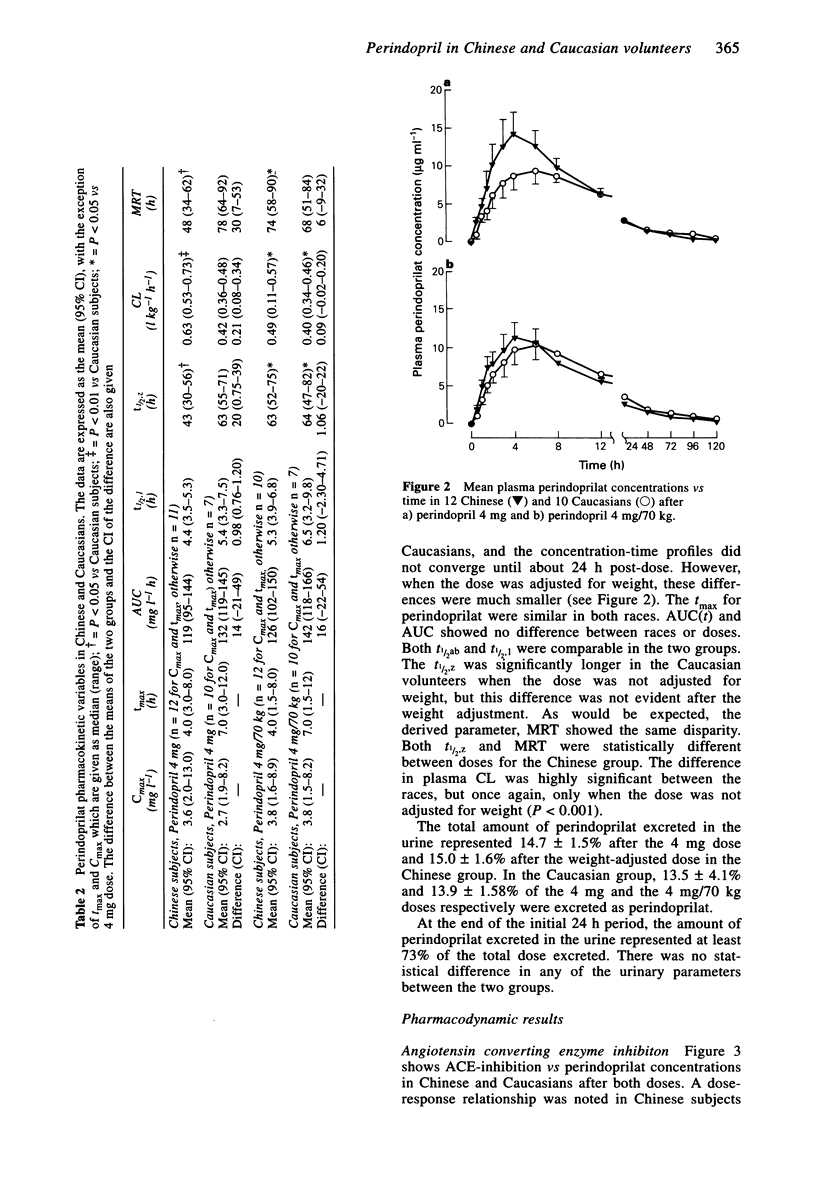
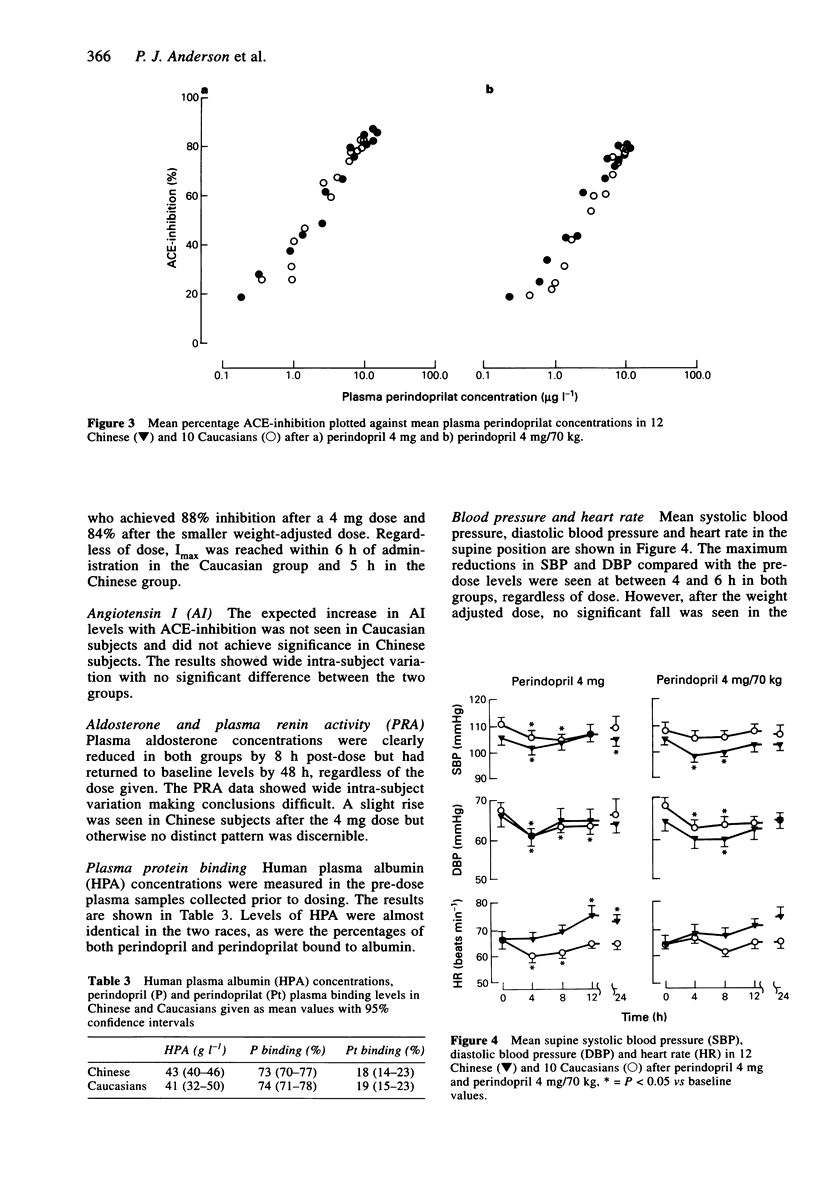
1. The pharmacokinetics of perindopril and perindoprilat and the hormonal and haemodynamic responses following a single oral dose were studied in 12 Chinese and 10 Caucasian healthy, normotensive volunteers on two occasions. Perindopril was given on the first occasion as a 4 mg dose and then after at least 10 days as a weight-adjusted dose of 4 mg/70 kg. Plasma was sampled for assay of perindopril, perindoprilat, plasma renin activity (PRA), aldosterone, angiotensin I (AI) and ACE activity. Urine was collected for perindopril and perindoprilat assay. A radioimmunoassay technique was used to measure the prodrug and its active metabolite. 2. The time to maximum concentration (tmax) for perindopril was shorter for the Chinese group after the 4 mg dose (median 0.5, range 0.5-1.5 h vs median 1.0, 0.5-1.5 h P < 0.05) and also tended to be shorter after the weight-adjusted dose (median 0.5, range 0.5-1.0 h vs median 1.0, range 0.5-3.0 h). Cmax and AUC tended to be higher after the 4 mg dose in the Chinese group who had a lower body weight than the Caucasians. 3. The tmax of perindoprilat tended to be shorter for both doses and there was a tendency towards a higher Cmax after the 4 mg dose in the Chinese group but there was no statistically significant difference between the two groups. 4. There were no differences in the levels of PRA, plasma AI, plasma aldosterone or the degree of ACE-inhibition for either dose in the two ethnic groups. 5. Blood pressure was measured at intervals up to 24 h post-dose in both the supine and standing positions. Perindopril reduced blood pressure acutely with respect to the pre-dose level with good tolerability in both groups.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belz G. G., Kirch W., Kleinbloesem C. H. Angiotensin-converting enzyme inhibitors. Relationship between pharmacodynamics and pharmacokinetics. Clin Pharmacokinet. 1988 Nov;15(5):295–318. doi: 10.2165/00003088-198815050-00003. [DOI] [PubMed] [Google Scholar]
- Bree F., Nguyen P., Urien S., Riant P., Albengres E., Fenner H., Tillement J. P. Blood distribution of tenoxicam in humans: a particular HSA drug interaction. Fundam Clin Pharmacol. 1989;3(3):267–279. doi: 10.1111/j.1472-8206.1989.tb00456.x. [DOI] [PubMed] [Google Scholar]
- Devissaguet J. P., Ammoury N., Devissaguet M., Perret L. Pharmacokinetics of perindopril and its metabolites in healthy volunteers. Fundam Clin Pharmacol. 1990;4(2):175–189. doi: 10.1111/j.1472-8206.1990.tb00486.x. [DOI] [PubMed] [Google Scholar]
- Feely J., Grimm T. A comparison of drug protein binding and alpha 1-acid glycoprotein concentration in Chinese and Caucasians. Br J Clin Pharmacol. 1991 May;31(5):551–552. doi: 10.1111/j.1365-2125.1991.tb05579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R. J., Brown A. N., Kler L., Fasanella d'Amore T., Nussberger J., Waeber B., Brunner H. R. Pharmacokinetics of the converting enzyme inhibitor cilazapril in normal volunteers and the relationship to enzyme inhibition: development of a mathematical model. J Cardiovasc Pharmacol. 1987 Jan;9(1):32–38. [PubMed] [Google Scholar]
- Kappas A., Alvares A. P., Anderson K. E., Pantuck E. J., Pantuck C. B., Chang R., Conney A. H. Effect of charcoal-broiled beef on antipyrine and theophylline metabolism. Clin Pharmacol Ther. 1978 Apr;23(4):445–450. doi: 10.1002/cpt1978234445. [DOI] [PubMed] [Google Scholar]
- Lecocq B., Funck-Brentano C., Lecocq V., Ferry A., Gardin M. E., Devissaguet M., Jaillon P. Influence of food on the pharmacokinetics of perindopril and the time course of angiotensin-converting enzyme inhibition in serum. Clin Pharmacol Ther. 1990 Mar;47(3):397–402. doi: 10.1038/clpt.1990.45. [DOI] [PubMed] [Google Scholar]
- Lees K. R., Kelman A. W., Reid J. L., Whiting B. Pharmacokinetics of an ACE inhibitor, S-9780, in man: evidence of tissue binding. J Pharmacokinet Biopharm. 1989 Oct;17(5):529–550. doi: 10.1007/BF01071348. [DOI] [PubMed] [Google Scholar]
- Lees K. R., Reid J. L. Haemodynamic and humoral effects of oral perindopril, an angiotensin converting enzyme inhibitor, in man. Br J Clin Pharmacol. 1987 Feb;23(2):159–164. doi: 10.1111/j.1365-2125.1987.tb03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfadyen R. J., Lees K. R., Reid J. L. Perindopril. A review of its pharmacokinetics and clinical pharmacology. Drugs. 1990;39 (Suppl 1):49–63. doi: 10.2165/00003495-199000391-00009. [DOI] [PubMed] [Google Scholar]
- Richer C., Thuillez C., Giudicelli J. F. Perindopril, converting enzyme blockade, and peripheral arterial hemodynamics in the healthy volunteer. J Cardiovasc Pharmacol. 1987 Jan;9(1):94–102. [PubMed] [Google Scholar]
- Seedat Y. K., Parag K. B. A comparison of lisinopril and atenolol in black and Indian patients with mild-to-moderate essential hypertension. S Afr Med J. 1987 Feb 7;71(3):149–153. [PubMed] [Google Scholar]
- Seedat Y. K. Trial of atenolol and chlorthalidone for hypertension in black South Africans. Br Med J. 1980 Nov 8;281(6250):1241–1243. doi: 10.1136/bmj.281.6250.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuillez C., Richard C., Richer C., Loueslati H., Perret L., Auzépy P., Giudicelli J. F. Peripheral haemodynamic effects of perindopril compared in patients with congestive heart failure and in normal volunteers. J Hypertens Suppl. 1988 Dec;6(3):S41–S43. [PubMed] [Google Scholar]
- Waeber B., Nussberger J., Perret L., Santoni J. P., Brunner H. R. Experience with perindopril in normal volunteers. Clin Exp Hypertens A. 1989;11 (Suppl 2):507–519. [PubMed] [Google Scholar]
- Zhou H. H., Adedoyin A., Wilkinson G. R. Differences in plasma binding of drugs between Caucasians and Chinese subjects. Clin Pharmacol Ther. 1990 Jul;48(1):10–17. doi: 10.1038/clpt.1990.111. [DOI] [PubMed] [Google Scholar]
- Zhou H. H., Koshakji R. P., Silberstein D. J., Wilkinson G. R., Wood A. J. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N Engl J Med. 1989 Mar 2;320(9):565–570. doi: 10.1056/NEJM198903023200905. [DOI] [PubMed] [Google Scholar]
- van den Berg H., Resplandy G., de Bie A. T., Floor W., Bertrand M., Arts C. J. A new radioimmunoassay for the determination of the angiotensin-converting enzyme inhibitor perindopril and its active metabolite in plasma and urine: advantages of a lysine derivative as immunogen to improve the assay specificity. J Pharm Biomed Anal. 1991;9(7):517–524. doi: 10.1016/0731-7085(91)80172-6. [DOI] [PubMed] [Google Scholar]


