Abstract
The virologic and cellular factors that are involved in transmission of human immunodeficiency virus type 1 (HIV-1) across the female genital tissue are poorly understood. We have recently developed a human cervical tissue-derived organ culture model to study heterosexual transmission of HIV-1 that mimics the in vivo situation. Using this model we investigated the role of phenotypic characteristics of HIV-1 and identified the cell types that are first infected during transmission. Our data indicate that the cell-free R5 HIV-1 was more efficiently transmitted than cell-free X4 HIV-1. Cell-free and cell-associated HIV-1 had comparable transmission efficiency regardless of whether the virus was of R5 or X4 type. We have demonstrated that memory CD4+ T cells and not Langerhans cells were the first HIV-1 RNA-positive cells detected at the epithelial-submucosal junction 6 h after virus exposure. Multicolor laser confocal microscopy demonstrated a globular distribution of HIV-1 gag-pol mRNA in the cytoplasm, and the distribution of CD4 and the CD45RO isoform was irregular on the cellular membrane. At 96 h postinoculation, in addition to memory CD4+ T cells, HIV-1 RNA-positive Langerhans cells and macrophages were also detected. The identification of CD4+ T cells in the tissue at 6 h was confirmed by flow cytometric simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization assay on immune cells isolated from disaggregated tissue. Furthermore, PMPA {9-[2-(phosphonomethoxy)propyl] adenine}, an antiretroviral compound, and UC781, a microbicide, inhibited HIV-1 transmission across the mucosa, indicating the utility of the organ culture to screen topical microbicides for their ability to block sexual transmission of HIV-1.
Heterosexual transmission accounts for the majority of worldwide human immunodeficiency virus type 1 (HIV-1) infections in women (17, 20, 21). Very little is known about the role of biologic and molecular characteristics of HIV-1 during sexual transmission. Since semen has been shown to contain free infectious HIV-1 and HIV-1-infected cells (19, 26, 32-35), transmission could potentially occur via cell-free and cell-associated HIV-1. Studies on the viral envelope sequence in men during primary infection suggest that virus transmitted during sexual contact was mostly homogeneous and had macrophage- tropic and non-syncytium-inducing phenotypes (35, 37). However, recent data of Long et al. (16) indicate that heterogeneous HIV-1 was found in women, whereas homogeneous virus was detected in men soon after sexual contact, indicating that the mechanism of sexual transmission of HIV-1 in women could be different than that in men.
There are no in vivo data to indicate the cell types that first become infected in the reproductive tract of women. Although in chronically HIV-1-infected women T cells, macrophages, and Langerhans cells in cervical tissue are infected with HIV-1 (25, 26), analysis of lymph nodes in HIV-infected men during the acute phase of infection indicates that actively virus replication occurs in activated and resting CD4+ T cells (36). A number of studies have demonstrated that productively HIV-1-infected cells in vivo are memory T cells (CD4+ CD45RO+) that respond to antiretroviral therapy (22, 24). There is controversy in the simian immunodeficiency virus (SIV)-rhesus monkey model system about the types of cells that become first infected during sexual transmission. Spira et al. (28) found that cells which initially become infected in the female genital tract are Langerhans cells present in the lamina propria, but the identity of these cells could not be determined by specific immunohistochemical staining on contiguous tissues. Moreover, this study could not determine whether these HIV-1 DNA-containing cells were productively infected. On the other hand, using simultaneous in situ hybridization and immunohistochemical staining, Zhang et al. (36) recently found that CD4+ T (both activated and resting) cells and not dendritic cells were predominantly the first cell types that became productively infected after 3 days of intravaginal inoculation of SIV in monkeys. However, Hu et al. (13) recently demonstrated the presence of intracellular SIV RNA in dendritic cells in HLA DR+-enriched genital cell suspensions within 60 min of intravaginal exposure of SIV.
One of the problems in studying HIV-1 transmission in humans is the lack of a suitable in vitro model. In vitro models based on primary and transformed cells derived from female genital tissues do not provide the natural cellular structures of the female genital organ that may be important in viral transmission. Recently, we have described a cervical tissue-derived organ culture model to study HIV transmission across the mucosal surface (6). Since this model uses tissues rather than a monolayer of cells (primary or transformed), it provides the natural tissue architecture, including epithelial cells, submucosa, and immune cells, such as T cells, macrophages, and Langerhans cells.
In this report we have used this organ culture model to study virological factors involved in transmission. Furthermore, we have determined that memory CD4+ T cells were the first cells that became infected during HIV-1 transmission across the cervical mucosa. In addition, our data indicate that the organ culture can be used to screen potential microbicides for their ability to block HIV-1 transmission across the mucosa.
MATERIALS AND METHODS
Virus culture.
Cell-free HIV-1 BAL and IIIB were grown in phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMC). PBMC infected with HIV-1 BAL and IIIB were prepared by infecting five to ten million phytohemagglutinin-stimulated PBMC with cell-free HIV-1 BAL or IIIB virus (1 × 105 to 2 × 105 pg of p24) for 1 h and cultured as described previously (1). After 5 to 10 days of infection when high levels of p24 were detected in the culture supernatant, infected cells were harvested and cryopreserved in aliquots to be used as cell-associated HIV-1. Titers of both cell-free and cell-associated HIV-1 (PBMC infected with BAL or IIIB) were determined in cMAGI cells (5).
Organ culture.
The organ culture was set up as previously described (6). Briefly, after we measured the thickness of the tissues, we placed 6.0-mm circular pieces of tissue from HIV-negative, premenopausal women in the top chamber of a 12-well transwell with the epithelial layer oriented on top. The thickness of the tissues varied between 2.1 and 2.6 mm. A 3% solution of agarose was then added to the area surrounding the tissue in the top well, which upon solidification created a tight seal around the tissue. The modified raft (MR) medium (Dulbecco modified Eagle medium supplemented with 25% Ham F-12 medium, 4 mg of hydrocortisone/ml, 0.5 ng of epidermal growth factor/ml, 10% fetal bovine serum, and 10,000 U of penicillin-streptomycin/ml) was then added to the top and bottom chambers of the transwell. A transwell with agarose only in the top chamber served as a negative control, whereas a transwell with the membrane only served as a positive control. To study the transmission of virus, a defined amount of pretitered cell-free HIV-1 or HIV-1-infected PBMC was added to the top chambers of the tissue and control wells in MR medium and then incubated at 34°C for 1 day. After incubation, infected cells or cell-free virus were removed, the top of the tissue was thoroughly washed while still in the well, and the incubation was continued for a total of 3 to 5 days in culture medium. At various days after inoculation, 0.5 ml of MR medium was harvested from the bottom chamber and tested for infectivity in cMAGI cells (5).
On the last day of culture, leakiness of the organ culture system was routinely monitored by examining the transmission of blue dextran, a 2 × 106-molecular-weight polysaccharide, through the tissue well and the agarose control well into the bottom well as described previously (6). The amount of blue dextran transmitted was <1% and even lower than that in the agarose control well.
Tissue-based simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization.
Immunophenotyping and ultrasensitive fluorescence in situ hybridization were performed as previously described (3, 22). Briefly, the tissue sections were air dried, rehydrated in phosphate-buffered saline, and labeled with optimized concentrations of phycoerythrin-conjugated antibodies specific for the cell type of interest (activated T cells [CD4 and CD45RO] and Langerhans cells [CD1a]; PharMingen, San Diego, Calif.). Cells were fixed in PermiFlow (Invirion, Inc., Frankfort, Mich.) and HIV-1 mRNA was detected in the tissues with the ViroTect In Cell HIV-1 Detection System (Invirion). The probe was hybridized to the target sequence for 60 min at 43°C in a GeneAmp 1000 slide cycler (PE Applied Biosystems, Foster City, Calif.). Multiparameter analysis of cell surface receptors and HIV gag-pol mRNA was performed on a laser confocal microscope (Olympus, Melville, N.Y.).
Flow cytometric simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization assay on immune cells from disaggregated explant biopsies.
A representative portion of cervical explant tissue was disaggregated by using the MediMachine (Becton Dickinson, San Jose, Calif.) as per the manufacturer's instructions. Immune cells were separated from cervical squamous epithelial cells by using a 50-μm (pore size) filcon. Immune cells were pelleted by centrifugation and stained with CD4-APC (PharMingen) or CD68-biotin. Cells were fixed in PermiFlow (Invirion) and HIV-1 mRNA was detected by using the ViroTect in-cell HIV-1 detection system (Invirion). Biotin-labeled cells were detected with streptavidin-PE (PharMingen). Flow cytometry was performed on a FACScalibur (BDIS, San Jose, Calif.).
Testing of antiviral activity of compounds in organ culture.
The antiviral activities of compounds were tested in our standard organ culture with cell-free HIV-1 BAL. To test PMPA {9-[2-(phosphonomethoxy)propyl] adenine}, different concentrations of PMPA were added to the tissue wells and incubated for 1 to 2 h. After incubation, medium of the tissue well was replaced by 104 50% tissue culture infective dose(s) (TCID50) of cell-free HIV-1 BAL in the presence of the same concentrations of PMPA for an additional 24 h, after which tissues were washed to remove virus, and incubation was continued for 3 days in the presence of respective concentrations of PMPA. Suppression of viral transmission was measured by monitoring the viral infectivity of transmitted virus from the bottom well by the cMAGI assay. For testing UC781, the assay was conducted as described for PMPA, except that HIV-1 BAL was preincubated with UC781 at different concentrations for 1 h and then added to the tissue well.
RESULTS
Evaluation of the integrity of the organ culture system.
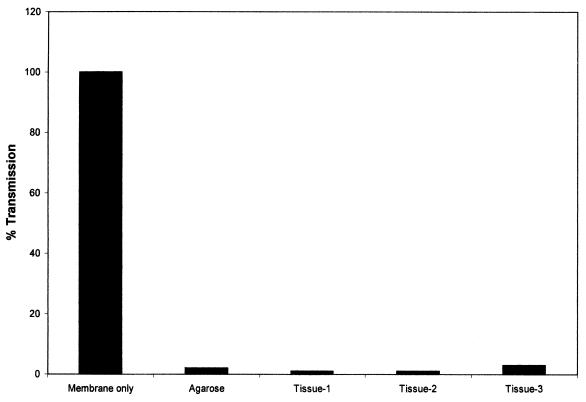
We previously evaluated the integrity of the organ culture system by examining the transmission of blue dextran across the epithelium. No transmission of blue dextran dye was observed when the dye was added onto the tissue at 1, 3, and 5 days after incubation and this was monitored for 24 h thereafter (6). To further rule out the leakiness of the organ culture system, we examined the transmission of fluorescent beads of 7.2 μm across the mucosa. For this purpose, a standard organ culture was set up with tissue and control agarose wells exposed to fluorescent beads for the next 24 h; the beads were then removed, and the incubation was continued for 3 days. A larger- pore-size (12-μm) membrane instead of the standard 3-μm membrane containing transwells was used for this experiment. Transmission of the beads was monitored by flow cytometry. The results presented in Fig. 1 indicate that very few beads compared to those of the control agarose well were transmitted in the tissue well. In addition, we performed transmission electron microscopy of the tissue before and after culture. The results (Fig. 2) did not reveal any discontinuity of the basal layer at day 0 and after 1 and 3 days of culture in our standard organ culture condition. All of these data together demonstrate no leakiness of the organ culture used in this study.
FIG. 1.
Transmission of fluorescently labeled beads across the cervical tissue after 24 h in culture. Transmission was measured by flow cytometry of the culture medium from the bottom well.
FIG. 2.
Transmission electron micrograph of cervical tissue at day 0 and after 1 and 3 days of culture in our standard organ culture condition. The arrow indicates the basal layer junction between the epithelial layer and the squamous cell layer.
Effect of viral phenotypes and cell association on transmission.
To determine whether HIV-1 transmission was dependent on viral phenotypes, we examined the transmission of the well-characterized R5 virus BAL and an X4 virus IIIB in our organ culture model. For this purpose, the infectivity titer of cryopreserved cell-free and cell-associated HIV BAL or IIIB was determined by serial dilution by an infectivity assay in cMAGI cells (5). Cervical tissues were then exposed for 24 h to 1 × 104 to 2 × 104 TCID50 of pretitered cell-free HIV-1 BAL, cell-free IIIB, PBMC infected with BAL (PBMC-BAL) or PBMC infected with IIIB (PBMC-IIIB), after which the tissues were washed to remove viral inoculum, and incubation was continued for 3 to 5 days. Viral transmission was monitored by measuring HIV-1 infectivity in the supernatant from the bottom well by the cMAGI assay. The percentage of transmission was calculated by the number of blue-stained cMAGI cells obtained from the culture supernatant of the tissue well divided by the number of blue-stained cMAGI cells obtained from the supernatant of the membrane-only well. We could not use HIV p24 measurement as a readout of viral transmission in the bottom well, because soluble p24 that is often present in the virus preparation could freely move through the agarose and would increase the background. Figure 3 shows representative results of HIV-1 transmission from cell-free and cell-associated HIV-1 BAL and IIIB. Cumulative data of all tissues tested are presented in Table 1. The results indicate that the kinetics of viral transmission of cell-free BAL and IIIB were similar with maximum transmission occurring within 1 day. While the maximum transmission of virus from IIIB-infected PBMC was on day 1, considerably slower transmission occurred from HIV-1 BAL-infected PBMC with maximum transmission on day 5. There was no transmission in the agarose control wells with either cell-free virus or infected PBMC. The lower rate of viral transmission from HIV-1 BAL-infected PBMC was probably not due to delay in the production of virus from infected cells, because the kinetics of virus production from HIV-1 IIIB-infected PBMC was comparable with that from HIV-1 BAL-infected PBMC in cell culture (P. Gupta, personal communication). These results clearly indicate that HIV-1 transmission across the tissue does occur regardless of whether virus is cell free or cell associated.
FIG. 3.
Kinetics of HIV-1 transmission from cell-free and cell-associated virus across the mucosa of the cervical tissue. (A) Cell-free BAL; (B) PBMC infected with BAL; (C) cell-free IIIB; (D) PBMC infected with IIIB. Percentage of transmission was calculated by the number of blue-stained cMAGI cells obtained from the culture supernatant of the tissue well divided by the number of blue-stained cMAGI cells obtained from the supernatant of the membrane-only well.
TABLE 1.
Transmission from cell-free and cell-associated HIV-1 IIIB and BAL across the mucosa of cervical tissues
| Source of HIV-1 exposure | Total no. of tissues tested | Median maximal HIV-1 transmission, % (range)a | Day of maximum transmission |
|---|---|---|---|
| BAL cell-free | 39 | 14.5 (1-64) | 1 |
| BAL PBMC | 12 | 10.0 (2.1-41) | 5 |
| IIIB cell-free | 12 | 6.0 (2.7-22) | 1 |
| IIIB PBMC | 16 | 6.0 (1-43) | 1 |
The median values of the percent transmissions in all tissues on the day of maximum transmission. The numbers of blue-stained cells in tissue wells and membrane-only control wells corresponding to the median values are as follows: for BAL cell-free, 40 in tissue wells with 275 in control wells (14.5%); BAL-PBMC, 21 in tissue wells with 210 in the control well (10.0%); IIIB cell-free, 17 in the tissue well with 233 in the control well (6.0%); and IIIB PBMC, 37 in the tissue well with 616 in the control well (6.0%).
To determine the relationship between viral phenotypes or cell association and transmission, the percentage of transmission at the peak point in all tissues was stratified by quartile ranging from the lowest to the highest level of maximum transmission that gave a value of 28% transmission at the cut point of the top quartile. When using 28% as a cut point in the quartile analysis, 11 of 39 tissues exposed to cell-free HIV-1 BAL had a transmission rate greater than 28%. In contrast, 1 of 12 tissues exposed to PBMC infected with HIV-1 BAL, 0 of 12 tissues exposed to cell-free HIV-1 IIIB and 1 of 16 tissues exposed to PBMC-infected with HIV-1 IIIB had transmission rates greater than 28%. Using these cut points for contingency table analysis, we found a significant difference in viral transmission between cell-free HIV-1 BAL and cell-free IIIB (P = 0.035; Fisher exact test). However, similar quartile analysis of transmission at the peak point showed no difference between cell-free and cell-associated HIV, regardless of whether it was BAL or IIIB (P = 0.32 and 0.35, respectively; Fisher exact test).
Identification of initial cell types that become infected during transmission.
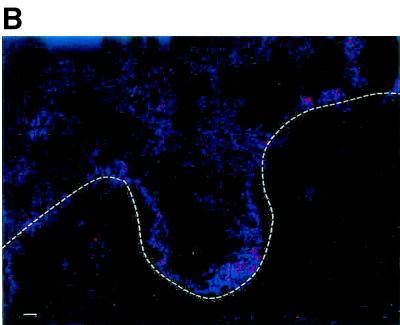
Since R5 viruses are more prevalent in most HIV-1-infected persons than X4 viruses, we have used HIV-1 BAL, an R5 virus, to determine the cell types that are initially infected during mucosal transmission. For this purpose, a standard cervical tissue-based organ culture was set up with HIV-1 BAL as described above. At 6, 24, and 96 h after viral exposure tissues were frozen and then analyzed by a modified simultaneous ultrasensitive fluorescence in situ HIV-1 RNA detection and immunophenotyping assay (3, 6, 22). The photomicrographic results are shown in Fig. 4. The first HIV-1 RNA-positive cells appeared in the epithelial-submucosal junction at 6 h, and this cell had the phenotype and morphology of a T cell (arrows). Numerous HIV-1 RNA-positive T cells were then identified after 24 h, and infected Langerhans cells (arrowheads) were seen only after 96 h (Fig. 4A). Multicolor laser confocal microscopy with three-dimensional recreation with a biotinylated anti-CD4 antibody subsequently stained with streptavidin-Cy5 (blue, Fig. 4A, inset) and an anti-CD45RO antibody conjugated to phycoerythrin (red, Fig. 4A, inset) demonstrated a globular distribution of HIV-1 gag-pol mRNA (green, Fig. 4A, inset) in the cytoplasm of lymphocytes. Further, the distribution of CD4 and the CD45RO isoform was irregular on the cell membrane. Infected Langerhans cells were identified by using CD1a antibody staining with simultaneous in situ hybridization and characteristic cellular morphology. Tissues stained with isotype control antibody or control labeled sense oligonucleotide did not show any RNA-positive cells (Fig. 4B). The quantitative analysis of the infected cells is shown in Table 2. Since the numbers of CD68-stained macrophages extremely low, they are not shown in Fig. 4 but results are presented in Table 2. The first HIV-1 RNA-positive cells were memory CD4+ T cells (0.2%) detected within 6 h of exposure, as indicated by a few HIV-1-expressing cells (Fig. 4). By 24 h the number of HIV-1 RNA-expressing memory CD4 cells increased to 2.7%, and a small amount in macrophages (0.8%) was located on or immediately beneath the epithelial layer. However, no dendritic cells were detected as HIV-1 RNA positive by 24 h. Only at 96 h after exposure was productive infection of Langerhans dendritic cells (0.7%), along with memory CD4+ T cells (2.3%) and macrophages (1.2%), detected.
FIG. 4.
Identification of cell types that first become infected in cervical tissue during transmission of HIV-1. Tissues from a single patient were exposed to cell-free HIV-1 BAL for 6, 24, and 96 h and then analyzed for the identification of cell types expressing HIV-1 gag-pol mRNA by simultaneous two-parameter immunophenotyping and ultrasensitive fluorescence in situ hybridization as previously described (3, 22). (A) Tissues stained with CD4, CD45, and CD1a antibodies and hybridized with HIV-1 gag-pol probes. T cells are indicated by arrows, and Langerhans cells are indicated by arrowheads. The inset (scale bar = 10 μm) is a three-dimensional, three-parameter, laser confocal image of a productively infected CD4+ CD45RO+ T cell at 24 h postinfection. This infected cell demonstrated a globular distribution of HIV-1 gag-pol mRNA (green) in the cytoplasm. Further, the distribution of CD4 (red) and the CD45RO (blue) isoform was irregular on the cell membrane. (B) Control tissue from the same individual as in panel A, stained with anti-immunoglobulin-phycoerythrin and anti-immunoglobulin-Cy5, and hybridized under the same conditions as in panel A with a sense probe cocktail. Scale bar, 20 μm. The dashed line indicates the epithelial-submucosal junction.
TABLE 2.
Quantitation of in situ signals in cervical tissues during HIV-1 transmission
| Time (h) after HIV-1 exposure | Percent HIV-1 RNA+ ina:
|
||
|---|---|---|---|
| CD4+ cells | CD68+ cells | CD1a+ cells | |
| 6 | 0.2 | 0 | 0 |
| 24 | 2.7 | 0.8 | 0.1b |
| 96 | 2.3 | 1.2 | 0.7 |
Values are averages of at least 10 high-power fields analyzed per specimen.
0.1% value constitutes the background signal.
Confirmation of infected immune cells in cervical explant biopsies by flow cytometry.
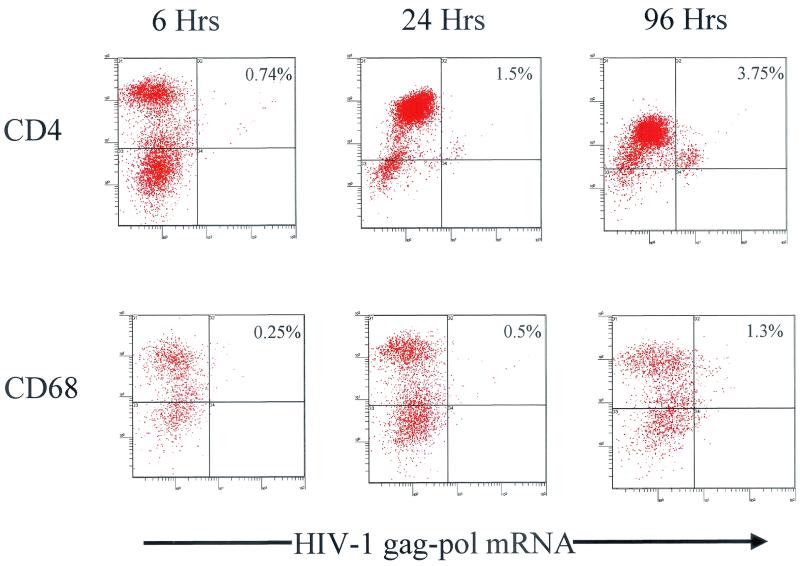
To confirm the results of laser confocal image analysis, we examined HIV-1 RNA-expressing cells directly in immune cells isolated from cervical tissues by using a mechanical disaggregation system that does not require enzymatic digestion. Such a nonproteolytic digestion was critical to preserve antigens for subsequent immunophenotyping stain. After recovery of immune cells, we stained these cells with CD4 and CD68 to unequivocably identify the T cells and macrophages. Unfortunately, we could not stain the immune cells for Langerhans cells by CD1a staining; because of their size, they are excluded from the immune cell fraction during filtration through the 50-μm filter used to eliminate epithelial cells from the analysis. After the immunophenotypic labeling, ultrasensitive fluorescence in situ hybridization was performed and the cells were analyzed by flow cytometry. Quadrants were adjusted based on nonspecific immunoglobulin controls. Dot plots were prepared based on gated-based forward and orthogonal light scatter characteristics, which allowed us to distinguish between T cells, macrophages, and dendritic cells prior to setting a gate on CD4. Similar to the tissue-based in situ studies, distinct HIV-1 RNA-positive T cells (0.74%) were primarily detected as early as 6 h which increased with time to 1.5% and 3.75% at 24 and 96 h, respectively (Fig. 5). Interestingly, a quantitative loss of CD4 in the lymphocyte gate was demonstrated in the fluorescence-activated cell sorting dot plots over time (Fig. 5), which supports previous in vivo and in vitro studies showing a loss of CD4 expression with HIV-1 infection (12, 23). Productive infection of macrophages was clearly demonstrated by 24 h, but not to the extent of productively infected T cells, and this increased over time (Fig. 5). As an internal control for the technique and instrument setup, a quantitative loss of CD68 was not seen, further supporting the loss of CD4 demonstrated in T cells.
FIG. 5.
Representative two-color dot plots of productively infected T cells and macrophages from cells isolated from cervical explant tissue postinfection at 6, 24, and 96 h. The background signal was 0.3%.
Effect of antiretroviral compounds on HIV-1 transmission.
To determine whether the organ culture system can be used to test potential anti-HIV compounds for their ability to block HIV-1 transmission across the mucosa, we examined the antiviral properties of PMPA, an inhibitor of HIV-1 and SIV replication (27, 29, 30). PMPA, a nucleotide analog, blocks viral replication by inhibiting reverse transcriptase at a preintegrational step. For this purpose, different concentrations of PMPA were added to the tissue wells and incubated for 1 to 2 h. After incubation, medium of the tissue well was replaced by 104 TCID50 of cell-free HIV-1 BAL in the presence of the same concentrations of PMPA and viral transmission was measured as described in Materials and Methods. Since maximum transmission from BAL occurs after 24 h of virus exposure, we determined the effect of PMPA on viral transmission at 24 h after exposure. The results shown in Fig. 6A indicate that PMPA blocked HIV-1 transmission in a dose-dependent manner. More than 70% inhibition was achieved at a concentration of ≥5.0 μg of PMPA per ml.
FIG. 6.
Effect of antiretroviral compound PMPA (A) and microbicide UC781 (B) on the transmission of cell-free HIV-1 BAL across the genital mucosa. The data represent the number of blue-stained cells at the peak point (day 1).
To determine whether the HIV-1 transmission in the organ culture system can be used to test microbicides for their ability to block HIV-1 transmission, we examined the antiviral properties of UC781, which has been shown to be virucidal for HIV-1 (2, 4). The experimental setup was the same as described for PMPA, except HIV-1 BAL was preincubated with UC781 at different concentrations for 1 h and then added to the tissue well. The results shown in Fig. 6B indicate a dose-dependent inhibition of HIV-1 transmission. Hematoxylin and eosin (H&E) staining of the tissues after 1 day in culture did not reveal any cytotoxicity due to exposure to UC781.
DISCUSSION
In this report we described a human cervical tissue-derived organ culture to study HIV-1 transmission. We previously provided biochemical, histological, and in situ molecular data to demonstrate that the transmission of HIV-1 observed in the organ culture system was not due to leakiness of the system. These data included the lack of transmission from blue dextran and UV-inactivated HIV-1, the maintenance of structural integrity of H&E-stained tissue as determined by low- and high-power microscopy, the detection of HIV-1 RNA-positive T cells in the submucosa, and a correlation of coreceptor utilization of HIV-1 in the tissues during viral transmission with the coreceptor properties of inoculating virus. In the present study we provided further evidence for the integrity of the organ culture by showing the physical integrity of the basal layer by transmission electron microscopy. Furthermore, we have shown the lack of transmission from a bigger particle size (7.2 μm) than blue dextran across the mucosa. An increase in the level of HIV-1 RNA-positive T cells and dendritic cells in the tissue during the 96 h of culture further demonstrates the specificity of HIV-1 transmission in the organ culture. Although the modified raft medium used in the present study contained hydrocortisone, subsequent studies have indicated similar levels of HIV-1 transmission in medium lacking hydrocortisone (data not shown).
Using the organ culture system we demonstrated HIV-1 transmission from both cell-free and cell-associated virus of different phenotypic properties across the genital mucosa. A relatively low level of viral transmission (6 to 12.5%) observed in the organ culture probably reflects the in vivo situation, wherein it is estimated that for every single vaginal exposure to infected semen the likelihood of an infection being established is only ca. 0.01% (15). However, HIV-1 transmission from cell-free virus in our organ culture system (1 day) is faster than that observed in the monkey (3 days) after intravaginal inoculation of SIV. A comparable level of viral transmission from cell-free and cell-associated HIV-1 suggests that both forms of virus that are present in semen can be transmitted across the female genital organ. However, the high level of cell-free HIV-1 and low level of cell-associated HIV-1 often found in semen (11, 15) would favor transmission from cell-free HIV-1 compared to cell-associated HIV-1. By using cell-free virus, we examined the effect of phenotypes on HIV-1 transmission across the mucosa. We have demonstrated significant levels of transmission from both R5 and X4 HIV-1, although the transmission efficiency was higher from the cell-free R5 virus. Based on these data and those of Long et al. (16), we hypothesize that at least in women there is no preferential transmission of macrophage-tropic HIV-1, although there could be a difference in transmission efficiency between strains of HIV-1 with different phenotypic properties.
Studies from a group of men acutely infected with HIV-1 led to the suggestion of a selective transmission of a homogeneous macrophage-tropic virus during sexual transmission in men (37). Recently, this suggestion gained support from the studies of Meng et al. (18), who demonstrated that intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ T cells. However, since biologic and molecular analysis of HIV-1 in semen indicated that the majority of the viral population present in semen is of the R5 type (7, 8, 10, 34), the transmission of R5 viruses in men may be a reflection of the existing pool of R5 variants of HIV-1 present in donor semen and not due to selective transmission. On rare occasions when X4 viruses are transmitted, the subjects develop AIDS in less than 2 years (1).
By using the in situ hybridization assay for HIV-1 RNA in the tissue of the organ culture, we demonstrated that within 6 h of exposure to HIV-1, memory CD4+ T cells and not the Langerhans cells located immediately below the epithelial cell layer were the first HIV-1-infected cells detected in this reproductive organ. Because of the detection of a few HIV-1 RNA-positive CD4+ T cells in the tissue 6 h after virus exposure, we have confirmed such an observation by examining the immune cells directly for CD4+ T cells expressing HIV-1 RNA by a flow cytometric simultaneous immunophenotyping and ultrasensitive fluorescence in situ hybridization assay. HIV-1 RNA-expressing Langerhans cells were detected between 1 and 4 days after infection. The lack of HIV-1 RNA-positive Langerhans cells at early time points after virus exposure could not be due to the migration of these cells from the tissue during culture because a sufficient number of CD1a+ Langerhans cells were present during the first 24 h of the expression. However, we could not rule out the possibility that Langerhans cells first became transiently infected with HIV-1, which was then transferred to the adjacent activated CD4+ T cells. A few HIV-1-infected macrophages were detected in the first 3 days after infection, although we did not extend the culture to 7 days to address the findings of Greenhead et al. (9), who found that the majority of HIV-1-infected cells are of macrophage lineage after 7 days in culture. These authors also could not detect any HIV-1-infected dendritic cells. However, by using an explant system, Kawamura et al. (14) implicated Langerhans cells as the first cells to become infected during sexual transmission of HIV-1. The difference in results between our study and that of Kawamura et al. (14) could be due to differences between the two model systems: our organ cultures are designed to study transmission, whereas explant cultures are HIV-1 infection models. Our data contrast with the studies of Hu et al. (13) in the SIV-rhesus monkey model, in which SIV-infected dendritic cells were detected as the first infected cell types shortly after intravaginal exposure to SIV. However, it is difficult to compare our data with the transmission data from the SIV-macaque model because human cervical biopsies from sexually active women with or without known sexually transmittted diseases generally have greatly elevated numbers of activated T cells compared to non-sexually active women (22) and macaques (B. K. Patterson, unpublished data). These data indicate that the cellular and viral factors responsible for the sexual transmission of HIV-1 could be different than those of SIV.
Identification of memory T cells as the first HIV-1-infected cells in cervical tissue after HIV exposure has an important implication on the biology of sexual transmission of HIV-1. This finding provides an alternative pathway for the dissemination of infection to the body. Since T cells support higher replication than do dendritic cells, early infection of T cells in genital mucosa would allow virus to expand rapidly and get transferred to the dendritic cells, which would then carry HIV-1 to draining lymph nodes, where the infection would be passed to CD4+ T lymphocytes that would spread the virus systemically throughout the body. These findings may have practical implications for developing strategies to block HIV sexual transmission.
The fact that PMPA, a known antiviral agent for HIV-1 and SIV (27, 29, 30), was able to block HIV-1 transmission across the mucosa provides further support for the specificity of HIV-1 transmission in the organ culture system. Since our organ culture system mimics in most part the in vivo situation, this system could also be useful to screen potential topical microbicides for their antiviral activity before they are used in clinical trials. The ability of UC781, a known microbicide of HIV-1 (2, 4), to block HIV-1 transmission in the organ culture system supports such a contention. Studies are ongoing to test a number of such topical microbicides in this organ culture system.
Acknowledgments
We thank Sharon Hillier for her continued support in microbicide work, Gilead Sciences for providing PMPA, Mike Parniak for providing UC781, Donna Beer Stolz for making the electron microscopic picture, Lawrence Kingsley for providing biostatistical analysis, Lindsey Mock for procuring tissue, and Judy Malenka for secretarial assistance.
This work was funded by the grants RO1-AI48402 (P.G.), PO1-AI39061 (Sharon Hillier), and RO1-AI47065 and PO1 HD40539-01 (B.K.P.) from the National Institutes of Health.
REFERENCES
- 1.Balachandran, R., P. Thampatty, A. Enrico, C. Rinaldo, and P. Gupta. 1991. Human immunideficiency virus isolates from asymptomatic homosexual men and from AIDS patients have distinct biologic and genetic properties. Virology 180:229-238. [DOI] [PubMed] [Google Scholar]
- 2.Balzarini, J., L. Naesens, E. Verbeken, M. Laga, L. Van Damme, M. Parniak, L. Van Mellaert, J. Anne, and E. De Clercq. 1998. Preclinical studies on thiocarboxanilide UC781 as a virucidal agent. AIDS 12:1129-1138. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani, H., E. Popck, P. Garcia, J. Anderson, A. L. Spectz, A. Landay, Z. Flener, and B. K. Patterson. 2000. Upregulation of CCR5 expression in the placenta is associated with human immunodeficiency virus-1 vertical transmission. Am. J. Pathol. 157:1811-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borkow, G., J. Barnard, T. M. Nguyen, A. Belmonte, M. A. Wainberg, and M. A. Parniak. 1997. Chemical barrier to human immunodeficiency virus type 1 (HIV-1) infection: retrovirucidal activity of UC781, a thiocarboxanilide nonnucleoside inhibitor of HIV-1 reverse transcriptase. J. Virol. 71:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chackerian, B., E. Long, P. Luciw, and J. Overbaugh. 1997. Human immunodeficiency virus type 1 coreceptors participate in postentry stages of the virus replication cycle and function in simian immunodeficiency virus infection. J. Virol. 71:3932-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins, K. B., B. K. Patterson, G. J. Naus, D. V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475-479. [DOI] [PubMed] [Google Scholar]
- 7.Coombs, R., C. Speck, J. Hughes, W. Lee, R. Sampoleo, S. Ross, J. Dragavon, G. Peterson, T. Hooton, A. Collier, L. Corey, L. Koutsky, and J. N. Krieger. 1998. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J. Infect. Dis. 177:320-330. [DOI] [PubMed] [Google Scholar]
- 8.Delwart, E., J. Mullins, P. Gupta, G. J. Learn, M. Holodny, D. Katzenstein, B. Walker, and M. Singh. 1998. Human immunodeficiency virus type 1 populations in blood and semen. J. Virol. 72:617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenhead, P., P. Hays, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta, P., C. Leroux, B. K. Patterson, L. Kingsley, C. Rinaldo, M. Ding, Y. Chen, K. Kulka, W. Buchanan, B. McKeon, and R. Montelaro. 2000. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasi species between blood and semen. J. Infect. Dis. 182:79-87. [DOI] [PubMed] [Google Scholar]
- 11.Gupta, P., J. Mellors, L. Kingsley, S. Riddler, M. Singh, S. Schreiber, M. Cronin, and C. Rinaldo. 1997. High viral load in semen of human immunodeficiency virus type 1-infected men at all stages of disease and its reduction by therapy with protease and nonnucleoside reverse transcriptase inhibitors. J. Virol. 71:6271-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoxie, J. A., J. D. Alpers, J. L. Rackoqski, K. Huebner, B. S. Haggerty, A. J. Cederbaum, and J. C. Reed. 1986. Alteration in T4 (CD4) protein and mRNA synthesis in cells infected by HIV. Science 234:1123-1127. [DOI] [PubMed] [Google Scholar]
- 13.Hu, J., M. D. Gardner, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawamura, T., S. Cohen, D. L. Borris, E. A. Aquilino, S. Glushakova, L. B. Margolis, J. M. Orenstein, R. E. Offord, R. A. Neurath, and A. Blauvelt. 2000. Candidate microbicides block HIV-1 infection of human immature Langerhans cells within epithelial tissue explants. J. Exp. Med. 192:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy, J. 1998. HIV and the pathogenesis of AIDS, p. 29-35. American Society for Microbiology, Washington, D.C.
- 16.Long, E. M., H. L. Martin, J. K. Kreiss, S. M. J. Rainwater, L. Lavreys, D. J. Lackson, J. Rakwar, K. Mandaliya, and J. Overbaugh. 2000. Gender differences in HIV-1 diversity at time of infection. Nat. Med. 6:71-75. [DOI] [PubMed] [Google Scholar]
- 17.Mann, J., D. Tarantola, and T. Netter. 1992. AIDS in the world. Harvard University Press, Cambridge, Mass.
- 18.Meng, G., X. Wei, X. Wu, M. T. Sellers, J. M. Decker, Z. Moldoveanu, J. M. Orenstein, M. F. Graham, J. C. Kappes, J. Mestecky, G. M. Shaw, and P. D. Smith. 2002. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5 cells. Nat. Med. 8:150-156. [DOI] [PubMed] [Google Scholar]
- 19.Mermin, J. H., M. Holodiny, D. A. Katzenstein, and T. C. Merigan. 1991. Detection of human immunodeficiency virus DNA and RNA in semen by the polymerase chain reaction. J. Infect. Dis. 164:769-772. [DOI] [PubMed] [Google Scholar]
- 20.Nkowane, B. 1991. Prevalence and incidence of HIV infection in Africa: a review of data published in 1990. AIDS 5:S7-S15. [PubMed] [Google Scholar]
- 21.Padian, N. S., S. C. Shiboski, and N. P. Jewell. 1991. Female-to-male transmission of human immunodeficiency virus. JAMA 266:1664-1667. [PubMed] [Google Scholar]
- 22.Patterson, B. K., M. A. Czerniewski, J. Pottage, M. Agnoli, H. Kessler, and A. Landay. 1999. Monitoring HIV therapy in immune cell subsets using ultrasensitive fluorescence in situ hybridization. Lancet 353:211-212. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, B. K., C. Goolsby, V. Hodara, P. Otto, K. Lohman, and S. Wolinsky. 1995. Flow cytometric detection of CD4+ cells harboring human immunodeficiency virus type 1 (HIV-1) DNA by dual immunophenotyping and PCR-driven in situ hybridization: evidence of epitope masking of the CD4 cell surface molecule in vivo. J. Virol. 69:4316-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patterson, B. K., S. McCallister, M. Schutz, J. N. Siegel, K. Shults, Z. Flener, and A. Landay. 2001. Persistence of intracellular HIV-1 mRNA correlates with HIV-1-specific immune responses in HIV-1-infected subjects on stable HAART therapy. AIDS 15:1635-1641. [DOI] [PubMed] [Google Scholar]
- 25.Pomerantz, R., S. de la Monte, C. Donegan, T. Rota, M. Vogt, and D. Craven. 1988. Human immunodeficiency virus (HIV) infection of uterine cervix. Ann. Intern. Med. 108:321-327. [DOI] [PubMed] [Google Scholar]
- 26.Pudney, J., and D. J. Anderson. 1991. Orchitis and human immunodeficiency virus type 1-infected cells in reproductive tissues from men with the acquired immune deficiency syndrome. Am. J. Pathol. 139:149-160. [PMC free article] [PubMed] [Google Scholar]
- 27.Silvera, P., P. Racz, K. Racz, N. Bischofberger, C. Crabbs, J. Yalley-Ogunro, J. Greenhouse, J. B. Jiang, and M. G. Lewis. 2000. Effect of PMPA and PMEA on the kinetics of viral load in simian immunodeficiency virus-infected macaques. AIDS Res. Hum. Retrovir. 16:791-800. [DOI] [PubMed] [Google Scholar]
- 28.Spira, A., P. Marx, B. Patterson, J. Mahoney, R. Koup, S. Wolinsky, and D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas, R. V., and A. Fridland. 1998. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl) PMPA agaainst various drug-resistant human immunodeficiency virus strains. Antimicrob. Agents Chemother. 42:1484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Rompay, K. K., J. M. Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pederson. 1999. 9-[2-(Phosphonomethoxy)propyl] adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother. 343:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Voorhis, B. J., A. Martinez, K. Meyer, and D. J. Anderson. 1991. Detection of human immunodeficiency virus type 1 in semen from seropositive men using culture and polymerase chain reaction deoxyribonucleic acid amplification technique. Fertil. Steril. 55:588-594. [PubMed] [Google Scholar]
- 32.Vernazza, P., J. Eron, M. Cohen, C. Vander Horst, L. Troian, and S. Fiscus. 1994. Detection of biologic characterization of infectious HIV-1 in semen of seropositive men. AIDS 8:1325-1329. [DOI] [PubMed] [Google Scholar]
- 33.Vernazza, P., J. Eron, and S. Fiscus. 1996. Sensitive method for the detection of infectious HIV in semen of seropositive individuals. J. Virol. Methods 56:33-40. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, H., G. Dornadula, M. Deumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1998. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]
- 35.Zhang, L. Q., P. MacKenzie, A. Cleland, E. C. Holmes, A. J. Leigh Brown, and P. Simmonds. 1993. Selection for specific sequences in the external envelop protein of human immunodeficiency virus type 1 upon primary infection. J. Virol. 67:3345-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, T., H. Mo, N. Wang, D. Nam, Y. Cao, R. Koup, and D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]