Abstract
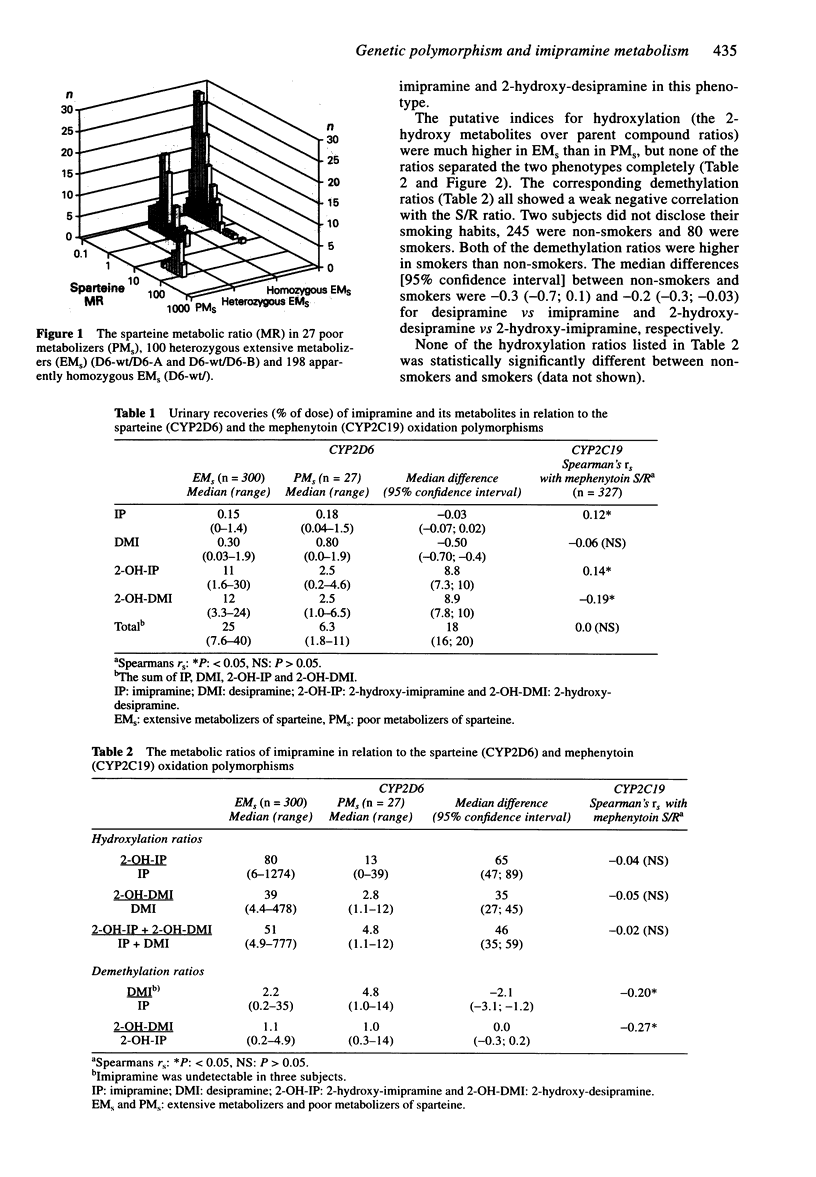
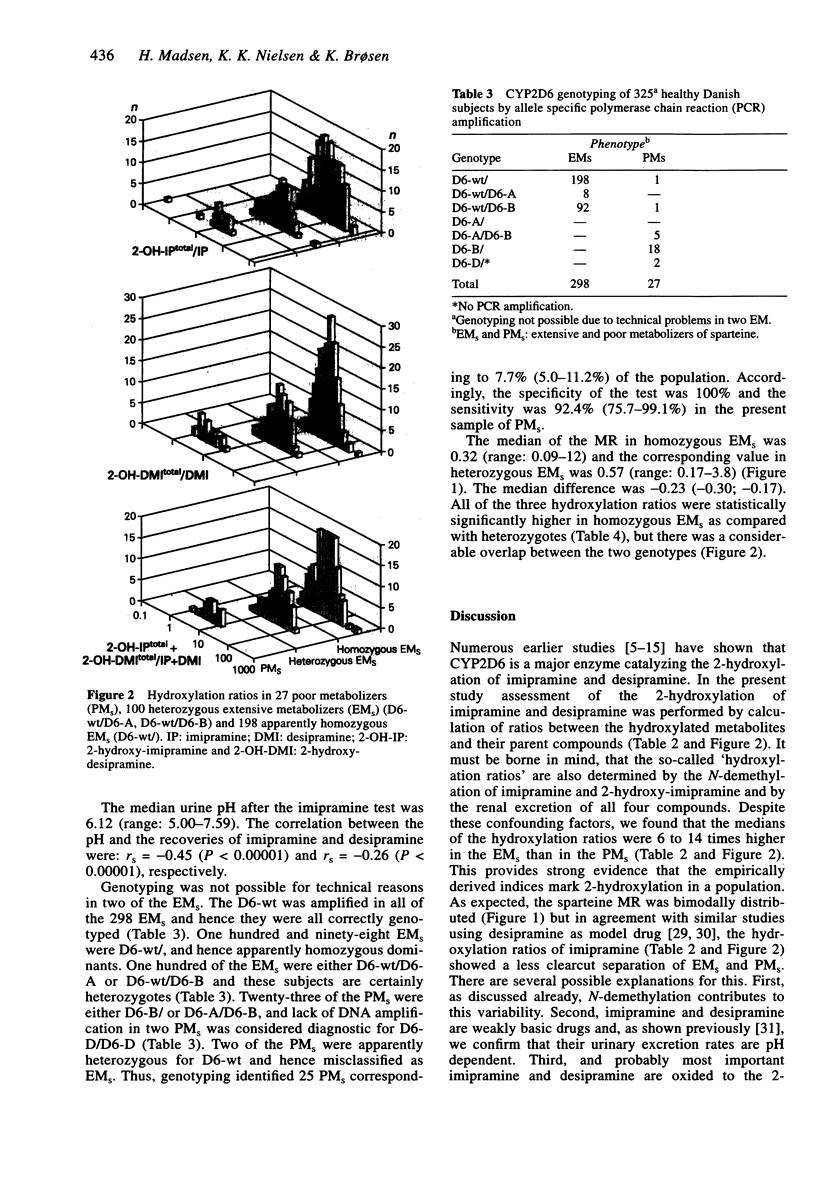
1. Sparteine and mephenytoin phenotyping tests were carried out in 327 healthy Danish subjects. Two weeks later each subject took 25 mg imipramine followed by urine collection for 24 h. The urinary content of imipramine, desipramine, 2-hydroxy-imipramine and 2-hydroxy-desipramine was assayed by h.p.l.c. 2. The medians of the hydroxylation ratios (i.e. 2-hydroxy-metabolite over parent compound) were 6 to 14 times higher in 300 extensive metabolizers of sparteine (EMs) as compared with 27 poor metabolizers (PMs), but none of the ratios separated the two phenotypes completely. 3. There were 324 EM of mephenytoin (EMM) and three PM (PMM) in the sample. The demethylation ratios between desipramine, 2-hydroxy-desipramine and their corresponding tertiary amines showed statistically significant correlations with the mephenytoin S/R isomer ratio (Spearman's rs: -0.20 and -0.27, P < 0.05). 4. The demethylation ratios were higher in 80 smokers than in 245 non-smokers. This indicates that CYP1A2, which is induced by cigarette smoking, also catalyzes the N-demethylation of imipramine. 5. CYP2D6 genotyping was carried out by PCR in 325 of the subjects, and the D6-wt allele was amplified in 298 EMs, meaning that they were genotyped correctly. One PMs was D6-wt/D6-B, another PMs had the genotype D6-wt/ and hence both were misclassified as EMs. The remaining 25 PMs were D6-A/D6-B (n = 5), D6-B/ (n = 18) or D6-D/D6-D (no PCR amplification, n = 2).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Bechtel P., Iselius L., Gundert-Remy U. Hydroxylation polymorphisms of debrisoquine and mephenytoin in European populations. Eur J Clin Pharmacol. 1990;39(6):533–537. doi: 10.1007/BF00316090. [DOI] [PubMed] [Google Scholar]
- Bertilsson L., Aberg-Wistedt A. The debrisoquine hydroxylation test predicts steady-state plasma levels of desipramine. Br J Clin Pharmacol. 1983 Mar;15(3):388–390. doi: 10.1111/j.1365-2125.1983.tb01518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broly F., Gaedigk A., Heim M., Eichelbaum M., Morike K., Meyer U. A. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol. 1991 Oct;10(8):545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- Broly F., Meyer U. A. Debrisoquine oxidation polymorphism: phenotypic consequences of a 3-base-pair deletion in exon 5 of the CYP2D6 gene. Pharmacogenetics. 1993 Jun;3(3):123–130. [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. First-pass metabolism of imipramine and desipramine: impact of the sparteine oxidation phenotype. Clin Pharmacol Ther. 1988 Apr;43(4):400–406. doi: 10.1038/clpt.1988.50. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. Quinidine inhibits the 2-hydroxylation of imipramine and desipramine but not the demethylation of imipramine. Eur J Clin Pharmacol. 1989;37(2):155–160. doi: 10.1007/BF00558224. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Klysner R., Gram L. F., Otton S. V., Bech P., Bertilsson L. Steady-state concentrations of imipramine and its metabolites in relation to the sparteine/debrisoquine polymorphism. Eur J Clin Pharmacol. 1986;30(6):679–684. doi: 10.1007/BF00608215. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Nielsen P. N., Brusgaard K., Gram L. F., Skjødt K. CYP2D6 genotype determination in the Danish population. Eur J Clin Pharmacol. 1994;47(3):221–225. doi: 10.1007/BF02570501. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Otton S. V., Gram L. F. Imipramine demethylation and hydroxylation: impact of the sparteine oxidation phenotype. Clin Pharmacol Ther. 1986 Nov;40(5):543–549. doi: 10.1038/clpt.1986.221. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Otton S. V., Gram L. F. Sparteine oxidation polymorphism in Denmark. Acta Pharmacol Toxicol (Copenh) 1985 Nov;57(5):357–360. doi: 10.1111/j.1600-0773.1985.tb00058.x. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Skjelbo E., Rasmussen B. B., Poulsen H. E., Loft S. Fluvoxamine is a potent inhibitor of cytochrome P4501A2. Biochem Pharmacol. 1993 Mar 24;45(6):1211–1214. doi: 10.1016/0006-2952(93)90272-x. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Zeugin T., Meyer U. A. Role of P450IID6, the target of the sparteine-debrisoquin oxidation polymorphism, in the metabolism of imipramine. Clin Pharmacol Ther. 1991 Jun;49(6):609–617. doi: 10.1038/clpt.1991.77. [DOI] [PubMed] [Google Scholar]
- Chiba K., Saitoh A., Koyama E., Tani M., Hayashi M., Ishizaki T. The role of S-mephenytoin 4'-hydroxylase in imipramine metabolism by human liver microsomes: a two-enzyme kinetic analysis of N-demethylation and 2-hydroxylation. Br J Clin Pharmacol. 1994 Mar;37(3):237–242. doi: 10.1111/j.1365-2125.1994.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen J., Gram L. F., Kofod B., Rafaelsen O. J. Imipramine metabolism in man. A study of urinary metabolites after administration of radioactive imipramine. Psychopharmacologia. 1967 Aug 4;11(3):255–264. doi: 10.1007/BF00405231. [DOI] [PubMed] [Google Scholar]
- Coutts R. T., Su P., Baker G. B., Daneshtalab M. Metabolism of imipramine in vitro by isozyme CYP2D6 expressed in a human cell line, and observations on metabolite stability. J Chromatogr. 1993 Jun 2;615(2):265–272. doi: 10.1016/0378-4347(93)80340-a. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Iselius L., Alm C., Svensson J. O., Lee D., Johansson I., Ingelman-Sundberg M., Sjöqvist F. Polymorphic 2-hydroxylation of desipramine. A population and family study. Eur J Clin Pharmacol. 1993;44(5):445–450. doi: 10.1007/BF00315541. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Johansson I., Palmertz M. P., Ingelman-Sundberg M., Sjöqvist F. Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther. 1992 Jan;51(1):12–17. doi: 10.1038/clpt.1992.2. [DOI] [PubMed] [Google Scholar]
- Drøhse A., Bathum L., Brøsen K., Gram L. F. Mephenytoin and sparteine oxidation: genetic polymorphisms in Denmark. Br J Clin Pharmacol. 1989 May;27(5):620–625. doi: 10.1111/j.1365-2125.1989.tb03426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaedigk A., Blum M., Gaedigk R., Eichelbaum M., Meyer U. A. Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet. 1991 May;48(5):943–950. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. A., Faletto M. B., Romkes-Sparks M., Sullivan T., Kitareewan S., Raucy J. L., Lasker J. M., Ghanayem B. I. Evidence that CYP2C19 is the major (S)-mephenytoin 4'-hydroxylase in humans. Biochemistry. 1994 Feb 22;33(7):1743–1752. doi: 10.1021/bi00173a017. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Bjerre M., Kragh-Sørensen P., Kvinesdal B., Molin J., Pedersen O. L., Reisby N. Imipramine metabolites in blood of patients during therapy and after overdose. Clin Pharmacol Ther. 1983 Mar;33(3):335–342. doi: 10.1038/clpt.1983.42. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Kofod B., Christiansen J., Rafaelsen O. J. Imipramine metabolism: pH-dependent distribution and urinary excretion. Clin Pharmacol Ther. 1971 Mar-Apr;12(2):239–244. doi: 10.1002/cpt1971122part1239. [DOI] [PubMed] [Google Scholar]
- Gram L. F. Metabolism of tricyclic antidepressants. A review. Dan Med Bull. 1974 Oct;21(6):218–231. [PubMed] [Google Scholar]
- Gram L. F., Sondergaard I., Christiansen J., Petersen G. O., Bech P., Reisby N., Ibsen I., Ortmann J., Nagy A., Dencker S. J. Steady-state kinetics of imipramine in patients. Psychopharmacology (Berl) 1977 Nov 15;54(3):255–261. doi: 10.1007/BF00426573. [DOI] [PubMed] [Google Scholar]
- Heim M., Meyer U. A. Genotyping of poor metabolisers of debrisoquine by allele-specific PCR amplification. Lancet. 1990 Sep 1;336(8714):529–532. doi: 10.1016/0140-6736(90)92086-w. [DOI] [PubMed] [Google Scholar]
- Jackson P. R., Tucker G. T., Lennard M. S., Woods H. F. Polymorphic drug oxidation: pharmacokinetic basis and comparison of experimental indices. Br J Clin Pharmacol. 1986 Nov;22(5):541–550. doi: 10.1111/j.1365-2125.1986.tb02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I., Lundqvist E., Bertilsson L., Dahl M. L., Sjöqvist F., Ingelman-Sundberg M. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11825–11829. doi: 10.1073/pnas.90.24.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine A., Gautier J. C., Azoulay D., Kiffel L., Belloc C., Guengerich F. P., Maurel P., Beaune P., Leroux J. P. Major pathway of imipramine metabolism is catalyzed by cytochromes P-450 1A2 and P-450 3A4 in human liver. Mol Pharmacol. 1993 May;43(5):827–832. [PubMed] [Google Scholar]
- Nielsen K. K., Brøsen K. High-performance liquid chromatography of imipramine and six metabolites in human plasma and urine. J Chromatogr. 1993 Jan 29;612(1):87–94. doi: 10.1016/0378-4347(93)80371-a. [DOI] [PubMed] [Google Scholar]
- Sanz E. J., Villén T., Alm C., Bertilsson L. S-mephenytoin hydroxylation phenotypes in a Swedish population determined after coadministration with debrisoquin. Clin Pharmacol Ther. 1989 May;45(5):495–499. doi: 10.1038/clpt.1989.63. [DOI] [PubMed] [Google Scholar]
- Sesardic D., Boobis A. R., Edwards R. J., Davies D. S. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1988 Oct;26(4):363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelbo E., Brøsen K., Hallas J., Gram L. F. The mephenytoin oxidation polymorphism is partially responsible for the N-demethylation of imipramine. Clin Pharmacol Ther. 1991 Jan;49(1):18–23. doi: 10.1038/clpt.1991.4. [DOI] [PubMed] [Google Scholar]
- Skjelbo E., Brøsen K. Inhibitors of imipramine metabolism by human liver microsomes. Br J Clin Pharmacol. 1992 Sep;34(3):256–261. doi: 10.1111/j.1365-2125.1992.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjelbo E., Gram L. F., Brøsen K. The N-demethylation of imipramine correlates with the oxidation of S-mephenytoin (S/R-ratio). A population study. Br J Clin Pharmacol. 1993 Mar;35(3):331–334. [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Gough A. C., Leigh P. N., Summers B. A., Harding A. E., Maraganore D. M., Sturman S. G., Schapira A. H., Williams A. C., Maranganore D. M. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet. 1992 Jun 6;339(8806):1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- Spina E., Birgersson C., von Bahr C., Ericsson O., Mellström B., Steiner E., Sjöqvist F. Phenotypic consistency in hydroxylation of desmethylimipramine and debrisoquine in healthy subjects and in human liver microsomes. Clin Pharmacol Ther. 1984 Nov;36(5):677–682. doi: 10.1038/clpt.1984.239. [DOI] [PubMed] [Google Scholar]
- Spina E., Steiner E., Ericsson O., Sjöqvist F. Hydroxylation of desmethylimipramine: dependence on the debrisoquin hydroxylation phenotype. Clin Pharmacol Ther. 1987 Mar;41(3):314–319. doi: 10.1038/clpt.1987.33. [DOI] [PubMed] [Google Scholar]
- Tyndale R., Aoyama T., Broly F., Matsunaga T., Inaba T., Kalow W., Gelboin H. V., Meyer U. A., Gonzalez F. J. Identification of a new variant CYP2D6 allele lacking the codon encoding Lys-281: possible association with the poor metabolizer phenotype. Pharmacogenetics. 1991 Oct;1(1):26–32. doi: 10.1097/00008571-199110000-00005. [DOI] [PubMed] [Google Scholar]
- Vinks A., Inaba T., Otton S. V., Kalow W. Sparteine metabolism in Canadian Caucasians. Clin Pharmacol Ther. 1982 Jan;31(1):23–29. doi: 10.1038/clpt.1982.4. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Stevens J. C., Becker G. W., VandenBranden M. Isolation and characterization of human liver cytochrome P450 2C19: correlation between 2C19 and S-mephenytoin 4'-hydroxylation. Arch Biochem Biophys. 1993 Oct;306(1):240–245. doi: 10.1006/abbi.1993.1506. [DOI] [PubMed] [Google Scholar]
- Zanger U. M., Vilbois F., Hardwick J. P., Meyer U. A. Absence of hepatic cytochrome P450bufI causes genetically deficient debrisoquine oxidation in man. Biochemistry. 1988 Jul 26;27(15):5447–5454. doi: 10.1021/bi00415a010. [DOI] [PubMed] [Google Scholar]
- de Morais S. M., Wilkinson G. R., Blaisdell J., Nakamura K., Meyer U. A., Goldstein J. A. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994 Jun 3;269(22):15419–15422. [PubMed] [Google Scholar]


