Abstract
1. We have assessed the interaction of the antimalarial halofantrine with cytochrome P450 (CYP) enzymes in vitro, with the use of microsomes from human liver and recombinant cell lines. 2. Rac-halofantrine was a potent inhibitor (IC50 = 1.06 microM, Ki = 4.3 microM) of the 1-hydroxylation of bufuralol, a marker for CYP2D6 activity. Of a group of structurally related antimalarials tested, only quinidine (IC50 = 0.04 microM) was more potent. 3. Microsomes prepared from recombinant CYP2D6 and CYP3A4 cell lines were shown to catalyse halofantrine N-debutylation. 4. The metabolism of halofantrine to its N-desbutyl metabolite by human liver microsomes showed no correlation with CYP2D6 genotypic or phenotypic status and there was no consistent inhibition by quinidine. 5. The rate of halofantrine metabolism showed a significant correlation with both CYP3A4 protein levels (r = 0.88, P = 0.01) and the rate of felodipine metabolism (r = 0.86, P = 0.013), a marker substrate for CYP3A4 activity. Inhibition studies showed that ketoconazole is a potent inhibitor of halofantrine metabolism (IC50 = 1.57 microM). 6. In conclusion, we have demonstrated that halofantrine is a potent inhibitor of CYP2D6 in vitro and can also be metabolised by the enzyme. However, in human liver microsomes it appears to be metabolised largely by CYP3A4.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basco L. K., Gillotin C., Gimenez F., Farinotti R., Le Bras J. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br J Clin Pharmacol. 1992 May;33(5):517–520. doi: 10.1111/j.1365-2125.1992.tb04081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson H. M., Goa K. L. Halofantrine. A review of its antimalarial activity, pharmacokinetic properties and therapeutic potential. Drugs. 1992 Feb;43(2):236–258. doi: 10.2165/00003495-199243020-00009. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F., Haghfelt T., Bertilsson L. Extensive metabolizers of debrisoquine become poor metabolizers during quinidine treatment. Pharmacol Toxicol. 1987 Apr;60(4):312–314. doi: 10.1111/j.1600-0773.1987.tb01758.x. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Hansen J. G., Nielsen K. K., Sindrup S. H., Gram L. F. Inhibition by paroxetine of desipramine metabolism in extensive but not in poor metabolizers of sparteine. Eur J Clin Pharmacol. 1993;44(4):349–355. doi: 10.1007/BF00316471. [DOI] [PubMed] [Google Scholar]
- Brøsen K. The pharmacogenetics of the selective serotonin reuptake inhibitors. Clin Investig. 1993 Dec;71(12):1002–1009. doi: 10.1007/BF00180032. [DOI] [PubMed] [Google Scholar]
- Cosgriff T. M., Boudreau E. F., Pamplin C. L., Doberstyn E. B., Desjardins R. E., Canfield C. J. Evaluation of the antimalarial activity of the phenanthrenemethanol halofantrine (WR 171,669). Am J Trop Med Hyg. 1982 Nov;31(6):1075–1079. doi: 10.4269/ajtmh.1982.31.1075. [DOI] [PubMed] [Google Scholar]
- Forrester L. M., Henderson C. J., Glancey M. J., Back D. J., Park B. K., Ball S. E., Kitteringham N. R., McLaren A. W., Miles J. S., Skett P. Relative expression of cytochrome P450 isoenzymes in human liver and association with the metabolism of drugs and xenobiotics. Biochem J. 1992 Jan 15;281(Pt 2):359–368. doi: 10.1042/bj2810359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez F., Gillotin C., Basco L. K., Bouchaud O., Aubry A. F., Wainer I. W., Le Bras J., Farinotti R. Plasma concentrations of the enantiomers of halofantrine and its main metabolite in malaria patients. Eur J Clin Pharmacol. 1994;46(6):561–562. doi: 10.1007/BF00196116. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. Human cytochromes P450: problems and prospects. Trends Pharmacol Sci. 1992 Sep;13(9):346–352. doi: 10.1016/0165-6147(92)90107-h. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Idle J. R. Pharmacogenetic phenotyping and genotyping. Present status and future potential. Clin Pharmacokinet. 1994 Jan;26(1):59–70. doi: 10.2165/00003088-199426010-00005. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Meyer U. A. Molecular genetics of the debrisoquin-sparteine polymorphism. Clin Pharmacol Ther. 1991 Sep;50(3):233–238. doi: 10.1038/clpt.1991.131. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Brian W. R., Iwasaki M., Sari M. A., Bärnhielm C., Berntsson P. Oxidation of dihydropyridine calcium channel blockers and analogues by human liver cytochrome P-450 IIIA4. J Med Chem. 1991 Jun;34(6):1838–1844. doi: 10.1021/jm00110a012. [DOI] [PubMed] [Google Scholar]
- Halpert J. R., Guengerich F. P., Bend J. R., Correia M. A. Selective inhibitors of cytochromes P450. Toxicol Appl Pharmacol. 1994 Apr;125(2):163–175. doi: 10.1006/taap.1994.1061. [DOI] [PubMed] [Google Scholar]
- Kagimoto M., Heim M., Kagimoto K., Zeugin T., Meyer U. A. Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem. 1990 Oct 5;265(28):17209–17214. [PubMed] [Google Scholar]
- Karbwang J., Na Bangchang K., Bunnag D., Harinasuta T., Laothavorn P. Cardiac effect of halofantrine. Lancet. 1993 Aug 21;342(8869):501–501. doi: 10.1016/0140-6736(93)91631-u. [DOI] [PubMed] [Google Scholar]
- Kolars J. C., Schmiedlin-Ren P., Schuetz J. D., Fang C., Watkins P. B. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J Clin Invest. 1992 Nov;90(5):1871–1878. doi: 10.1172/JCI116064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koymans L. M., Vermeulen N. P., Baarslag A., Donné-Op den Kelder G. M. A preliminary 3D model for cytochrome P450 2D6 constructed by homology model building. J Comput Aided Mol Des. 1993 Jun;7(3):281–289. doi: 10.1007/BF00125503. [DOI] [PubMed] [Google Scholar]
- Koymans L., Vermeulen N. P., van Acker S. A., te Koppele J. M., Heykants J. J., Lavrijsen K., Meuldermans W., Donné-Op den Kelder G. M. A predictive model for substrates of cytochrome P450-debrisoquine (2D6). Chem Res Toxicol. 1992 Mar-Apr;5(2):211–219. doi: 10.1021/tx00026a010. [DOI] [PubMed] [Google Scholar]
- Kronbach T. Bufuralol, dextromethorphan, and debrisoquine as prototype substrates for human P450IID6. Methods Enzymol. 1991;206:509–517. doi: 10.1016/0076-6879(91)06120-r. [DOI] [PubMed] [Google Scholar]
- Kronbach T., Mathys D., Gut J., Catin T., Meyer U. A. High-performance liquid chromatographic assays for bufuralol 1'-hydroxylase, debrisoquine 4-hydroxylase, and dextromethorphan O-demethylase in microsomes and purified cytochrome P-450 isozymes of human liver. Anal Biochem. 1987 Apr;162(1):24–32. doi: 10.1016/0003-2697(87)90006-6. [DOI] [PubMed] [Google Scholar]
- Kunze K. L., Trager W. F. Isoform-selective mechanism-based inhibition of human cytochrome P450 1A2 by furafylline. Chem Res Toxicol. 1993 Sep-Oct;6(5):649–656. doi: 10.1021/tx00035a009. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
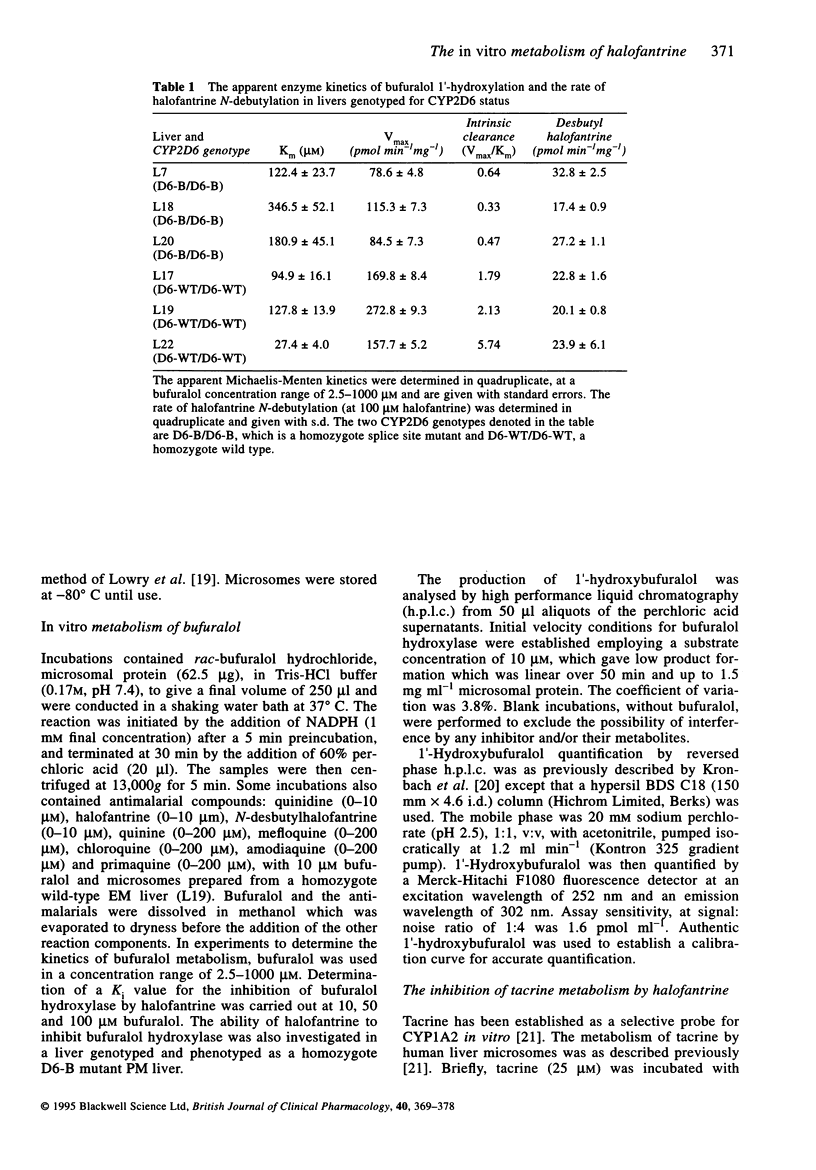
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Madden S., Back D. J., Orme M. L. Metabolism of the contraceptive steroid desogestrel by human liver in vitro. J Steroid Biochem. 1990 Feb;35(2):281–288. doi: 10.1016/0022-4731(90)90285-z. [DOI] [PubMed] [Google Scholar]
- Mberu E. K., Muhia D. K., Watkins W. M. Measurement of halofantrine and its major metabolite desbutylhalofantrine in plasma and blood by high-performance liquid chromatography: a new methodology. J Chromatogr. 1992 Oct 2;581(1):156–160. doi: 10.1016/0378-4347(92)80461-x. [DOI] [PubMed] [Google Scholar]
- Milton K. A., Ward S. A., Edwards G. Determination of halofantrine and its principal metabolite desbutylhalofantrine in biological fluids by reversed-phase high-performance liquid chromatography. J Chromatogr. 1988 Dec 9;433:339–344. doi: 10.1016/s0378-4347(00)80618-0. [DOI] [PubMed] [Google Scholar]
- Mura C., Gerard N., Vincent-Viry M., Galteau M. M., Jacqz-Aigrain E., Krishnamoorthy R. Molecular heterogeneity of the XbaI defined 44kb allele of the CYP2D locus within the Caucasian population. Br J Clin Pharmacol. 1993 Feb;35(2):161–165. doi: 10.1111/j.1365-2125.1993.tb05681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M. D., Brøsen K., Gram L. F. A dose-effect study of the in vivo inhibitory effect of quinidine on sparteine oxidation in man. Br J Clin Pharmacol. 1990 Mar;29(3):299–304. doi: 10.1111/j.1365-2125.1990.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F., ter Kuile F. O., Luxemburger C., Woodrow C., Kyle D. E., Chongsuphajaisiddhi T., White N. J. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993 Apr 24;341(8852):1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Otton S. V., Inaba T., Kalow W. Competitive inhibition of sparteine oxidation in human liver by beta-adrenoceptor antagonists and other cardiovascular drugs. Life Sci. 1984 Jan 2;34(1):73–80. doi: 10.1016/0024-3205(84)90332-1. [DOI] [PubMed] [Google Scholar]
- Shimada T., Yamazaki H., Mimura M., Inui Y., Guengerich F. P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994 Jul;270(1):414–423. [PubMed] [Google Scholar]
- Smith C. A., Gough A. C., Leigh P. N., Summers B. A., Harding A. E., Maraganore D. M., Sturman S. G., Schapira A. H., Williams A. C., Maranganore D. M. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson's disease. Lancet. 1992 Jun 6;339(8806):1375–1377. doi: 10.1016/0140-6736(92)91196-f. [DOI] [PubMed] [Google Scholar]
- Smith D. A., Jones B. C. Speculations on the substrate structure-activity relationship (SSAR) of cytochrome P450 enzymes. Biochem Pharmacol. 1992 Dec 1;44(11):2089–2098. doi: 10.1016/0006-2952(92)90333-e. [DOI] [PubMed] [Google Scholar]
- Strobl G. R., von Kruedener S., Stöckigt J., Guengerich F. P., Wolff T. Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modeling and inhibition studies. J Med Chem. 1993 Apr 30;36(9):1136–1145. doi: 10.1021/jm00061a004. [DOI] [PubMed] [Google Scholar]
- Su P., Coutts R. T., Baker G. B., Daneshtalab M. Analysis of imipramine and three metabolites produced by isozyme CYP2D6 expressed in a human cell line. Xenobiotica. 1993 Nov;23(11):1289–1298. doi: 10.3109/00498259309059439. [DOI] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Watkins P. B., Turgeon D. K., Saenger P., Lown K. S., Kolars J. C., Hamilton T., Fishman K., Guzelian P. S., Voorhees J. J. Comparison of urinary 6-beta-cortisol and the erythromycin breath test as measures of hepatic P450IIIA (CYP3A) activity. Clin Pharmacol Ther. 1992 Sep;52(3):265–273. doi: 10.1038/clpt.1992.140. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Stevens J. C. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22(1):1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- Yamazaki H., Guo Z., Persmark M., Mimura M., Inoue K., Guengerich F. P., Shimada T. Bufuralol hydroxylation by cytochrome P450 2D6 and 1A2 enzymes in human liver microsomes. Mol Pharmacol. 1994 Sep;46(3):568–577. [PubMed] [Google Scholar]
- Yokota H., Tamura S., Furuya H., Kimura S., Watanabe M., Kanazawa I., Kondo I., Gonzalez F. J. Evidence for a new variant CYP2D6 allele CYP2D6J in a Japanese population associated with lower in vivo rates of sparteine metabolism. Pharmacogenetics. 1993 Oct;3(5):256–263. doi: 10.1097/00008571-199310000-00005. [DOI] [PubMed] [Google Scholar]


